
Expired activity
Please go to the PowerPak
homepage and select a course.
Advances in Age-Related Macular Degeneration and Diabetic Macular Edema: Pharmacist Focus on Emerging Therapies
INTRODUCTION
Age-related macular degeneration (AMD) and diabetic macular edema (DME) are distinct ophthalmic conditions of the macula (center of the retina) that adversely affect vision and can lead to total vision loss. Although the conditions each have their own unique pathology, they share commonalities in pathophysiology (eg, degradation of the blood-retinal barrier leading to fluid accumulation and eventual deterioration of the macula), which appears to be linked to inflammation where vascular endothelial growth factor (VEGF) plays a major role in disease onset and progression.1 VEGF promotes the inflammatory response in AMD and DME by increasing vascular permeability at the blood-retinal barrier, while blockage of VEGF signaling pathways decrease macular edema.1 As a result, established and emerging treatments for AMD and DME focus on anti-VEGF pharmacological therapies.
AGE-RELATED MACULAR DEGENERATION OVERVIEW
The hallmark of AMD is progressive vision loss, particularly central vision, and the rate of vision loss varies by individual. A number of factors influence the potential risk to develop AMD. Major demographic characteristics include age, gender, and race/ethnicity.2,3 Environmental and behavioral factors include obesity (body mass index [BMI] >30), smoking (2-4 times more likely to develop AMD), unhealthy lifestyle, and low dietary intake of several vitamins and nutrients.2,4 Researchers are also identifying genetic and molecular risk factors to use in predictive models.4
Pathophysiology and Etiology
While the name of the condition clearly indicates that age is the predominant factor in the development and progression of AMD, a number of other factors and cellular processes (eg, inflammation, drusogenesis, lipofuscinogenesis, neovascularization) play important roles.5 The role of inflammation in AMD, an increasingly common suspect in many disease states, may also be linked via the complement cascade and drusogenesis, which is the process of developing drusen (lipid deposits) that are hallmarks of AMD. Lipofuscinogenesis refers to the process of accumulating lipofuscin, a pigment generated by cellular metabolism that accumulates in lysosomes. The growth of blood vessels (neovascularization) under the macula is a sign of wet AMD (described below), and this process may also be affected by inflammation. As a regulator of vascular permeability, VEGF is a major driver of AMD processes and is the target of effective pharmacotherapies.5,6
Based on the progression and severity of the disease process, AMD can be classified as early, intermediate, or advanced. These classifications are based on the presence and size of drusen that appear under the retina and contribute to the progressive loss of central vision.5,7,8 AMD is also differentiated into “wet” or “dry,” although this nomenclature is increasingly being supplanted by neovascular AMD (nAMD; primarily the exudative stage) for “wet AMD” and geographic atrophy for “dry AMD.”8
Epidemiology
Prevalence
Per the National Eye Institute of the National Institutes of Health (NIH), the overall 2010 prevalence rate of AMD was 2.09%.3 The prevalence rate varies based on age and race/ethnicity. For example, the 2010 prevalence of AMD in those 50 to 54 years of age was 0.36%, while the rate was 11.73% in persons over 80 years old. The prevalence rates for all persons over 50 years old based on race/ethnicity highlight that White persons are at greater risk of AMD with a rate of 2.5% compared to rates ranging from 0.90% to 0.94% for other races/ethnicities (FIGURE 1).3 Prevalence rates also vary based on gender, potentially affected by longer life expectancy of females, whereby 65% of 2010 cases of AMD were in females and 35% in males.3 While 2.07 million cases of AMD were identified in 2010, the number is projected to increase to 3.66 million in 2030 and 5.44 million in 2050.3
| FIGURE 1. National Eye Institute 2010 Prevalence Rates of AMD3 |
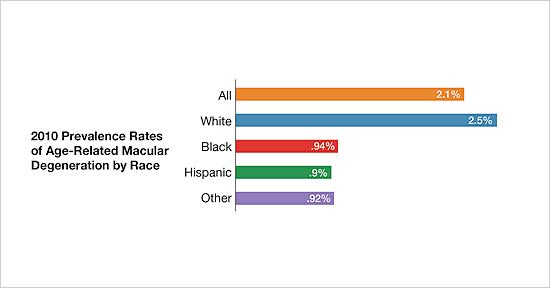 |
Global numbers of AMD cases and estimates of future cases highlight the burden of the condition. Analyses by Wong et al, published in 2014, estimated a worldwide prevalence of 8.7% and projected 196 million cases in 2020, rising to 288 million cases in 2040, with greater numbers projected for Asia based on the higher population numbers.9 In addition, AMD is a leading cause of blindness from 6% to 9% of cased globally translating to approximately 1.8 million cases of blindness in 2020.10,11 In some countries the impact may be even greater where AMD accounted for 54% cases of blindness in Caucasian persons in the US (2004 estimate) and 50% of similar cases in the United Kingdome (2013 estimate).11 Of note, the prevalence of blindness associated with AMD declined by 28% (95% uncertainty interval [UI], -30·0 to -25·6) from 1990 to 2020, and this decline may be attributed to the use of anti-VEGF pharmacotherapy.10
Incidence
In their prospective cohort study using data from the Multi-Ethnic Study of Atherosclerosis, Fisher et al analyzed the incidence of AMD across racial/ethnic groups.12 They determined an overall incidence (8-year age and sex standardized) of 4.1% for early and 2.3% for late AMD. When delineating between racial/ethnic groups, the lowest incidence was found in Blacks (1.6% for early AMD, 0.4% for late AMD) followed by Hispanics (3.3% for early AMD, 0.8% for late AMD), then Chinese (4.5% for early AMD, 2.2% for late AMD), and the highest incidence in Whites (5.3% for early AMD, 4.1% for late AMD). An explanation for the observed variations was not determined by the available data in their study.12 Similar results have been reported in other studies.
