
Expired activity
Please go to the PowerPak
homepage and select a course.
Optimizing Outcomes in Advanced Non-Small Cell Lung Cancer:
Focus on Novel Biomarkers and Targeted Therapies
INTRODUCTION
Lung cancer is the leading cause of cancer-related death in the United States, with an estimated 235,000 new cases in 2021 and nearly 132,000 deaths.1 Non-small cell lung cancer (NSCLC) represents approximately 85% of all these cases. Unfortunately, over 60% of such tumors are locally advanced or metastatic at diagnosis, resulting in poor outcomes.2 Use of the older “one-size-fits-all” approach with chemotherapy has proven only marginally effective in these patients and is often highly toxic. In contrast, the increasing number of oncogenic driver genomic alterations and immune biomarkers identified in advanced NSCLC has greatly increased our understanding and molecular classification of this disease.3 This has resulted in the successful clinical development of numerous agents targeting these alterations (ie, precision oncology). For many patients, this molecularly targeted approach has proven more effective than traditional systemic chemotherapy.4
The increasing number of NSCLC subtypes identified and the rapidly expanding number of available targeted agents and immunotherapies present challenges for pharmacists to remain current on these topics. Adding to this complexity is the need for biomarker testing to ensure targetable alterations are identified and that patients are appropriately matched with the appropriate targeted agent. Currently, at least 20 different therapies targeting 8 distinct molecular biomarkers are recommended by National Comprehensive Cancer Network (NCCN) clinical practice guidelines for treatment of advanced NSCLC, and these numbers are likely to increase.5
Pharmacists must be aware of current evidence-based clinical practice guidelines for targeted treatment of advanced NSCLC, since lack of adherence to guidelines is associated with poorer outcomes and higher costs.6 They must also keep abreast of the most appropriate targeted therapies, based on biomarker assessment. This monograph reviews recently approved novel therapies for advanced or metastatic NSCLC that target specific oncogenic driver genomic alterations, and recommendations regarding biomarker testing to optimize selection of the most appropriate therapy. Treatment-related adverse events and their management, as well as the importance of adherence to orally administered targeted agents, are discussed.
ESTABLISHED THERAPIES FOR ADVANCED NSCLC
Historically, platinum-based doublet chemotherapy and other chemotherapeutics have been used for treatment of advanced NSCLC. However, outcomes tended to be poor, with a median progression-free survival (PFS) of 4 to 6 months and median overall survival (OS) of less than 12 months.7,8 Advances in the identification and molecular characterization of NSCLC subtypes have led to improved understanding of the pathobiology of this disease and its subtypes. Concurrent development of inhibitors targeting these defects, including agents directed against specific genomic alterations and immune checkpoint proteins, has resulted in a gradual shift away from the use of nonspecific chemotherapy to more selective targeted therapies for patients with particular alterations. In many cases this has generated higher response rates—and in some cases, improved survival—as well as reduced toxicity compared with systemic chemotherapy. The development of next-generation NSCLC inhibitors continues to provide more effective treatment options for patients with specific alterations.9,10 Until recently, however, targeted therapies for less common alterations had been lacking.
The realization that specific molecular alterations could be effectively targeted in advanced NSCLC has driven the need for expanded biomarker testing that can provide prognostic and predictive information, and guide clinicians in the selection of the most effective therapy for each patient. These biomarkers are based on known genomic alterations (including mutations, translocations, gene fusions, and other rearrangements) and expression of immune checkpoint proteins.
CURRENT TREATMENT GUIDELINES FOR FIRST-LINE THERAPY OF ADVANCED NSCLC
For patients with advanced or metastatic NSCLC and confirmed genomic alterations, current NCCN treatment guidelines recommend therapeutic options in the first-line setting and beyond based on molecular alterations that may be present as detected by biomarker testing.5 The number of recommended first-line targeted agents for patients with advanced or metastatic NSCLC is rapidly expanding as more effective agents and regimens are identified. Preferred first-line therapies for patients with advanced or metastatic NSCLC bearing one of the more common oncogenic genomic alterations include:
- EGFR mutations (eg, exon 19 deletion, L858R): osimertinib; additional options include erlotinib, afatinib, gefitinib, dacomitinib, erlotinib plus bevacizumab, and erlotinib plus ramucirumab
- ALK rearrangements: alectinib, brigatinib, lorlatinib (possibly ceritinib or crizotinib)
- MET exon 14 skipping mutations: capmatinib, tepotinib (possibly crizotinib)
- ROS1 rearrangements: entrectinib, crizotinib (possibly ceritinib)
- BRAF V600E mutations: dabrafenib plus trametinib (possibly vemurafenib)
- NTRK1/2/3 gene fusions: larotrectinib, entrectinib
- RET rearrangements: selpercatinib, pralsetinib (possibly cabozantinib or vandetanib).5
Immune checkpoint inhibitors (ICIs) may be appropriate for some patients with advanced NSCLC who lack one of these targetable driver mutations. Recommended first-line ICI-based treatment is based on tumor programmed death-ligand 1 (PD-L1) expression level (as measured by tumor proportion score or TPS). Use of specific ICIs and regimens (eg, pembrolizumab, atezolizumab, cemiplimab, nivolumab/ipilimumab) varies depending on PD-L1 expression level (≥50% or 1%-49%) and tumor histology (nonsquamous or squamous).5 Since the list of recommended therapies is continually evolving, providers should refer to current guidelines for all preferred treatments and additional therapeutic options. Those patients who lack targetable driver mutations and who are not eligible for immunotherapy may receive platinum-based doublet chemotherapy.
BIOMARKER TESTING FOR ADVANCED NSCLC
Greater use of targeted agents and immunotherapy for NSCLC has required a concomitant increase in biomarker testing for these targets. The number of prognostic and predictive biomarkers in advanced NSCLC is rapidly evolving, resulting in a shift away from single-gene assays to broader genomic profiling. However, the need exists for greater awareness and adoption of such testing by health care professionals. An analysis of US community oncologist patient records indicated that rates of molecular testing for advanced NSCLC increased from 2016 to 2018 but were still suboptimal.11 Similarly, a retrospective community oncology study found that testing for ALK rearrangements in advanced NSCLC increased substantially from 2012 to 2019, yet approximately 20% of patients went untested, thus missing potentially effective therapy.12 In one study, patients with advanced NSCLC who underwent recommended biomarker testing had a significantly lower risk of mortality and treatment discontinuation compared with patients without testing data, highlighting the benefits of molecular testing.13 A panel of lung cancer specialists recently developed recommendations to optimize institutional genomic testing processes and to help overcome barriers to access and implementation of testing such as quality, cost, turnaround time, and provider education.14 Recommendations included improved triage of biopsy tissue, appointment of an institutional workflow navigator to facilitate adherence to clinical testing protocols, and better education of community-based oncology practices regarding testing procedures.
Current NCCN guidelines recommend broad molecular biomarker analyses for all patients with advanced or metastatic NSCLC to identify known oncogenic driver mutations that can inform prognosis and use of approved targeted therapy.5 Screening will continue to increase as new molecular alterations and biomarkers are discovered. Biomarker screening should consider not only targetable alterations for first-line therapy but also potential treatments for second-line therapy and beyond to avoid unnecessary repeat subsequent biopsies.15 All patients with adenocarcinoma, large cell, or NSCLC not otherwise specified should undergo screening for genomic alterations in EGFR and ALK (both category 1), KRAS, ROS1, BRAF, NTRK1/2/3, MET exon 14 skipping, and RET.5 Additionally, PD-L1 testing by immunohistochemistry (IHC) should be performed to detect patients who may be suitable candidates for ICI therapy. For those with squamous cell carcinoma, clinicians should consider molecular testing that includes EGFR, ALK, KRAS, ROS1, BRAF, NTRK1/2/3, MET exon 14 skipping, and RET alterations as well as PD-L1 testing.