DIABETIC MACULAR EDEMA OVERVIEW
With upwards of 13% of US adults having diabetes,13 the impact of diabetic complications correspondingly increases. Diabetic eye diseases (eg, diabetic retinopathy, DME, anterior ischemic optic neuropathy, retinal vein occlusion) are some of the more common long-term complications of diabetes, affecting up to 33% of persons with diabetes.14 DME—retinal thickening of the macula with edema—is the leading cause of vision loss in persons with diabetes.14,15 Prevention strategies (eg, long-term management of blood glucose, blood pressure, and cholesterol; annual eye exams; healthy diet and exercise habits) may help to mitigate the risk of developing DME. Such prevention strategies, particularly annual eye exams, are important since early stages of DME are asymptomatic.14 Similar to AMD, VEGF-mediated inflammation plays a major role in DME, and anti-VEGF pharmacotherapies are critical tools for treating the condition.
Pathophysiology and Etiology
DME can be defined by the amount of retinal thickening (≥250 𝜇m) and manifests from several pathophysiological processes that damage the retinal endothelium, thus impairing the blood-retinal barrier.15,16 The hyperglycemia of diabetes drives metabolic changes that lead to the disruption of the blood-retinal barrier and VEGF plays a major role in that process (FIGURE 2).17 Since a substantial percentage of DME cases are refractory to anti-VEGF treatment, the complex etiology of DME may be split into vasogenic and inflammatory.17
| FIGURE 2. Developmental Pathways of DME17 |
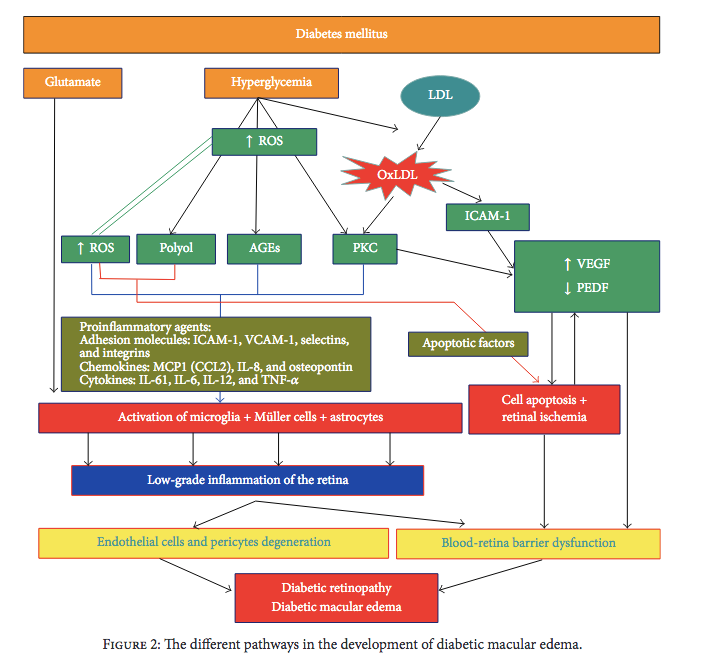 |
Abbreviations: AGEs: advanced glycation end-products; DME, diabetic macular edema; ICAM-1, intercellular adhesion molecule-1; IL- X, interleukin-X; LDL, low-density lipoprotein; MCP1 (CCL2), monocyte chemoattractant protein-1 (C-C motif ligand 1); OxLDL, oxidized low-density lipoprotein; PEDF, pigment epithelium-derived factor; PKC, protein kinase C; ROS, reactive oxidative species; TNF-α, tumor necrosis factor-alpha; VCAM-1, vascular cellular adhesion molecule; VEGF, vascular endothelial growth factor.
Copyright © 2016 Pedro Romero-Aroca et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
Epidemiology
A major driver of the incidence and prevalence of DME is the increasing prevalence of diabetes in the US and worldwide. The National Diabetes Statistic of 2020 by the Centers for Disease Control and Prevention (CDC) estimated that, in 2018, 13.0% of US adults (≥18 years of age) and 26.8% for US adults who are 65 years of age or older had diabetes (includes diagnosed and undiagnosed).13 In addition, the prevalence of diabetes (undiagnosed and diagnosed) in US adults 18 years of age and older has increased by approximately 26% between the years 1999 and 2016—from 9.5% (95% CI, 8.7-10.4) to 12.0% (95% CI, 11.1-12.9).13 The prevalence rates of DME vary between type 1 and type 2 diabetes. Estimates of DME in persons with type 1 diabetes range from 14.0% to 20.1%, while the estimates for persons with type 2 diabetes range from 6% to 25.4%.14,15
Current and Emerging Pharmacotherapy Approaches
Research into the pathophysiology of AMD and DME has been the driving force for developing pharmacotherapy options. Prior to the introduction of anti-VEGF drugs, the prevailing treatments for DME and AMD (primarily nAMD) focused on photodynamic therapy, which can only reduce the rate of vision loss.6,18 Anti-VEGF drugs, on the other hand, can achieve levels of vision restoration and are estimated to decrease blindness due to nAMD by approximately 50%. A summary of the currently approved drugs for nAMD and DME are found in TABLE 1.