Although patients with squamous tumors are less likely to have targetable alterations compared with nonsquamous tumors, the NCCN panel strongly recommends such screening for squamous cell cancers in case targetable biomarkers are present.5 (This is not a complete list of all NSCLC biomarkers since other rare alterations can occur such as HER2 mutations, high-level MET amplification, and NRG1 rearrangements). Numerous other potential biomarkers are currently under investigation in NSCLC and other tumors including IFN-g, IL-6, POLE mutations, tumor-infiltrating lymphocytes, and exosomes.16
Meta-analyses have found that the mutation frequencies of multiple oncogenes in patients with NSCLC, including EGFR, KRAS, ALK, and TP53, can vary significantly according to ethnicity, histology, and smoking status.17,18 For example, Caucasians have a lower frequency of EGFR mutations compared with Asians (20% vs 50%) but a higher rate of KRAS mutations (20%-30% vs ~5%).19 Such differences could have a prognostic impact, as seen in one study in which Asian ethnicity was one of several favorable prognostic factors in patients receiving first-line therapy with an EGFR inhibitor.20
Several techniques exist for detection and characterization of genomic alterations. Next-generation sequencing (NGS), which is often the preferred method, is used in clinical laboratories to identify rare, targetable driver mutations. Individual NGS assays may not detect all types of alterations, however. Real-time polymerase chain reaction (PCR) testing can be used to identify specific known mutations. Sanger (chain determination) sequencing can also detect point mutations and small deletions or insertions, but since it requires tumor enrichment it is not recommended for use in tumor samples with less than 25% to 30% of tumor after enrichment or for detection of low-frequency (eg, resistance) mutations.5 Other PCR methods, such as allele-specific PCR and qPCR, have also been used. Additional genomic tests can detect alterations such as microsatellite instability (MSI) and tumor mutation burden (TMB) that may be informative. Fluorescent in situ hybridization (FISH) can evaluate copy number, gene amplification, and structural alterations. IHC is used to detect cell surface expression of selected biomarkers such as PD-L1, EGFR, and HER2 that can inform selection of targeted therapy. NGS-based biomarker assessment for genomic alterations is usually performed on tumor tissue, although blood can be used if a surgical specimen or tumor biopsy is not available (so-called “liquid biopsy” to analyze cell-free circulating tumor DNA [ctDNA]).2
Plasma ctDNA analysis has been shown to be a valid tool that can enhance identification of driver mutations in patients with advanced NSCLC, in combination with tissue-based methods.21,22 Consequently, use of ctDNA, in addition to NGS testing of tumor tissue, is now recommended.23 Due to the ease of serial sampling, ctDNA can also be used for assessing mechanisms of resistance after tyrosine kinase inhibitor (TKI) therapy. In contrast, determination of PD-1/PD-L1 expression by IHC is performed only on tumor tissue.
Biomarker testing should be conducted as soon as possible following diagnosis to identify any genomic alterations or other targetable markers that could inform selection of the most appropriate therapy. This will help to maximize response, delay disease progression, and minimize undue toxicity from ineffective therapy. Inclusion of a pharmacist on the oncology molecular tumor board can aid with interpretation of genomic testing and making treatment recommendations based on these results.
NEWLY APPROVED TREATMENT OPTIONS DIRECTED AGAINST NOVEL MOLECULAR TARGETS
KRAS Inhibitors
Point mutations in the KRAS proto-oncogene cause dysregulated, constitutively activated tumor growth through activation of RAS-mediated downstream signaling pathways. Some studies suggest that the presence of KRAS mutations is prognostic for poor survival and may be associated with more aggressive metastatic disease.24,25KRAS mutations are the most common genomic driver event found in NSCLC, occurring in up to 30% of nonsquamous cases.26-28 These mutations are strongly associated with smoking history and are more common in adenocarcinomas than squamous cell carcinomas.29,30 In NSCLC, the majority of KRAS mutations occur at codons 12 and 13. KRAS G12C mutations in particular occur in approximately 13% of patients with lung adenocarcinoma and thus represent a clinically relevant therapeutic target. While RAS is the most commonly mutated oncogene in cancer, until recently development of effective therapies targeting the oncogenic RAS protein has been difficult since such mutations are associated with resistance to EGFR TKIs.31
Sotorasib
Sotorasib (AMG 510) is a first-in-class, highly selective, irreversible, small-molecule inhibitor that covalently binds to the KRAS G12C-mutant protein. This binding locks the protein in an inactive state to prevent KRAS-dependent oncogenic signaling, thus inhibiting tumor cell growth. In preclinical studies, sotorasib caused regression of KRAS G12C-mutated tumors and enhanced antitumor efficacy of chemotherapy and targeted agents.32 A phase 1/2 trial (CodeBreaK 100) evaluated sotorasib in 126 patients with locally advanced or metastatic KRAS G12C-mutant NSCLC with disease progression on prior ICI and/or platinum-based combination chemotherapy (and targeted therapy if EGFR, ALK, or ROS1 alterations were present). Sotorasib was administered orally at a dose of 960 mg once daily. Despite heavy pretreatment (up to 3 prior lines of therapy), clinical benefit was seen, with an objective response rate (ORR) of 37.1%, median PFS of 6.8 months, and median OS of 12.5 months (TABLE 1).33,34 The median time to response was 1.4 months and median duration of response was 11.1 months. The disease control rate was 80.6%, with a median best percentage reduction in tumor size of approximately 60%. The most common adverse events were diarrhea (50.8%), nausea (31.0%), fatigue (25.4%), arthralgia (21.4%), increased aspartate aminotransferase level (21.4%), and increased alanine aminotransferase level (20.6%).33,34
Sotorasib was granted accelerated approval (May 2021) for treatment of adults with KRAS G12C‑mutated locally advanced or metastatic NSCLC, as determined by a US Food and Drug Administration (FDA)‑approved test, following 1 or more lines of prior systemic therapy. (Note that since the recommended dosage of sotorasib is 960 mg/day but only comes in 120-mg tablets, patients must take 8 tablets at a time every day.) Current NCCN guidelines recommend only sotorasib as subsequent therapy for KRAS G12C-mutated advanced/metastatic NSCLC in patients with a performance status of 0 to 2.5
| TABLE 1. Pivotal Trials of Selected Novel Therapies for Advanced/Metastatic NSCLC34,35,37,43,48,55,56 |
| Study/Acronym |
Study Population (N) |
Regimen |
Efficacy |
Comments |
| ORR (%) |
Median DOR (mo) |
Median PFS (mo) |
Median OS (mo) |
| Tyrosine Kinase Inhibitors |
Skoulidis 2021
(CodeBreaK 100)34,35 |
Locally advanced or metastatic KRAS G12C-mutant NSCLC, progression on ICI and/or platinum-based combination chemotherapy, and targeted therapy if EGFR, ALK, or ROS1 alterations present; ≤3 prior lines of therapy (N = 126) |
Sotorasib 960 mg once daily |
37.1 (95% CI, 28.6-46.2) |
11.1 (95% CI, 6.9-NE) |
6.8 (95% CI, 5.1-8.2) |
12.5 (95% CI, 10.0-NE) |
· In exploratory subgroup analysis, patients with STK11-mutant, KEAP1-wild type disease had ORR of 50%
· Similar disease control rates seen in patients with or without stable brain metastases previously treated with radiation or surgery at baseline (77.5% vs 84.1%) |
Wolf 2020
(GEOMETRY mono-1)43 |
Advanced NSCLC with MET exon 14 skipping mutation (N = 97) |
Capmatinib 400 mg twice daily |
41 (95% CI, 29-53) in pretreated patientsa
68 (95% CI, 48-84) in treatment-naïve patients |
9.7 (95% CI, 5.6-13.0) in pretreated patients
12.6 (95% CI, 5.6-NE) in treatment-naïve patients |
5.4 (95% CI, 4.2-7.0) in pretreated patients
12.4 (95% CI, 8.2-NE) in treatment-naïve patients |
NR |
Responses in 7 of 13 (54%) patients with brain metastases, including 4 with complete resolution of brain metastases |
Paik 2020
(VISION)37 |
Advanced or metastatic NSCLC with confirmed MET exon 14 skipping mutation (N = 99) |
Tepotinib 500 mg once daily |
48 (95% CI, 36-61) in liquid-biopsy group
50 (95% CI, 37-63) in tissue-biopsy group |
9.9 (95% CI, 7.2-NE) in liquid-biopsy group
15.7 (95% CI, 9.7-NE) in tissue-biopsy group |
8.5 (95% CI, 6.7-11.0) in liquid-biopsy group
11.0 (95% CI, 5.7-17.1) in tissue-biopsy group |
17.1 (95% CI, 12.0-26.8) overall |
Responses observed in 11 of 99 (55%) patients with brain metastases |
| EGFR Inhibitors Targeting Exon 20 Insertion Mutations |
Park 2021
(CHRYSALIS)48 |
Advanced NSCLC harboring EGFR exon 20 insertion mutations, after progression on platinum-based chemotherapy (N = 81) |
Amivantamab 1050 mg or 1400 mg once weekly for the first cycle, then every other week for subsequent cyclesb |
40 (95% CI, 29-51) |
11.1 (95% CI, 6.9-not reached) |
8.3 (95% CI, 6.5-10.9) |
NR |
Responses observed in 7 of 18 (39%) patients with history of brain metastases |
Riely 2021
(Study 101)55 |
Locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations previously treated with systemic therapy (N = 28)c |
Mobocertinib 160 mg/day |
43 (95% CI, 24-63) |
13.9 (95% CI, 5.0-not reached) |
7.3 (95% CI, 4.4-15.6) |
NR |
Responses observed in 3 of 12 (25%) patients with baseline brain metastases |
| Zhou 202156 |
Locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations previously treated with systemic therapy
Platinum-pretreated patients (PPP cohort; n = 114) or EXCLAIM extension cohort of phase 1/2 trial (n = 96) |
Mobocertinib 160 mg/day |
PPP: 28 (95% CI, 20-37)
EXCLAIM: 25 (95% CI, 17-35) |
PPP: 17.5 (95% CI, 7.4-20.3)
EXCLAIM: Not reached (95% CI, 5.6-not reached) |
PPP: 7.3 (95% CI, 5.5-9.2)
EXCLAIM: 7.3 (95% CI, 5.5-9.1) |
PPP: 24.0 (95% CI, 14.6-28.8)
EXCLAIM: Not reached (95% CI, 13.1-not reached) |
Responses observed in 7 of 40 (18%) of PPP patients with baseline brain metastases |
Abbreviations: CI, confidence interval; DOR, duration of response; ICI, immune checkpoint inhibitor; NE, not estimable; NR, not reported; NSCLC, non-small cell lung cancer; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PPP, platinum-pretreated patients.