| TABLE 1 . FDA-Approved Drugs for nAMD and DME19,20,25,27,35,50 |
| Drug |
Indication (nAMD/DME) |
Dose |
Frequency |
| Dexamethasone |
DME |
0.7 mg |
Prescribing information does not specify frequency of dosing |
| Fluocinolone acetonide |
DME |
0.19 mg |
Every 36 months |
| Pegaptanib |
nAMD |
0.3 mg |
Every 6 weeks |
| Ranibizumab |
nAMD/DME |
nAMD: 0.5 mg
DME: 0.3 mg |
nAMD: monthly
DME: monthly |
| Aflibercept |
nAMD/DME |
nAMD: 2 mg
DME: 2 mg |
nAMD: 2 mg every 4 weeks x 3 months followed by 2 mg every 8 weeks
DME: 2 mg every 4 weeks x 5 injections followed by 2 mg every 8 weeks |
| Brolucizumab |
nAMD |
6 mg |
6 mg monthly x 3 doses followed by 6 mg every 8-12 weeks |
| Ranibizumab-nuna |
nAMD |
0.5 mg |
Monthly |
| Abbreviations: DME, diabetic macular edema; nAMD, neovascular age-related macular degeneration. |
Existing Agents With Clinical Trial Evidence That Led to Approval
Steroidal Therapies (for DME)
Two intravitreal steroid injectables are currently approved by the FDA for DME. The active ingredient in these products are dexamethasone or fluocinolone acetonide, and each agent uses a controlled-release polymer system to deliver the drugs.19,20 The dexamethasone product uses the biodegradable polymer, poly(D,L-lactide-co-glycolide), which ultimately erodes to lactic acid and glycolic acid.19 Clinical studies suggest that dexamethasone intravitreal injection can improve vision-related outcomes including best-corrected visual acuity, central retinal thickness, and fluorescein leakage.21,22 The fluocinolone acetonide product uses a nondegradable polymer insert to deliver the drug.20 Clinical studies also suggest that fluocinolone acetonide delivered via intravitreal insert can improve best-corrected visual acuity and foveal thickness.23,24
Intravitreal Anti-VEGF Therapies
Pegaptanib
The first anti-VEGF therapy to be approved by the FDA for nAMD was pegaptanib, a PEGylated RNA oligonucleotide aptamer, in 2004.25 The VEGF Inhibition Study in Ocular Neovascularization (VISION) Clinical Trials provided the data for FDA approval in which a greater percentage of patients receiving pegaptanib by intravitreal injection every 6 weeks for 48 weeks maintained visual acuity compared to patients receiving a sham injection.26 The effects of pegaptanib were not dose-dependent. The rate of visual acuity loss as quantitated by the percentage of patients with loss of fewer than 15 letters was 55% in the sham injection group, while results in the treatment groups were 70% (0.3-mg dose, P < .001), 71% (1.0-mg dose, P < .001), and 65% (3.0-mg dose, P = .03). Patients treated with pegaptanib also displayed improvements in other vision-related outcomes including reduced risk of severe visual acuity loss and reduced risk of progression to legal blindness.26
Ranibizumab
Ranibizumab, a recombinant humanized monoclonal antibody fragment, became the second anti-VEGF therapy approved by the FDA for nAMD in 2006, with other indications for DME, macular edema from retinal vein occlusion, diabetic retinopathy, and myopic choroidal neovascularization.27 The efficacy of ranibizumab for nAMD was established in the Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration (MARINA) and Anti- VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-related Macular Degeneration (ANCHOR) trials.28,29 In the MARINA study at the 24-month endpoint, 92.0% of patients receiving 0.3 mg of ranibizumab and 90.0% of patients receiving 0.5 mg of ranibizumab lost fewer than 15 letters from baseline visual acuity compared to 52.9% of those in the sham injection group (P < .001 for both treatment groups compared to sham group).28 The ANCHOR study compared ranibizumab to an active control—verteporfin photodynamic therapy. Greater percentages of patients treated with 0.3 mg or 0.5 mg of ranibizumab lost fewer than 15 letters of visual acuity (90.0% and 89.9%, respectively) at the 24-month endpoint compared to 65.7% of patients in the control group.29
Aflibercept
Approved by the FDA in 2011 for nAMD, DME, diabetic retinopathy, and macular edema from retinal vein occlusion, aflibercept is a recombinant fusion protein with the soluble receptor domains of VEGR receptor 1 (VEGFR1) and VEGFR2 linked to the core molecule of human immunoglobulin G1 fragment (IgG1 Fc).30 Efficacy of aflibercept was determined in the VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD (VIEW 1 and VIEW 2) clinical trials using ranibizumab as the active control.31 Aflibercept was studied at 3 dosing regimens—0.5 mg every 4 weeks, 2 mg every 4 weeks, and 2 mg every 8 weeks—while ranibizumab was dosed at the recommended 0.5 mg every 4 weeks.31 Testing of the extended dosing interval of 8 weeks aimed to reduce the burden and risk of monthly injections. With an active control arm, the primary endpoint of the VIEW 1 and VIEW 2 studies was noninferiority of aflibercept compared to ranibizumab with respect to the percentage of patients maintaining vision acuity (fewer than 15 letters from baseline) at 52 weeks, and all 3 aflibercept dosing regimens achieved the primary endpoint. In both studies, 94.4% of ranibizumab (0.5 mg every 4 weeks) maintained vision acuity while the results for aflibercept were (VIEW 1 and VIEW 2 data, respectively): 95.9% and 96.3% for 0.5 mg every 4 weeks; 95.1% and 95.6% for 2 mg every 4 weeks; and 95.1% and 95.6% for 2 mg every 8 weeks.31
Aflibercept was also studied in DME with laser coagulation therapy as an active control in the Study of Intravitreal Aflibercept Injection in Patients With Diabetic Macular Edema (VISTADME) and Intravitreal Aflibercept Injection in Vision Impairment Due to DME (VIVIDDME) studies.32-34 In the analysis of 148 week results (original studies analyzed data at 52 weeks and 100 weeks),32,33 aflibercept at 2 mg every 4 weeks or every 8 weeks improved baseline in best-corrected visual acuity (measured by mean number of letters increased) compared to laser coagulation therapy. At the 148 weeks, the mean improvement were (VISTA and VIVID studies, respectively): 1.4 and 1.6 letters for the laser control; 10.4 and 10.3 letters for the 2 mg every 4 week group; 10.5 and 11.7 letters for the 2 mg every 8 week group (P < .001 for each aflibercept group compared to control).34
Brolucizumab-dbll
The most recently approved anti-VEGF therapy for nAMD is brolucizumab-dbll, a humanized single-chain antibody fragment that inhibits the VEGFR-1 and VEGFR-2 receptors by binding to VEGF-A isoforms.35 Efficacy of brolucizumab was established in the Efficacy and Safety of RTH258 Versus Aflibercept–Study 1 (HAWK) and Efficacy and Safety of RTH258 Versus Aflibercept–Study 2 (HARRIER) clinical trials, which used aflibercept as an active control.36 Brolucizumab was studied at 3-mg (HAWK only) and 6-mg doses using loading doses of 3 monthly injections followed by every 12 weeks dosing that was modified to every 8 week dosing if researchers observed disease progression in patients while the active control arm received 2 mg of aflibercept every 8 weeks. The studies concluded that brolucizumab was noninferior compared to aflibercept at 48 weeks with respect to the mean best-corrected visual acuity change from baseline as follows: HAWK (+6.8 letters for aflibercept, +6.1 letters for brolucizumab 3 mg, +6.6 letters for brolucizumab 6 mg) and HARRIER (+7.6 letters for aflibercept, +6.9 letters for brolucizumab 6 mg); P < .001 for all comparisons to active control.36