a Pretreated patients had received 1-2 lines of prior therapy.
b Initial amivantamab dose split over days 1 and 2 in first treatment cycle, then administered weekly for remainder of cycle 1 and subsequently every other week (days 1 and 15). Dosing adjusted based on weight (1050 mg for patients <80 kg or 1400 mg if ≥80 kg).
c Cohort treated with 160 mg/day mobocertinib. |
A subset analysis of patients with or without stable brain metastases previously treated with radiation or surgery at baseline indicated that durable intracranial responses and continued intracranial stabilization were achieved with sotorasib therapy, with similar disease control rates in both groups (77.5% vs 84.1%).35 Exploratory analyses of molecularly defined subgroups found that sotorasib-treated patients with STK11-mutant, KEAP1 wild-type tumors had an increased ORR (50%), median PFS (11.0 months), and median OS (15.3 months); this compared with an ORR of 23% for STK11-mutant, KEAP1-mutant disease; 14% for STK11 wild-type, KEAP1-mutant; and 42% for STK11 wild-type, KEAP1 wild-type disease.34 The higher response rate seen in patients with STK11-mutated NSCLC is noteworthy since such alterations confer primary resistance to docetaxel and PD-1/PD-L1 inhibitors in patients with KRAS-mutated NSCLC.36 Since the study was not statistically powered for this analysis, further research is needed to confirm the significance of STK11 and KEAP1 mutational status in correlation with response to sotorasib in NSCLC.
To further improve efficacy and inhibit development of resistance to KRAS inhibitors, ongoing trials (NCT04303780, NCT04933695) are evaluating sotorasib in patients with advanced or metastatic NSCLC bearing KRAS G12C mutations, either as a single agent (as first-line therapy) or combined with docetaxel (as second-line or greater therapy) (TABLE 2).
| TABLE 2. Selected Ongoing Phase 2 and 3 Trials of Novel Therapies for Advanced or Metastatic NSCLC |
| Study Population |
Regimen |
Phase |
Setting |
N |
NCT Numbera (Study Acronym) |
Primary Outcome Measure(s) |
Target Primary Completion Date |
| Tyrosine Kinase Inhibitors |
| KRAS G12C-mutated advanced or metastatic NSCLC |
Sotorasib vs docetaxel |
3 |
Second-line or greater |
345 |
NCT04303780 (CodeBreak 200) |
PFS |
November 2021 |
| KRAS G12C-mutated metastatic NSCLC with <1% PD-L1 expression and/or STK11 mutation |
Sotorasib |
2 |
First-line |
170 |
NCT04933695 |
ORR |
July 2023 |
| Locally advanced or metastatic NSCLC harboring MET exon 14 skipping mutationb |
Capmatinib vs docetaxel |
3 |
Second- or third-line |
90 |
NCT04427072 (GeoMETry-III) |
PFS |
July 2023 |
| Advanced or metastatic NSCLC with EGFR mutations sensitive to EGFR TKI, T790M negative, and MET gene amplification following progression on first/second-generation EGFR TKIs or osimertinib |
Capmatinib + osimertinib vs platinum + pemetrexed-based doublet chemotherapy |
3 |
Second-line |
245 |
NCT04816214 (GEOMETRY-E) |
PFS |
January 2025 |
| Advanced or metastatic NSCLC with activating EGFR mutation and MET amplification, and resistance to first-line osimertinib |
Tepotinib monotherapy or tepotinib + osimertinib |
2 |
Second-line |
120 |
NCT03940703 (INSIGHT 2) |
ORR, safety |
November 2022 |
| EGFR Inhibitors Targeting Exon 20 Insertion Mutations |
| EGFR exon 20 insertion-mutated locally advanced or metastatic NSCLC |
Amivantamab + carboplatin/pemetrexed vs carboplatin/pemetrexed |
3 |
First-line or greater |
300 |
NCT04538664 (PAPILLON) |
PFS |
January 2022 |
| EGFR-mutated locally advanced or metastatic NSCLC after osimertinib failure |
Amivantamab + lazertinib + carboplatin vs carboplatin/pemetrexed vs amivantamab + carboplatin/pemetrexed |
3 |
Second- or third-line |
500 |
NCT04988295 (MARIPOSA-2) |
PFS |
May 2023 |
| EGFR-mutated metastatic NSCLC with progressive or new CNS metastases on previous treatment |
Amivantamab + lazertinib |
2 |
Second-line or greater |
40 |
NCT04965090 |
CNS ORR |
July 2023 |
| EGFR exon 19 deletion- or exon 21 L858R substitution-mutated locally advanced or metastatic NSCLC |
Amivantamab + lazertinib vs osimertinib vs lazertinib |
3 |
First-line |
1000 |
NCT04487080 (MARIPOSA) |
PFS |
April 2024 |
| Recurrent or metastatic NSCLC with EGFR exon 20 insertion mutation |
Mobocertinib or platinum-pemetrexed chemotherapy followed by maintenance pemetrexed |
3 |
First-line |
318 |
NCT04129502 (EXCLAIM-2) |
PFS |
June 2022 |
Abbreviations: CNS, central nervous system; EGFR, epidermal growth factor receptor; NCT, National Clinical Trial; NSCLC, non-small cell lung cancer; ORR, objective response rate; PD-L1, programmed cell death-ligand 1; PFS, progression-free survival; TKI, tyrosine kinase inhibitor.
a See study information with NCT identifier at ClinicalTrials.gov.