Treat-and-Extend and As-needed Approaches
The clinical trials for the anti-VEGF therapies used fixed-dose intervals for treatments, some of which were adjusted based on disease progression. The frequency of treatments for anti-VEGF therapies imposes burdens on patients and caregivers. The burdens include direct costs for the treatments and travel as well as indirect costs such as reduced productivity (or possibly salary) due to time off from work.37,38 The increased dual burdens of costs and frequent visits can contribute to nonadherence with treatments, which can contribute to decreased efficacy of nAMD therapy.39 Efforts to reduce the burdens associated with anti-VEGF intravitreal therapy include extending treatment intervals either by as-needed approach or treat-and-extend (T&E) approaches.40,41 Data from the retrospective study by Skelly et al,42 the prospective Aflibercept Treat and extend for Less frequent Administration Study (ATLAS),43 and Canadian Treat-and-Extend Analysis Trial With Ranibizumab in Patients With Neovascular Age-Related Macular Disease (CANTREAT)40 studies suggest that a majority of patients requires T&E dosing intervals of <12 weeks (69%, 62%, and 57%, respectively). 40,42,43 The mean numbers of injections that patients received over a 2-year period were 13.5 for Skelly et al, 14.5 for ATLAS, and 17.6 for CANTREAT.40,42,43 While these studies suggest that dosing frequency may be reduced while maintaining desirable outcomes, improvements in treatment paradigms would be welcomed.
Emerging Therapies
Faricimab
One of the therapies currently in development is faricimab, which is unique in that it is an antibody drug with two targets—VEGF-A and angiopoietin-2 (Ang-2) isoform. The anti-VEGF-A fragment targets the well-known VEGF pathway while adding an anti-Ang-2 fragment that targets Ang-2 of the angiopoietin-tyrosine kinase endothelial (Ang-Tie) receptors pathway.44,45 The Ang-Tie pathway appears to be involved in vascular homeostasis and permeability, while adding an additional biochemical target may increase efficacy while decreasing dosing frequency.45 Recently, the FDA accepted the Biologics License Applications for faricimab in treating nAMD and DME based on preliminary results suggest that faricimab at dosing intervals of every 3 to 4 months produces similar visual acuity gains to aflibercept dosed at every 2-month intervals.46
Port Delivery System With Ranibizumab
Innovative drug delivery devices may also increase efficacy while decreasing dosing frequency. An implantable port delivery system (PDS) that can be refilled is currently under investigation using ranibizumab as the active ingredient.47 Inserted posterior to the limbus of the eye, the implant is not visible to the patient and can be refilled at intervals customized to the patient. Results from a phase 2 study compared monthly ranibizumab monthly intravitreal injections (0.5 mg) to 3 concentrations of ranibizumab in the PDS (10 mg/mL, 40 mg/mL, or 100 mg/mL).47 The concentration of ranibizumab affected the median time to first refill of the insert with 8.7, 13.0, and 15.0 months for the 10-mg/mL, 40-mg/mL, and 100-mg/mL cohorts, respectively. Patients in the 100-mg/mL group had the best response in the PDS cohorts at month 9. The adjusted mean best-corrected visual acuity from baseline results were -3.2 letters, -0.5 letters, and +5.0 letters for the 10-mg/mL, 40-mg/mL, and 100-mg/mL cohorts, respectively, compared to +3.9 letters for the ranibizumab 0.5-mg monthly injection cohort.47
Ranibizumab-nuna and Biosimilars
Since many of the anti-VEGF drugs are biologics (ie, large-molecular-weight drugs produced by biotechnology), biosimilars of those products are expected to become available for use in the US. The term, biosimilars, describes a regulatory class of drug products that are developed and produced to have no “clinically meaningful” differences compared to the reference biologic in regard to safety, purity, and potency while also having similarity to the reference biologic with respect to efficacy.48 Recently, the FDA approved the first biosimilar for nAMD. Ranibizumab-nuna, a biosimilar of ranibizumab, was approved with indications for nAMD, macular edema following retinal vein occlusion, and myopic choroidal neovascularization.49,50
The anti-VEGF monoclonal antibody drug, bevacizumab, is currently approved for a limited set of specific cancers.51 With VEGF as bevacizumab’s target, the high price tags of anti-VEGF therapies, and the availability of bevacizumab biosimilars, ophthalmologists became interested in the off-label use of bevacizumab for nAMD.52 Reports suggest that some commercial payer have also pushed for the off-label use of bevacizumab biosimilars, although the American Academy of Ophthalmology registered its opposition based on lack of clinical testing in nAMD and concerns about formulation ingredients in some products.53 In an apparent attempt to fill the void of bevacizumab ophthalmologic formulations, bevacizumab-vikg (ONS-5010) is currently under investigation for nAMD, DME, and branch retinal vein occlusion.54
Gene Therapies, Advanced Drug Delivery Systems, and New Molecular Targets
Ongoing research into the next generation of nAMD and DME therapies include gene therapy, advanced drug delivery systems or drug-polymer conjugates to decrease dosing frequency, and new molecular targets to increase efficacy of treatments. Gene therapy approaches have the potential for one time treatments of nAMD, DME, and related conditions.55 One such gene therapy currently in phase 2 trials, is RGX-314 for subretinal injection, which delivers the gene for a soluble anti-VEGF monoclonal antibody fragment similar to ranibizumab.55,56 Another gene therapy in early stages of development, ADVM-022, follows a similar approach as it is designed to deliver the gene for aflibercept.55,57
Extending the time between doses may be achievable through advanced drug delivery systems. One such approach, KSI-301, combines an anti-VEGF monoclonal antibody with a high-molecular-weight phosphorylcholine polymer, and phase 1b results suggest that the approach can extend treatment intervals to every 4 to 6 months.58 Another drug delivery approach, GB-102, uses a tyrosine kinase inhibitor (TKI), sunitinib maleate, encapsulated in biodegradable nanoparticles with the goal of twice yearly injections. On the horizon will likely be additional therapeutics that focus on new(er) targets for nAMD and DME. These include the previously mentioned Ang-Tie pathway and the platelet derived growth factor pathway.55,58
CONSIDERATION FOR PHARMACISTS
With an understanding of current and emerging pharmacotherapies, pharmacists can apply their other health care roles to persons with nAMD and DME. While there is a dearth of specific data on the impact of pharmacist intervention on nAMD and DME, the study by Weber et al suggest that pharmacist management of cardiovascular risk in persons with diabetes may provide benefits in progression towards diabetic retinopathy.59 Other major pharmacist roles will continue to be important in overall disease management for persons with nAMD or DME.
Patient-Centered Therapy Selection
Patients under treatment for nAMD and DME may benefit from a patient-centered approach to therapy selection, so a brief synopsis on that approach would be beneficial. Along with a host of prior research, clinicians at the Department of Family Medicine at Western University in Ontario, Canada, popularized and championed the idea of patient-centered clinical method, a style of medical practice that shifts the paradigm of the physician-patient relationship. As the phrase specifies, the patient becomes the focus or reference point with the clinician empowering the patient with shared decision-making.60 The patient-centered clinical method can be summarized by the 4 interactive components and their corresponding goals59:
- Exploring Health, Disease, and the Illness Experience
Goal: Understand each patient’s unique perception of health and illness
- Understanding the Whole Person
Goal: Integrate clinical concepts with an understanding of the whole person
- Finding Common Ground
Goal: Define the medical problem, establish goals of treatment, and identify the roles to be assumed by the patient and clinician
- Enhancing the Patient-Centered Relationship
Goal: Build on the patient-clinician relationship with compassion, empathy, a sharing of power, healing, and hope.59
Implementing patient-centered practices, including therapy selection, can improve several health outcomes including adverse drug events.61,62 The range of current and emerging therapy options for AMD and DME provides an excellent opportunity to put patient-centered therapy selection into practice, particularly considering the burdens borne by patients and caregivers in regard to clinic visits for treatments.
Patient Education and Improvement of Adherence
Putting patient-centered therapy selection into practice will also require effective patient and caregiver education on available therapies. The frequency of office/clinic visits and the need for a caregiver to transport patients for intravitreal treatments puts substantial burdens on the patient and caregiver(s). Both patients and caregivers often have to take time off from work, which can lead to lost income and other burdens.37,38 The hurdles experienced by patients and the caregivers on whom they rely often lead to undertreatment where over half of patients lost treatment are often lost to treatment at the 5-year mark.63,64
In their study of patients receiving ranibizumab, Boulanger-Scemama et al found that more than half (57%) of study subjects were lost to follow up after 5 years with the main reasons for discontinuation of therapy being distance between home and clinic (51.7%), dissatisfaction with therapeutic outcomes (34.5%), and the overall burden of frequency of follow-up visits (24.1%).63 In a larger study of 9007 patients, Obeid et al found 22.2% of patients lost to follow-up with major factors in discontinuation of increased age, race other than white, lower gross income, longer distance to clinic, and disease involvement in a single eye.65
Pharmacists can apply a range of general strategies, which are not specific to nAMD or DME, to help improve patient education and improve adherence to treatment. After identifying at-risk patients, pharmacists can advise patients to schedule routine eye exams (particularly important for persons with diabetes). Counseling and patient education are important components of the pharmacist’s duties, where those activities can constitute 9% to 37% of a pharmacist’s workday.66 Once a therapy option is determined, pharmacists can continue to encourage patients to adhere to treatment and they can monitor therapy for safety and efficacy. Often, pharmacists will assist patients with navigating the financial aspects of pharmacotherapy, including those described below.
Financial Considerations
The high costs of nAMD and DME therapies directly impact patients as well as Medicare and Medicaid.41 On the individual side of the equation, patients will likely continue to see options to lower out-of-pocket expenses. Patient assistance programs include those directly from biopharmaceutical manufacturers as well as programs from independent organizations, many of which are nonprofits that may receive some funding from biopharmaceutical manufacturers. The Foundation of the American Society of Retina Specialists provides a list of patient assistance programs applicable for persons with nAMD or DME (https://www.asrs.org/patients/patient-assistance-resources).67 Patients can also feel the financial impact when Medicare drug costs rise via increased out-of-pocket expenses.68 In addition, since the anti-VEGF drugs are administered by a physician, they are generally covered under Medicare Part B where patient copayments are typically 20% of the drug cost plus the cost of physician services.69
Formulary and Payor Considerations
On the payor/formulary side of the drug pricing equation, the average wholesale prices (AWPs) of the anti-VEGF therapies are good starting points to consider. According to the Micromedex Red Book, the per-dose AWP (as of September 8, 2021) for anti-VEGF drugs were: $2220 per 2-mg/0.05-mL dose of aflibercept, $2220 per 6 mg/0.05 mL of brolucizumab, and $2340 per 0.5 mg/0.05 mL of ranibizumab.70 With those high prices, the off-label use of bevacizumab may be unsurprising given its AWP of approximately $50 to $75 per 1.25-mg dose.70 A recent change to Medicare policies may also impact payor/formulary considerations as well as patient costs and outcomes. Starting in January 2019, the Centers for Medicare & Medicaid Services (CMS) granted Medicare Advantage plans the ability to implement step therapy for Part B drugs, such as anti-VEGF drugs.71 This change in policy, ostensibly implemented to lower drug costs, raised concerns among ophthalmologists that implementing step therapy requirements could negatively impact patients, by delaying optimal therapy.41 Although anti-VEGF therapy is typically administered in the office setting, payors may utilize specialty pharmacies to provide the injections to providers rather than allowing the process of buy and bill to occur at the provider level.