b EGFR wild-type and ALK rearrangement-negative. |
MET-Specific Inhibitors
The MET proto-oncogene encodes a receptor tyrosine kinase, and binding of its ligand (hepatocyte growth factor [HGF]) to the kinase induces MET downstream signaling through the RAS-RAF and PI3K pathways. Activation of the MET pathway, which occurs in some tumors, can result from protein overexpression, gene amplification, and MET exon 14 skipping mutations. These skipping alterations arise from genomic alterations (including point mutations, insertions or deletions, and large-scale whole-exon deletions) that can cause loss of MET exon 14 transcription, resulting in dysregulated MET signaling, tumor growth, and metastasis.37MET exon 14 skipping mutations occur in 3% to 4% of patients with NSCLC and are associated with poor outcomes. Such patients tend to be older (age ≥70 years), female, and less likely to have a history of smoking, and many have bone, liver, or brain metastases.38
Tumors with MET alterations are sensitive to MET inhibition, providing the rationale for targeting this protein in NSCLC bearing such alterations.39 In contrast to nonselective MET inhibitors, MET-selective TKIs cause little off-target toxicity.40 Two selective MET inhibitors, capmatinib and tepotinib, have been approved for treatment of advanced or metastatic NSCLC with a MET exon 14 skipping alteration. Multiple other MET inhibitors are currently in clinical trials for MET-altered advanced NSCLC.41
Capmatinib
Capmatinib (INCB28060) is an oral, highly selective MET TKI that blocks MET phosphorylation and activation of downstream effectors. Capmatinib has demonstrated preclinical activity in MET-dependent cancer cell lines and MET-driven tumors in xenograft models.42 A multicohort phase 2 trial (GEOMETRY mono-1) of capmatinib was conducted in patients with MET-dysregulated advanced NSCLC. Oral capmatinib was administered at a dose of 400 mg twice daily. In patients with MET exon 14 skipping mutations, the ORR was 68% in treatment-naïve patients and 41% in those with 1 to 2 lines of prior therapy; median duration of response was 12.6 months and 9.7 months, respectively (TABLE 1).43 The median PFS was 12.4 months and 5.4 months, respectively (median OS not reported). The majority of patients (82% in the treatment-naïve group and 68% in the previously treated group) had achieved a response at their first tumor evaluation. Responses were also seen in patients with MET amplification with a gene copy number of 10 or higher (40% ORR for treatment-naïve patients, 29% for previously treated). Additionally, intracranial responses were noted in 7 of 13 patients with a MET exon 14 skipping mutation, including 4 with complete resolution of brain metastases. The most common all-grade adverse events regardless of causality were peripheral edema (51%), nausea (45%), vomiting (28%), blood creatinine increased (24%), dyspnea (23%), and fatigue (22%).42,43
Capmatinib was approved for treatment of adults with metastatic NSCLC whose tumors have a mutation that leads to MET exon 14 skipping as detected by an FDA-approved test.5 This inhibitor is now included in current NCCN treatment guidelines as a preferred first-line treatment option for advanced/metastatic NSCLC with a MET exon 14 skipping alteration, as well as for subsequent therapy following disease progression on initial systemic therapy. Because the observed ORR in patients with MET amplification was lower than the prespecified threshold for clinically relevant activity, capmatinib was not approved for use in this patient population, although it is listed as a treatment option in the NCCN guidelines.5
Two ongoing phase 3 trials are further evaluating capmatinib as second-line treatment of advanced NSCLC. In one study (NCT04427072), this agent is being compared to docetaxel in patients with a MET exon 14 skipping mutation (who are also EGFR wild-type and lacking an ALK rearrangement). A second trial (NCT04816214) is evaluating the combination of capmatinib and osimertinib in patients with an EGFR activating mutation and MET amplification, following disease progression on an EGFR TKI (TABLE 2).
Tepotinib
The oral ATP-competitive, MET-selective TKI tepotinib (EMD 1214063) was shown to have potent antitumor activity in NSCLC xenograft models.44 A phase 2 trial (VISION) evaluated the safety and efficacy of tepotinib in patients with advanced or metastatic NSCLC and a confirmed MET exon 14 skipping mutation, detected by liquid or tissue biopsy. Over half of patients (56%) had received at least 1 prior course of therapy for advanced/metastatic disease. Tepotinib 500 mg was administered orally once daily. The ORR was 48% in the liquid-biopsy group and 50% in the tissue-biopsy group, with an overall median duration of response of 11.1 months (TABLE 1).37 The median PFS was 8.5 months for patients in the liquid-biopsy group and 11.0 months for those in the tissue-biopsy group; the combined median OS was 17.1 months. For the 11 patients with brain metastases, a response rate of 55% and median PFS of 10.9 months were observed. The most common adverse events (all-grade) were peripheral edema (63%), nausea (26%), diarrhea (22%), blood creatinine increased (18%), and hypoalbuminemia (16%), while the most common grade ≥3 adverse event was peripheral edema (7%).37
Based on these results, tepotinib was approved in February 2021 for treatment of adults with metastatic NSCLC bearing MET exon 14 skipping alterations as detected by an FDA-approved test. 5 (Note that the approved dose of tepotinib is 450 mg daily, which is based on the active moiety dose rather than the 500 mg hydrochloride hydrate dose used in the pivotal trial.) Since prospective testing for MET exon 14 skipping mutations in the VISION trial used tumor tissue or ctDNA, the tepotinib prescribing information indicates that mutation testing in plasma specimens is allowed but only in patients for whom a tumor biopsy cannot be obtained. NCCN clinical practice guidelines include tepotinib as a preferred first-line treatment option (in addition to capmatinib) for patients with advanced/metastatic NSCLC and a MET exon 14 skipping alteration, and also for subsequent therapy following disease progression on initial systemic therapy.5
While resistance to first-generation EGFR TKIs can be acquired through secondary EGFR mutations such as T790M or by aberrant MET receptor activity, the combination of an EGFR TKI and MET inhibitor can overcome such resistance in animal tumor models, suggesting the clinical utility of this approach.44 A phase 2 trial found that the combination of tepotinib and gefitinib was more effective than platinum-based doublet chemotherapy in patients with EGFR-mutated advanced NSCLC with MET overexpression or MET amplification having acquired resistance to EGFR inhibition, particularly in those with high-level MET overexpression.45
Several ongoing trials (NCT03940703) are evaluating tepotinib for advanced MET-driven NSCLC (TABLE 2). These include use of tepotinib alone or combined with osimertinib in patients with MET amplification and an activating EGFR mutation with acquired resistance to osimertinib, and another study in which tepotinib is combined with a different TKI as second-line therapy in patients with or without measurable CNS disease.
EGFR Inhibitors Targeting Exon 20 Insertion Mutations
Alterations in the EGFR gene are detected in 10% to 15% of patients with advanced NSCLC.46 EGFR mutations in lung adenocarcinomas are more common in Asian patients (up to 50%) compared with Caucasians (up to 20%).47 Approximately 85% of EGFR-mutated tumors are characterized as “sensitizing” EGFR mutations (eg, exon 19 deletions or exon 21 L858R point substitutions) that are sensitive to established EGFR TKIs such as erlotinib, gefitinib, afatinib, dacomitinib, and osimertinib. EGFR exon 20 insertion mutations are the third most prevalent activating EGFR mutation, comprising up to 12% of all EGFR mutations. They are characterized by in-frame insertions and duplications near the C-helix of the EGFR kinase domain.48 These alterations induce an active kinase confirmation resulting in resistance to standard EGFR TKIs (response rates ≤9%) and are associated with a poor prognosis.48
Patients with advanced NSCLC bearing EGFR exon 20 insertion mutations may receive chemotherapy, immunotherapy, or an EGFR TKI approved for other mutations (such as erlotinib, gefitinib, afatinib, dacomitinib, and osimertinib). However, these TKIs often result in response rates of less than 30% and a short survival in this patient population. Newer EGFR TKIs have been developed, but only poziotinib has shown clinical benefit for this subgroup (ORR 27.6%).49 More effective treatment options are needed for patients with EGFR exon 20 insertion mutations.
Amivantamab-vmjw
Amivantamab-vmjw (JNJ-61186372) is a novel, fully human bispecific antibody targeting both EGFR and MET proteins, thus inhibiting 2 key NSCLC oncogenic driver pathways.50 Binding of amivantamab to these receptors blocks ligand binding and promotes degradation of receptor-antibody complexes, antibody-dependent cellular cytotoxicity by natural killer cells, and Fc-dependent trogocytosis by macrophages.51 Preclinical data indicate that amivantamab treatment of NSCLC bearing EGFR exon 20 insertion mutations downregulates EGFR and MET, inhibits tumor growth, and induces antitumor immunity.50 Targeting both EGFR and MET may therefore offer a novel approach to overcoming resistance to standard EGFR TKIs in patients with advanced NSCLC. Amivantamab has been shown to inhibit various types of NSCLC EGFR driver mutations including exon 19 deletions, L858R mutation, exon 20 insertions, EGFR-TKI resistance mutations (T790M, C797S), and drug resistance arising from MET amplification.51
A clinical trial (CHRYSALIS) evaluated amivantamab in 81 patients with advanced NSCLC harboring EGFR exon 20 insertion mutations, following disease progression on platinum-based chemotherapy.48 In the phase 2 part of the study, amivantamab was administered IV twice on week 1, then once weekly during the first 28-day cycle, then every other week in subsequent cycles (FIGURE 1). Dosing was based on weight, with 1050 mg amivantamab administered to patients <80 kg or 1400 mg amivantamab for those ≥80 kg. To mitigate infusion-related reactions, patients received prophylactic premedications and the first dose was administered over 2 days. The ORR was 40%, with a median duration of response of 11.1 months; 75% of responses were observed at the first disease assessment (TABLE 1). The median PFS was 8.3 months and median OS was 22.8 months (OS data are not yet mature). Seven of 18 (39%) patients with a history of brain metastases responded to amivantamab, although those with active or untreated brain metastases were excluded from the study.48
The most common adverse events (all-grade) were rash (including dermatitis acneiform, pruritus, and dry skin; 86%), infusion-related reactions (66%), and paronychia (45%), while the most common grade ≥3 adverse events were hypokalemia (5%) as well as rash, pulmonary embolism, diarrhea, and neutropenia (4% each).52 Median time to rash onset was 14 days (range: 1 to 276 days), and 85% were grade 1/2. Infusion-related reactions included dyspnea, flushing, fever, chills, nausea, vomiting, chest discomfort, and hypotension; most occurred on cycle 1 day 1 (93%) or day 2 (4%). Median time to onset of infusion-related reactions following start of infusion was 1 hour (range: 0.1 to 18 hours), and the majority were grade 1/2 in severity.52
These results led to approval of amivantamab (May 2021) for treatment of adults with locally advanced or metastatic NSCLC and EGFR exon 20 insertion mutations as detected by an FDA-approved test, with disease progression on or after platinum-based chemotherapy.52 Current NCCN guidelines indicate use of amivantamab as subsequent therapy for EGFR exon 20 insertion mutation-positive locally advanced or metastatic NSCLC.5 Additional analysis of the entire enrolled patient population in the CHRYSALIS trial, including those without previous chemotherapy treatment, is planned pending sufficient follow-up.
Mobocertinib
Mobocertinib (TAK-788, AP32788) is an oral selective TKI targeting the EGFR, HER2, and HER4 proteins. In animal models, mobocertinib demonstrated greater inhibition of NSCLC tumor xenografts bearing EGFR exon 20 insertion mutations compared with other EGFR inhibitors.53 Preclinical activity was also seen against HER2 exon 20 insertion mutants, with synergistic growth inhibition when combined with ado-trastuzumab emtansine.54 A phase 1/2 trial evaluated mobocertinib in 136 patients with NSCLC refractory to standard therapies. For the 28 patients with EGFR exon 20 insertion mutations who received mobocertinib 160 mg daily, the confirmed ORR was 43% with a median duration of response in confirmed responders of 13.9 months (TABLE 1).55 The median PFS was 7.3 months (median OS not reported). Responses were seen across a diverse array of EGFR exon 20 insertion mutation variants (more than 50 such variants are known). Clinical responses were also noted in patients with baseline CNS metastases: the ORR was 25% in patients with baseline brain metastases versus 56% in those without (median PFS 3.7 months and 10.2 months, respectively).55
The safety profile of mobocertinib was consistent with that seen with other EGFR inhibitors. Among all patients treated at the 160 mg dose, the most common (any-grade) treatment-related adverse events (>25%) were diarrhea (83%), nausea (43%), rash (33%), and vomiting (26%); the only grade ≥3 treatment-related adverse event higher than 5% was diarrhea (21%).55 Based on these results, mobocertinib was granted accelerated approval for treatment of adults with locally advanced or metastatic NSCLC having EGFR exon 20 insertion mutations as detected by an FDA-approved test, following disease progression on or after platinum-based chemotherapy.56
A subsequent analysis evaluated mobocertinib in a phase 1/2 trial of previously treated patients with EGFR exon 20 insertion mutation-positive advanced NSCLC, which included a cohort of platinum-pretreated patients (PPP) (n = 114) with a subset of these patients being a part of the EXCLAIM cohort (n = 86).57 Patients received mobocertinib 160 mg once daily. In the PPP cohort, the ORR was 28%, with a median duration of response of 17.5 months; the median PFS was 7.3 months and median OS was 24.0 months. Responses were observed in patients with and without baseline brain metastases (ORR 18% and 34%, respectively). In the EXCLAIM cohort, the ORR was 25% (median duration of response not reached) and median PFS was 7.3 months (median OS not reached). In this trial, prolongation of the QT interval occurred in 11% and 8% of patients in these 2 cohorts, respectively. As noted in the prescribing information, since mobocertinib can cause life-threatening, heart rate–corrected QT (QTc) interval prolongation, including Torsades de pointes, QTc and electrolytes must be monitored at baseline and periodically during treatment, and more frequently in patients with risk factors for QTc prolongation.56
An ongoing phase 3 trial (NCT04129502) is comparing mobocertinib monotherapy to platinum-pemetrexed chemotherapy (followed by maintenance pemetrexed) as first-line treatment for patients with recurrent or metastatic NSCLC and an EGFR exon 20 insertion mutation (TABLE 2).
CASE STUDY
VB is a 66-year-old Caucasian female and former smoker who was diagnosed with stage IV NSCLC adenocarcinoma with bone metastases in November 2020. A brain MRI was performed and was negative. Genomic testing analysis via tissue biopsy and liquid biopsy demonstrated the following results: EGFR-negative, ALK-negative, ROS1-negative, MET-negative, RET-negative, BRAF-negative, KRAS G12C-positive, STK11-mutated, KEAP1-negative, NTRK-negative, and PD-L1 15%. Her Eastern Cooperative Oncology Group (ECOG) performance status is 1. VB presents to the medical oncologist’s office to discuss her treatment options.
Past Medical History: Anxiety, depression, gastroesophageal reflux disease (GERD), and cerebrovascular accident (CVA)
Family History: No pertinent family history
Social History: Former smoker (quit 35 years ago). No illicit drug use, drinks wine occasionally
Current Medications:
Atorvastatin 80 mg by mouth once daily
Losartan 100 mg by mouth once daily
Omeprazole 40 mg by mouth daily
Venlafaxine 75 mg by mouth daily
Warfarin 5 mg by mouth daily
Laboratory and Physical Findings:
Weight: 59.1 kg
Body surface area: 1.69 m2
White blood cell (WBC) count: 7.6 x 103/µL
Absolute neutrophil count: 5.44 x 103/µL
Hemoglobin: 13.0 g/dL
Platelets: 286 x 109/L
Serum creatinine: 0.89 mg/dL
Sodium: 137 mEq/L
Potassium: 4.5 mmol/L
Total bilirubin: 0.4 mg/dL
Aspartate aminotransferase (AST): 23 U/L
Alanine aminotransferase (ALT): 10 U/L
Case Discussion
With VB being newly diagnosed with metastatic NSCLC adenocarcinoma, the team should always perform genomic testing first to determine whether there are any actionable alterations that can be targeted. If no targetable biomarkers are detected, or if one is found but the appropriate agent is reserved for use in the second-line setting or beyond (eg, sotorasib, amivantamab, mobocertinib), the patient should be evaluated as to whether they are a candidate for immunotherapy with or without chemotherapy. Since VB is negative for EGFR, ALK, ROS1, MET, RET, BRAF, and NTRK alterations (all of which are associated with agents that can be used in the first-line setting), VB will proceed with immunotherapy with or without chemotherapy based on her PD-L1 status. Her PD-L1 TPS is 15%, and she does not have any contraindications to immunotherapy based on her past medical history. Per NCCN guidelines,5 since VB’s PD-L1 TPS is <50%, the preferred first-line regimen would be chemotherapy plus immunotherapy consisting of carboplatin, pemetrexed, and pembrolizumab due to her adenocarcinoma histology. VB proceeds to receive 4 cycles of carboplatin, pemetrexed, and pembrolizumab, then transitions to maintenance pemetrexed and pembrolizumab. She receives 9 cycles of maintenance treatment, but unfortunately her most recent scans show disease progression.
The medical oncologist asks for your recommendation for the next treatment option. As the pharmacist, it is important to always review the genomic analysis to see if there are any targeted agents that can be utilized in the second-line setting. You review the genomic analysis report and note the patient’s tumor is KRAS G12C-positive, STK11-mutated, and KEAP1-negative. Per NCCN guidelines and FDA-approved indication,5 sotorasib can be utilized for patients with KRAS G12C-mutated metastatic NSCLC who have received at least 1 prior systemic therapy; VB meets this criteria, as she has progressed on her initial treatment regimen.