Prior Authorizations
As long as prior authorizations are required by payors for certain medications, specific strategies may be helpful to streamline and improve the processes. Towards that end, an Academy of Managed Care Pharmacy (AMCP) forum drafted recommendations to improve prior authorization policies and procedures.72 The forum included stakeholders from a range of perspectives including academia, health plans, integrated delivery systems, pharmacy benefit managers, employers, federal government agencies, national health care provider organizations, and patient advocacy organizations. The recommendations of the forum participants are summarized in 9 categories72:
- Patient safety and appropriate medication use
- Clinical decision-making
- Evidence-based review criteria
- Automated decision support
- Transparency and advanced notice
- Emergency access
- Provider collaboration
- Need for timeliness and avoiding disruptions in therapy
- Cost-effectiveness and value.72
A group of industry and professional organizations similarly gathered to suggest areas of improvement in the prior authorization process. They identified 5 such areas: selective application of prior authorization; prior authorization program review and volume adjustment; transparency and communication regarding prior authorization; continuity of patient care; and automation to improve transparency and efficiency.73
Whether or not such efforts lead to actual improvement will be determined with time. In the meantime, pharmacists and other health care professionals will continue to navigate the prior authorization process. Managing prior authorization can subsume extensive amounts of resources in time, personnel, and other costs. Cases studies suggest that pharmacy professionals can provide substantial benefits when they manage prior authorizations. In a recent published case focusing on the role of specialty pharmacy, Hecht et al demonstrated that pharmacists can significantly reduce the prior authorization decision time and the time to first fill.74 When managed by the specialty pharmacy, the mean prior authorization time (1.9 days) and the mean time to first prescription fill (6.6 days) were significantly shorter when compared to the times when managed by a dermatology provider’s office (mean prior authorization time of 20.9 days, P < .001; mean time to first prescription fill of 16.2 days, P < .001).74
SUMMARY
The advent of anti-VEGF therapies shepherded in a new era in the treatment of nAMD and DME with 5 FDA-approved anti-VEGF drugs—pegaptanib, ranibizumab, aflibercept, brolucizumab, and the biosimilar ranibizumab-nuna. Ongoing research into the pathophysiology of the conditions and novel drug delivery systems are aimed at decreasing the number of doses and/or dose frequency of the intravitreal injections to increase adherence and increase therapeutic outcomes. Pharmacist knowledge of the current and emerging therapies for nAMD and DME is critical for providing optimal patient care.
REFERENCES
- Das UN. Diabetic macular edema, retinopathy and age-related macular degeneration as inflammatory conditions. Arch Med Sci. 2016;12(5):1142-1157. doi: 10.5114/aoms.2016.61918
- Lim LS, Mitchell P, Seddon JM, et al. Age-related macular degeneration. Lancet. 2012;379(9827):1728-1738. doi: 10.1016/S0140-6736(12)60282-7
- National Eye Institute. Age-related macular degeneration (AMD) data and statistics. Published 2019. Accessed September 30, 2021. https://www.nei.nih.gov/learn-about-eye-health/outreach-campaigns-and-resources/eye-health-data-and-statistics/age-related-macular-degeneration-amd-data-and-statistics
- Heesterbeek TJ, Lorés-Motta L, Hoyng CB, et al. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol Opt. 2020;40(2):140-170. doi: 10.1111/opo.12675
- Patel P, Sheth V. New and innovative treatments for neovascular age-related macular degeneration (nAMD). J Clin Med. 2021;10(11):2436. doi: 10.3390/jcm10112436
- Adamis AP, Brittain CJ, Dandekar A, Hopkins JJ. Building on the success of anti-vascular endothelial growth factor therapy: a vision for the next decade. Eye. 2020;34(11):1966-1972. doi: 10.1038/s41433-020-0895-z
- VanDenLangenberg AM, Carson MP. Drusen bodies. In: StatPearls [Internet]. StatPearls Publishing; 2021. Updated May 9, 2021. Accessed September 19, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559087/
- Fleckenstein M, Keenan TDL, Guymer RH, et al. Age-related macular degeneration. Nat Rev Dis Primers. 2021;7(1):31. doi: 10.1038/s41572-021-00265-2
- Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Heal. 2014;2(2):e106-e116. doi:10.1016/S2214-109X(13)70145-1
- GBD 2019 Blindness and Vision Impairment Collaborators, Vision Loss Expert Group of the Global Burden of Disease Study; Bourne RRA, Steinmetz JD, Saylan M, et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e144-e160. doi:10.1016/S2214-109X(20)30489-7
- Keenan TDL, Cukras CA, Chew EY. Age-related macular degeneration: epidemiology and clinical aspects. Adv Exp Med Biol. 2021;1256:1-31. doi:10.1007/978-3-030-66014-7_1
- Fisher DE, Klein BEK, Wong TY, et al. Incidence of age-related macular degeneration in a multi-ethnic United States population: the multi-ethnic study of atherosclerosis. Ophthalmology. 2016;123(6):1297-1308. doi:10.1016/j.ophtha.2015.12.026.
- Centers for Disease Control and Prevention (CDC). National Diabetes Statistics Report, 2020. Published 2020. Accessed October 6, 2021. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- Holekamp NM. Overview of diabetic macular edema. Am J Manag Care. 2016;22(10 suppl):s284-s291.