Potential Case Recommendations and Considerations
An important consideration before initiating a patient on oral targeted agents is to evaluate laboratory results to see if any dose adjustments are warranted based on renal or hepatic impairment, to screen for drug-drug interactions, and to educate the patient on appropriate administration of the medication. All of VB’s laboratory results are within normal limits, so no dose adjustments are needed for sotorasib. After reviewing VB’s medication list, a notable drug-drug interaction was identified between sotorasib and omeprazole. Omeprazole can decrease the serum concentration of sotorasib, so it is recommended that proton pump inhibitors (PPIs) and histamine-2 receptor antagonists(H2RAs) be avoided. Since the patient has GERD and may require acid suppression, antacids may be used. If antacids are needed, sotorasib can be administered 4 hours before or 10 hours after administration of an antacid. Proper administration of the medication is important to review with the patient. Although sotorasib is only taken once daily, the patient must take eight 120-mg tablets to reach the desired dose of 960 mg. If a patient is unable to swallow or has difficulty swallowing several tablets at a time, an oral sotorasib mixture may be prepared.
ROLE OF PHARMACISTS IN ENSURING OPTIMAL OUTCOMES IN ADVANCED NSCLC
As key members of the multidisciplinary oncology team, pharmacists can play a pivotal role in enhancing outcomes for patients with NSCLC who may receive these and other targeted agents. Pharmacists are uniquely qualified to improve cancer care through adverse event monitoring and mitigation. In light of the high cost of many new cancer therapies and a restrictive reimbursement environment, these health care professionals can work with patients and providers to improve reimbursement and reduce drug-related expenses. Pharmacists routinely advise oncologists on the optimal NSCLC therapy based on a patient’s fitness, comorbidities, potential treatment-related adverse effects, and concomitant medications. Oncology and specialty pharmacists in particular are pivotal in educating patients to help them maintain their therapy as prescribed and understand and monitor for potential side effects.
Toxicity Management
Toxicity profiles of targeted agents for NSCLC are generally more favorable in comparison to those associated with cytotoxic chemotherapy, although some adverse events with these newer drugs can be significant and even fatal. Toxicities may be exacerbated when such agents are used in combination with other anticancer drugs. Effective monitoring and proactive management of treatment-related adverse events are therefore critical for maintaining therapy and optimizing outcomes with targeted therapy for NSCLC. Poor adverse event management can result in suboptimal adherence and early discontinuation, leading to worse outcomes, and drug-related toxicities can significantly impact patient quality of life. Recommended dosing, indications for dose modifications, potential drug interactions, and key treatment-related adverse effects for new approved targeted agents are listed in TABLE 3. (Clinicians should refer to prescribing information for individual drugs for a complete list of all treatment-related toxicities and dose modifications since these can vary by product, even within the same class.)52,56,58-60
| TABLE 3. Key Characteristics of Approved New Agents for Advanced NSCLC52,56,58-60 |
| Drug |
Formulation |
Recommended Dosing and Administration |
Recommended Dose Modification Indicationa |
Metabolism |
Drug Interactions |
Key Adverse Effects |
Adverse Event Monitoring and Management |
| Sotorasib58 |
120-mg tablets |
960 mg orally once daily, with or without food |
· Grade 2 AST or ALT with symptoms, or Grade ≥3 AST or ALT
· ILD (any grade)
· Grade ≥3 nausea, vomiting, or diarrhea |
· Major CYP3A substrate
· Moderate CYP3A4 inducer |
· Avoid coadministration with PPIs and H2RAs
· Avoid strong CYP3A4 inducers, and with CYP3A4 substrates for which minimal concentration changes may lead to therapeutic failures of substrate
· Avoid P-gp substrates for which minimal concentration changes may lead to serious toxicities |
· Hepatotoxicity
· ILD/pneumonitis
· Diarrhea
· Nausea/vomiting |
· Monitor LFTs prior to and during treatment as clinically indicated (more frequently if transaminase and/or bilirubin elevations occur)
· Monitor for new or worsening pulmonary symptoms indicative of ILD/pneumonitis
· Antiemetic or antidiarrheal therapy as needed |
| Capmatinib59 |
150- and 200-mg tablets |
400 mg orally twice daily with or without food |
· Increased ALT and/or AST without increased total bilirubin (Grade ≥3); increased ALT and/or AST with increased total bilirubin in absence of cholestasis or hemolysis; increased total bilirubin without concurrent increased ALT and/or AST (Grade ≥2)
· ILD (any grade) |
· Major CYP3A4 substrate
· Minor P-gp substrate
· Moderate CYP1A2 inhibitor
· BCRP/ABCG2 inhibitor
· P-gp/ABCB1 inhibitor |
· Avoid coadministration with strong and moderate CYP3A inducers, and with strong CYP3A inhibitors |
· Hepatotoxicity
· ILD/pneumonitis
· Peripheral edema
· Nausea/vomiting
· Noncardiac chest pain
· Photosensitivity |
· Monitor LFTs during treatment
· Monitor for new or worsening pulmonary symptoms indicative of ILD/pneumonitis
· Antiemetic or antidiarrheal therapy as needed
· Protect against UV exposure to decrease risk of photosensitivity |
| Tepotinib60 |
225-mg tablets |
450 mg orally once daily with food |
· Increased ALT and/or AST without increased total bilirubin (Grade ≥3); increased ALT and/or AST with increased total bilirubin in absence of cholestasis or hemolysis; increased total bilirubin without concurrent increased ALT and/or AST
· ILD/pneumonitis (any grade) |
· Minor CYP3A4 substrate · Minor P-gp/ABCB1 substrate · P-gp/ABCB1 inhibitor |
· Avoid concomitant use of dual strong CYP3A inhibitors and P-gp inhibitors, strong CYP3A inducers, and certain P-gp substrates |
· Hepatotoxicity
· ILD/pneumonitis
· Peripheral edema
· Diarrhea
· Musculoskeletal pain |
· Monitor LFTs prior to and during treatment as clinically indicated (more frequently if transaminase and/or bilirubin elevations occur)
· Monitor for new or worsening pulmonary symptoms indicative of ILD/pneumonitis |
| Amivantamab52 |
350 mg/7 mL (50 mg/mL) solution |
1050 mg for baseline body weight <80 kg;
1400 mg if ≥80 kg
IV infusion weekly for 4 weeks, with initial dose as a split infusion in week 1 on day 1 and day 2, then every 2 weeks thereafter |
· Infusion-related reactions (any grade)
· ILD (any grade)
· Dermatologic adverse reactions (Grade ≥2) |
· N/A |
· None |
· Infusion-related reactions
· ILD/pneumonitis
· Dermatologic toxicity (rash, paronychia)
· Ocular toxicity
· Photosensitivity |
· Premedicate with antihistamines, antipyretics, and glucocorticoids (required prior to week 1, days 1 and 2; optional for subsequent doses), and use split dosing schedule to reduce risk of IRRs
· Monitor for dermatologic toxicities; treat with topical corticosteroids and topical and/or oral antibiotics |
| Mobocertinib56 |
40-mg capsules |
160 mg orally once daily, with or without
food |
· Not recommended for mild-to-moderate renal impairment (eGFR 30-89 mL/min/1.73 m2)
· Not recommended for mild (total bilirubin ≤ ULN and AST > ULN or total bilirubin >1-1.5x ULN and any AST) or moderate (total bilirubin ≥1.5-3x ULN and any AST) hepatic impairment |
· Major CYP3A4 substrate
· Minor P-gp/ABCB1 substrate
· Weak CYP3A4 inducer |
· Avoid concomitant use of strong or moderate CYP3A inhibitors or inducers
· May decrease plasma concentrations of CYP3A substrates
· Coadministration with drugs known to prolong QTc interval may increase risk of QTc interval prolongation
· Avoid concomitant use of hormonal contraceptives |
· Cardiotoxicity (decreased ejection fraction, cardiomyopathy, congestive heart failure, and QTc prolongation)
· ILD/pneumonitis
· Diarrhea
· Dermatologic toxicity (rash, paronychia) |
· Monitor electrolytes and QTc at baseline and during treatment (increase monitoring frequency in patients with QTc risk factors)
· Correct baseline abnormalities in electrolytes prior to initiating mobocertinib, especially potassium and magnesium, to prevent QTc prolongation
· Increase fluid and electrolyte intake, and consider use of antidiarrheals for diarrhea |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CYP, cytochrome P450; eGFR, estimated glomerular filtration rate; H2RAs, histamine-2 receptor antagonists; ILD, interstitial lung disease; IRRs, infusion-related reactions; LFTs, liver function tests; N/A, not applicable; NSCLC, non-small cell lung cancer; P-gp, P-glycoprotein; PPIs, proton pump inhibitors; QTc, heart rate-corrected QT interval; ULN, upper limit of normal; UV, ultraviolet.
a Refer to prescribing information for specific guidelines on dose modifications. |
Pharmacists can facilitate adverse event mitigation through regular monitoring and prompt management of drug-related adverse events, with subsequent interventions (eg, dose interruptions or reductions) as required. A recent study demonstrated that a pharmacist-facilitated laboratory monitoring system could significantly increase the number of laboratory tests for monitoring treatment-related adverse events and thus improve detection of toxicities in patients with lung cancer.61 Guidelines from the Hematology/Oncology Pharmacy Association (HOPA) recommend scheduled symptom-monitoring evaluations using patient-reported outcomes to facilitate adverse event reporting.62
Financial Toxicity
Many newer targeted therapies and immunotherapies are costly, and this can present a barrier to treatment for some patients and families.60 Pharmacists are uniquely positioned to address these challenges by assisting with prior authorizations, insurance claim appeals, and pharmaceutical patient assistance programs and rebates that can reduce out-of-pocket costs and ease financial toxicity. Manufacturers of the targeted agents discussed here offer patient assistance support programs that can help qualifying patients with benefit verification, claims appeals, insurance questions, and financial assistance for co-pays or individuals lacking insurance coverage (TABLE 4). By aiding with drug costs and prior authorizations and navigating manufacturer assistance programs, pharmacists can help avoid treatment delays and mitigate financial toxicity. Clinical pharmacist interventions have been shown to significantly reduce overall cost savings related to specialty hematology/oncology medications, including decreased use of chemotherapy and immunotherapy. Moreover, since most of these drugs are available only through specialty pharmacies, specialty pharmacists can help ensure that patients are filling their prescriptions on schedule and maintaining adherence.63
Adherence
For all orally administered targeted agents, patient adherence to the prescribed dosing regimen is essential to maximize efficacy. Yet oral oncolytics can present barriers to adherence due to low health literacy, patient forgetfulness, complex administration instructions, adverse effects, and high co-pays.64 A retrospective database study of patients receiving TKIs (mainly erlotinib and crizotinib) found acceptable adherence rates (>80%), although persistence declined over time.65 Notably, nearly half of all patients had stopped therapy at 60 days, and only 38% remained persistent at 120 days. A chart review of patients receiving oral oncolytics (primarily erlotinib) for NSCLC-identified factors related to lower adherence including low income, prior use of IV chemotherapy, lower baseline total health care costs, and high out-of-pocket costs for erlotinib.66 These results highlight the need for physicians and pharmacists to closely monitor patients to ensure adherence and continuity of treatment over the duration of planned therapy.
Patient education and good patient-provider communication and support are key for ensuring adherence. Pharmacists should follow pharmacy practice standards developed by HOPA on management of oral oncolytic therapy, including assessment of adherence and appropriate patient education.59 Quality Oncology Practice Initiative (QOPI) standards also recommend assessment and documentation of a patient’s ability to adhere to prescribed cancer therapy (including targeted agents and biologics) outside of the health care setting.67,68 However, a scoping literature review of adherence to oral cancer medications found that less than half of all interventions were effective, although some pharmacist-directed programs did improve adherence, particularly when integrated monitoring and routine provider follow-up were involved.69 Inclusion of an oncology pharmacist on the outpatient lung cancer team may improve adherence to oral targeted agents and thus lead to greater patient and provider satisfaction.70
Patient Education
Oncology pharmacists can play an important role in patient counseling and education, and therefore can make a significant contribution to patient care, especially regarding optimal use of novel targeted therapies and immunotherapy for NSCLC.71 This includes but is not limited to providing information on how these agents work, potential treatment-related adverse effects, addressing patient questions, and stressing the importance of adherence. By educating patients on common toxicities and the need for early adverse event identification and reporting, pharmacists can mitigate toxicities and thus minimize treatment interruptions or discontinuations.
Pharmacists are well positioned to answer patient questions on dosing, adherence, and potential drug interactions that could limit the absorption or bioavailability of certain anticancer therapies. Pharmacist-led patient education and interventions for NSCLC patients receiving EGFR TKIs was shown to be effective and reduced the discontinuation rate due to treatment-related adverse effects.72,73 In another study, consultation with pharmacists improved median relative dose intensity in patients with advanced NSCLC who were treated with erlotinib.74 Older individuals and patients with comorbidities may be at especially increased risk for drug-drug interactions due to polypharmacy and therefore could greatly benefit from pharmacist support. A pharmacist medication therapy management (MTM) program for elderly cancer patients (including some with NSCLC) was found to be effective in managing drug-related problems and significantly improved patient satisfaction.75
CONCLUSIONS
Identification and characterization of molecular alterations present in some patients with NSCLC has greatly improved our understanding of the complexity of this disease. This also has facilitated development of therapies, such as novel TKIs and bispecific antibodies, that target less common biomarkers for which prior therapies are not as effective. Many of these novel agents are now included as standard treatment options for patients with NSCLC bearing such alterations. Results from ongoing clinical trials are anticipated since these could further broaden the utility of these agents and enhance their use in patients with advanced NSCLC. The continued clinical validation of predictive molecular biomarkers in NSCLC will help clinicians better select the most appropriate targeted therapy for the right patient, and at the correct time.16,68
Pharmacists can make a significant contribution to the multidisciplinary oncology team by aiding in selection of the best therapy based on oncogenic driver mutations or other genomic alterations that may be present, assist in the management of adverse events and adherence, and help with accessibility and affordability of these high-cost medications for patients.
| FIGURE 1. Amivantamab Split Dosing in CHRYSALIS Phase 1 Study48 |
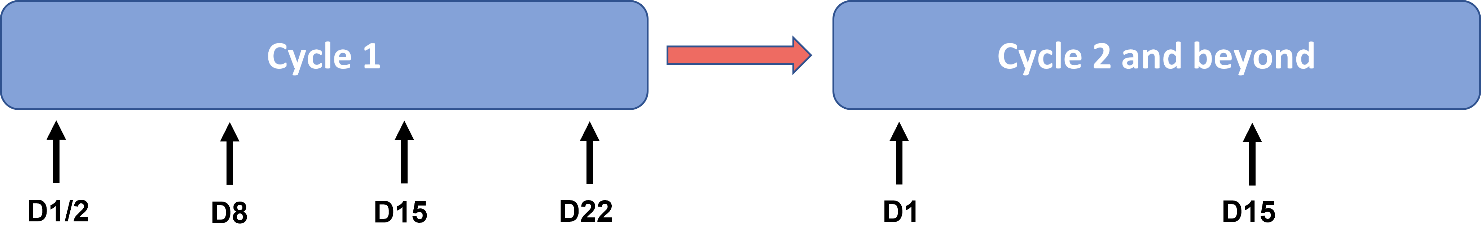 |
| In the CHRYSALIS phase 1 study, during the first treatment cycle of amivantamab the initial dose was split over days 1 and 2 and given with prophylactic premedication to mitigate infusion-related reactions. It was then administered weekly for the remainder of cycle 1. Subsequently, amivantamab was administered every other week (days 1 and 15). |
REFERENCES
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33.
- Augustus E, Zwaenepoel K, Siozopoulou V, et al. Prognostic and predictive biomarkers in non-small cell lung cancer patients on immunotherapy—the role of liquid biopsy in unraveling the puzzle. Cancers (Basel). 2021;13(7):1675.
- Majeed U, Manochakian R, Zhao Y, Lou Y. Targeted therapy in advanced non-small cell lung cancer: current advances and future trends. J Hematol Oncol. 2021;14(1):108.
- Abdel Karim N, Kelly K. Role of targeted therapy and immune checkpoint blockers in advanced non-small cell lung cancer: a review. Oncologist. 2019;24(9):1270-1284.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-Small Cell Lung Cancer. Version 5.2021. June 15, 2021. Accessed November 25, 2021. https://www.nccn.org/guidelines/recently-published-guidelines
- Tombleson R. Impact of emerging clinical trends on overall cost of care and the role of the managed care pharmacist. Am J Manag Care. 2021;27(suppl):S97-S103.
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer [published correction appears in N Engl J Med. 2007;356(3):318]. N Engl J Med. 2006;355(24):2542-2550.
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543-3551.
- Tartarone A, Lapadula V, Di Micco C, et al. Beyond conventional: the new horizon of targeted therapy for the treatment of advanced non small cell lung cancer. Front Oncol. 2021;11:632256.