- Bandello F, Battaglia Parodi M, Lanzetta P, et al. Diabetic macular edema. Dev Ophthalmol. 2017;58:102-138. doi:10.1159/000455277
- Udaondo P, Adan A, Arias-Barquet L, et al. Challenges in diabetic macular edema management: an expert consensus report. Clin Ophthalmol. 2021;15:3183-3195. doi:10.2147/OPTH.S320948
- Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, et al. Diabetic macular edema pathophysiology: vasogenic versus inflammatory. J Diabetes Res. 2016;2016:2156273. doi:10.1155/2016/2156273
- Mansour SE, Browning DJ, Wong Ket al. The evolving treatment of diabetic retinopathy. Clin Ophthalmol. 2020;14:653-678. doi:10.2147/OPTH.S236637
- Ozurdex (dexamethasone intraviteal implant). Package insert. Allergan USA, Inc; 2020.
- Iluvien (fluocinolone acetonide intraviteal implant) Package insert. Alimera Sciences, Inc; 2016.
- Haller JA, Kuppermann BD, Blumenkranz MS, et al. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128(3):289-296. doi:10.1001/archophthalmol.2010.21
- Boyer DS, Yoon YH, Belfort R, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904-1914. doi:10.1016/j.ophtha.2014.04.024
- Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125-2132. doi:10.1016/j.ophtha.2012.04.030
- Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626-635.e2. doi:10.1016/j.ophtha.2010.12.028
- Macugen (pegaptanib). Package insert. Bausch & Lomb, Inc; 2016.
- Gragoudas ES, Adamis AP, Cunningham ET, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805-2816. doi:10.1056/NEJMoa042760
- Lucentis (ranibizumab). Package insert. Genentech, Inc; 2018.
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431. doi:10.1056/nejmoa054481
- Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57-65. doi:10.1016/j.ophtha.2008.10.018
- Eylea (aflibercept). Package insert. Regeneron Pharmaceuticals, Inc; 2021.
- Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-2548. doi:10.1016/j.ophtha.2012.09.006
- Korobelnik JF, Do D V., Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247-2254. doi:10.1016/j.ophtha.2014.05.006
- Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122(10):2044-2052. doi:10.1016/j.ophtha.2015.06.017
- Heier JS, Korobelnik JF, Brown DM, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology. 2016;123(11):2376-2385. doi:10.1016/j.ophtha.2016.07.032
- Beovu (brolucizumab-dbll). Package insert. Novartis Pharmaceuticals Corporation; 2020.
- Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72-84. doi:10.1016/j.ophtha.2019.04.017
- McCloud C, Lake S. Understanding the patient’s lived experience of neovascular age-related macular degeneration: a qualitative study. Eye. 2015;29(12):1561-1569. doi:10.1038/eye.2015.167
- Gohil R, Crosby-Nwaobi R, Forbes A, et al. Caregiver burden in patients receiving ranibizumab therapy for neovascular age related macular degeneration. PLoS One. 2015;10(6). doi:10.1371/journal.pone.0129361
- Ricci F, Bandello F, Navarra P, et al. Neovascular age-related macular degeneration: therapeutic management and new-upcoming approaches. Int J Mol Sci. 2020;21(21):1-40. doi:10.3390/ijms21218242
- Kertes PJ, Galic IJ, Greve M, et al. Efficacy of a treat-and-extend regimen with ranibizumab in patients with neovascular age-related macular disease: a randomized clinical trial. JAMA Ophthalmol. 2020;138(3):244-250. doi:10.1001/jamaophthalmol.2019.5540
- Holekamp NM. Review of neovascular age-related macular degeneration treatment options. Am J Manag Care. 2019;25(10 suppl):S172-S181.
- Skelly A, Bezlyak V, Liew G, et al. Treat and extend treatment interval patterns with anti-VEGF therapy in nAMD patients. Vision (Basel). 2019;3(3). doi:10.3390/vision3030041
- DeCroos FC, Reed D, Adam MK, et al. Treat-and-extend therapy using aflibercept for neovascular age-related macular degeneration: a prospective clinical trial. Am J Ophthalmol. 2017;180:142-150. doi:10.1016/j.ajo.2017.06.002
- Regula JT, Lundh von Leithner P, Foxton R, et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol Med. 2016;8(11):1265-1288. doi:10.15252/emmm.201505889
- Nicolò M, Ferro Desideri L, Vagge A, Traverso CE. Faricimab: an investigational agent targeting the Tie-2/angiopoietin pathway and VEGF-A for the treatment of retinal diseases. Expert Opin Investig Drugs. 2021;30(3):193-200. doi:10.1080/13543784.2021.1879791
- FDA accepts application for Genentech’s faricimab for the treatment of wet age-related macular degeneration (AMD) and diabetic macular edema (DME). Business Wire. Published July 29, 2021. Accessed October 6, 2021. https://www.businesswire.com/news/home/20210728006055/en/FDA-Accepts-Application-for-Genentech’s-Faricimab-for-the-Treatment-of-Wet-Age-Related-Macular-Degeneration-AMD-and-Diabetic-Macular-Edema-DME
- Campochiaro PA, Marcus DM, Awh CC, et al. The Port Delivery System with ranibizumab for neovascular age-related macular degeneration: results from the randomized phase 2 Ladder clinical trial. Ophthalmology. 2019;126(8):1141-1154. doi:10.1016/j.ophtha.2019.03.036
- Declerck P, Danesi R, Petersel D, Jacobs I. The language of biosimilars: clarification, definitions, and regulatory aspects. Drugs. 2017;77(6):671-677. doi:10.1007/s40265-017-0717-1
- US Food & Drug Administration. FDA approves first biosimilar to treat macular degeneration disease and other eye conditions. Published September 17, 2021. Accessed October 8, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-biosimilar-treat-macular-degeneration-disease-and-other-eye-conditions
- Byooviz (ranibizumab-nuna). Package insert. Biogen, Inc; 2021.
- Avastin (bevacizumab). Package insert. Genentech, Inc; 2021.
- Jansen RM. The off-label use of medication: the latest on the Avastin-Lucentis debacle. Med Law. 2013;32(1):65-77.
- Hagen T. Ophthalmology group opposes payer recommendations for bevacizumab biosimilars. The Center for Biosimilars. Published August 1, 2021. Accessed October 6, 2021. https://www.centerforbiosimilars.com/view/ophthalmology-group-opposes-payer-recommendations-for-bevacizumab-biosimilars
- Outlook Therapeutics. ONS-5010/Lytenava overview. Published 2019. Accessed October 6, 2021. https://outlooktherapeutics.com/lytenava-overview/
- de Guimaraes TAC, Georgiou M, Bainbridge JWB, Michaelides M. Gene therapy for neovascular age-related macular degeneration: rationale, clinical trials and future directions. Br J Ophthalmol. 2021;105(2):151-157. doi:10.1136/bjophthalmol-2020-316195
- gov Identifier: NCT04514653. RGX-314 gene therapy administered in the suprachoroidal space for participants with neovascular age-related macular degeneration (nAMD) (AAVIATE). Updated August 21, 2020. Accessed October 6, 2021. https://clinicaltrials.gov/ct2/show/NCT04514653
- Kiss S, Grishanin R, Nguyen A, et al. Analysis of aflibercept expression in NHPs following intravitreal administration of ADVM-022, a potential gene therapy for nAMD. Mol Ther Methods Clin Dev. 2020;18:345-353. doi:10.1016/j.omtm.2020.06.007
- Hussain RM, Shaukat BA, Ciulla LM, et al. Vascular endothelial growth factor antagonists: promising players in the treatment of neovascular age-related macular degeneration. Drug Des Devel Ther. 2021;15:2653-2665. doi:10.2147/DDDT.S295223
- Weber ZA, Kaur P, Hundal A, et al. Effect of the pharmacist-managed cardiovascular risk reduction services on diabetic retinopathy outcome measures. Pharm Pract (Granada). 2019;17(1):1319. doi:10.18549/PharmPract.2019.1.1319
- Stewart M, Brown JB, Weston W, et al. Patient-Centered Medicine: Transforming the Clinical Method. 3rd ed. CRC Press; 2014.
- Poitras ME, Maltais ME, Bestard-Denommé L, et al. What are the effective elements in patient-centered and multimorbidity care? A scoping review. BMC Health Serv Res. 2018;18(1):446. doi:10.1186/s12913-018-3213-8
- di San Marco EC, Vegni E, Borghi L. Chronic illnesses, vulnerability, and uncertainty: how do recent challenges impact on patient-centered medicine? Int J Patient-Centered Healthc. 2019;9(1):50-63. Accessed October 6, 2021. https://www.researchgate.net/publication/339580551_Chronic_Illnesses_Vulnerability_and_Uncertainty_How_Do_Recent_Challenges_Impact_on_Patient-Centered_Medicine/fulltext/5e59cdc2299bf1bdb8445561/Chronic-Illnesses-Vulnerability-and-Uncertainty-How-Do-Recent-Challenges-Impact-on-Patient-Centered-Medicine.pdf
- Boulanger-Scemama E, Querques G, About F, et al. Ranibizumab for exudative age-related macular degeneration: a five year study of adherence to follow-up in a real-life setting. J Fr Ophtalmol. 2015;38(7):620-627. doi:10.1016/j.jfo.2014.11.015
- Gillies MC, Campain A, Barthelmes D, et al. Long-term outcomes of treatment of neovascular age-related macular degeneration: data from an observational study. Ophthalmology. 2015;122(9):1837-1845. doi:10.1016/j.ophtha.2015.05.010
- Obeid A, Gao X, Ali FS, et al. Loss to follow-up among patients with neovascular age-related macular degeneration who received intravitreal anti-vascular endothelial growth factor injections. JAMA Ophthalmol. 2018;136(11):1251-1259. doi:10.1001/jamaophthalmol.2018.3578
- Negaard BJ, Lyons KP, Nichol CL, Polgreen LA. What does a pharmacist do? A time and motion study. Res Soc Adm Pharm. 2020;16(9):1314-1317. doi:10.1016/j.sapharm.2019.03.007
- American Society of Retina Specialists. Patient assistance programs. Published 2021. Accessed October 8, 2021. https://www.asrs.org/patients/patient-assistance-resources
- Cubanski J, Neuman T. Price increases continue to outpace inflation for many Medicare Part D drugs. Kaiser Family Foundation (KFF). Published February 4, 2021. Accessed October 8, 2021. https://www.kff.org/medicare/issue-brief/price-increases-continue-to-outpace-inflation-for-many-medicare-part-d-drugs/
- Shah AR, Williams GA. Regulatory and economic considerations of retinal drugs. Dev Ophthalmol. 2015;55:376-380. doi:10.1159/000438965
- Average wholesale price (AWP). Micromedex Red Book. Published 2021. Accessed September 8, 2021. https://www.ibm.com/products/micromedex-red-book
- Centers for Medicare & Medicaid Services (CMS). Medicare Advantage prior authorization and step therapy for Part B drugs. Published August 7, 2018. Accessed October 8, 2021. https://www.cms.gov/newsroom/fact-sheets/medicare-advantage-prior-authorization-and-step-therapy-part-b-drugs.
- AMCP Partnership Forum: Optimizing prior authorization for appropriate medication selection. J Manag Care Spec Pharm. 2020;26(1):55-62. doi:10.18553/jmcp.2020.26.1.55
- American Medical Association. Consensus Statement on Improving the Prior Authorization Process. Published January 18, 2018. Accessed September 26, 2021. https://edhub.ama-assn.org/data/multimedia/10.1001ama.2018.0080supp1.pdf
- Hecht B, Frye C, Holland W, Holland CR, et al. Analysis of prior authorization success and timeliness at a community-based specialty care pharmacy. J Am Pharm Assoc. 2021;61(4S):S173-S177. doi:10.1016/j.japh.2021.01.001
Back to Top