- König D, Savic Prince S, Rothschild SI. Targeted therapy in advanced and metastatic non-small cell lung cancer. An update on treatment of the most important actionable oncogenic driver alterations. Cancers (Basel). 2021;13(4):804.
- Waterhouse DM, Tseng WY, Espirito JL, et al. Understanding contemporary molecular biomarker testing rates and trends for metastatic NSCLC among community oncologists. Clin Lung Cancer. 2021;22(6):e901-910.
- Bernicker EH, Xiao Y, Abraham A, et al. Adherence to National Comprehensive Cancer Network ALK testing guidelines for patients with advanced non-small cell lung cancer in U.S. community medical centers. Oncologist. 2021;26(6):e1050-e1057.
- John A, Yang B, Shah R. Clinical impact of adherence to NCCN guidelines for biomarker testing and first-line treatment in advanced non-small cell lung cancer (aNSCLC) using real-world electronic health record data. Adv Ther. 2021;38(3):1552-1566.
- Herbst RS, Aisner DL, Sonett JR, et al. Practical considerations relating to routine clinical biomarker testing for non-small cell lung cancer: focus on testing for RET Front Med (Lausanne). 2021;7:562480.
- Kerr KM, Bibeau F, Thunnissen E, et al. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer. 2021;154:161-175.
- Rossi G, Russo A, Tagliamento M, et al. Precision medicine for NSCLC in the era of immunotherapy: new biomarkers to select the most suitable treatment or the most suitable patient. Cancers (Basel). 2020;12(5):1125.
- Dearden S, Stevens J, Wu YL, et al. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol. 2013;24(9):2371-2376.
- Zhao F, Xu M, Lei H, et al. Clinicopathological characteristics of patients with non-small-cell lung cancer who harbor EML4-ALK fusion gene: a meta-analysis. PLoS One. 2015;10(2):e0117333.
- Gao B, Sun Y, Zhang J, et al. Spectrum of LKB1, EGFR, and KRAS mutations in Chinese lung adenocarcinomas. J Thorac Oncol. 2010;5(8):1130-1135.
- Gibson AJW, D'Silva A, Elegbede AA, et al. Impact of Asian ethnicity on outcome in metastatic EGFR-mutant non-small cell lung cancer. Asia Pac J Clin Oncol. 2019;15(6):343-352.
- Aggarwal C, Thompson JC, Black TA, et al. Clinical implications of plasma-based genotyping with the delivery of personalized therapy in metastatic non-small cell lung cancer. JAMA Oncol. 2019;5(2):173-180.
- Mack PC, Banks KC, Espenschied CR, et al. Spectrum of driver mutations and clinical impact of circulating tumor DNA analysis in non-small cell lung cancer: analysis of over 8000 cases. Cancer. 2020;126(14):3219-3228.
- Rolfo C, Mack P, Scagliotti GV, et al. Liquid biopsy for advanced NSCLC: a consensus statement from the International Association for the Study of Lung Cancer. J Thorac Oncol. 2021;16(10):1647-1662.
- Macerelli M, Caramella C, Faivre L, et al. Does KRAS mutational status predict chemoresistance in advanced non-small cell lung cancer (NSCLC)? Lung Cancer. 2014;83(3):383-388.
- Marabese M, Ganzinelli M, Garassino MC, et al. KRAS mutations affect prognosis of non-small-cell lung cancer patients treated with first-line platinum containing chemotherapy. Oncotarget. 2015;6(32):34014-34022.
- Riely GJ, Kris MG, Rosenbaum D, et al. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin Cancer Res. 2008;14(18):5731-5734.
- Boch C, Kollmeier J, Roth A, et al. The frequency of EGFR and KRAS mutations in non-small cell lung cancer (NSCLC): routine screening data for central Europe from a cohort study. BMJ Open. 2013;3(4):e002560.
- Biernacka A, Tsongalis PD, Peterson JD, et al. The potential utility of re-mining results of somatic mutation testing: KRAS status in lung adenocarcinoma. Cancer Genet. 2016;209(5):195-198.
- Martin P, Leighl NB, Tsao MS, et al. KRAS mutations as prognostic and predictive markers in non-small cell lung cancer. J Thorac Oncol. 2013;8(5):530-542.
- Reck M, Carbone DP, Garassino M, Barlesi F. Targeting KRAS in non-small-cell lung cancer: recent progress and new approaches. Ann Oncol. 2021;32(9):1101-1110.
- Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer. 2006;118:257-262.
- Canon J, Rex K, Saiki AY, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575(7781):217-223.
- Li BT, Skoulidis F, Falchook G, et al. CodeBreaK 100: Registrational phase 2 trial of sotorasib in KRAS p.G12C mutated non-small cell lung cancer. Abstract PS01.07. International Association for the Study of Lung Cancer 2020 World Conference on Lung Cancer. January 28-31, 2021. Accessed November 25, 2021. https://library.iaslc.org/conference-program?product_id=20&author=&category=&date=&session_type=&session=&presentation=PS01.07&keyword=&cme=undefined&
- Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRASG12C mutation. N Engl J Med. 2021;384(25):2371-2381.
- Ramalingam SS, Skoulidis F, Govindan R, et al. Efficacy of sotorasib in KRASG12C-mutated NSCLC with stable brain metastases: a post-hoc analysis of CodeBreaK100. Presented at: 2021 World Conference on Lung Cancer; September 8-14, 2021. Virtual. Abstract P52.03.
- Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;8:822-835.
- Paik PK, Felip E, Veillon R, et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383(10):931-943.
- Socinski MA, Pennell NA, Davies KD. MET exon 14 skipping mutations in non-small-cell lung cancer: an overview of biology, clinical outcomes, and testing considerations. JCO Precis Oncol. 2021;5:PO.20.00516.
- Cipriani NA, Abidoye OO, Vokes E, Salgia R. MET as a target for treatment of chest tumors. Lung Cancer. 2009;63(2):169-179.
- Salgia R, Sattler M, Scheele J, et al. The promise of selective MET inhibitors in non-small cell lung cancer with MET exon 14 skipping. Cancer Treat Rev. 2020;87:102022.
- De Mello RA, Neves NM, Amaral GA, et al. The role of MET inhibitor therapies in the treatment of advanced non-small cell lung cancer. J Clin Med. 2020;9(6):1918.
- Wu YL, Smit EF, Bauer TM. Capmatinib for patients with non-small cell lung cancer with MET exon 14 skipping mutations: a review of preclinical and clinical studies. Cancer Treat Rev. 2021;95:102173.
- Wolf J, Seto T, Han J-Y, et al. Capmatinib in MET exon 14–mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383(10):944-957.
- Friese-Hamim M, Bladt F, Locatelli G, et al. The selective c-Met inhibitor tepotinib can overcome epidermal growth factor receptor inhibitor resistance mediated by aberrant c-Met activation in NSCLC models. Am J Cancer Res. 2017;7(4):962-972.
- Wu YL, Cheng Y, Zhou J, et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): an open-label, phase 1b/2, multicentre, randomised trial [published correction appears in Lancet Respir Med. 2020 Jul;8(7):e59]. Lancet Respir Med. 2020;8(11):1132-1143.
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154-162.
- Yun J, Lee SH, Kim SY, et al. Antitumor activity of amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in diverse models of EGFR exon 20 insertion-driven NSCLC. Cancer Discov. 2020;10(8):1194-1209.
- Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39(30):3391-3402.
- Prelaj A, Bottiglieri A, Proto C, et al. Poziotinib in advanced NSCLC with EGFR or HER2 exon 20 insertion mutation: initial results from a single site expanded access program. Abstract 1388P. Ann Oncol. 2020;31(suppl 4):S882. Accessed November 25, 2021. https://www.annalsofoncology.org/article/S0923-7534(20)41698-9/fulltext#relatedArticles
- Moores SL, Chiu ML, Bushey BS, et al. A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors. Cancer Res. 2016;76(13):3942-3953.
- Vijayaraghavan S, Lipfert L, Chevalier K, et al. Amivantamab (JNJ-61186372), an Fc enhanced EGFR/cMet bispecific antibody, induces receptor downmodulation and antitumor activity by monocyte/macrophage trogocytosis. Mol Cancer Ther. 2020;19(10):2044.
- Rybrevant (amivantamab-vmjw) prescribing information. Janssen Biotech Inc; July 2021.
- Gonzalvez F, Vincent S, Baker TE, et al. Mobocertinib (TAK-788): a targeted inhibitor of EGFR exon 20 insertion mutants in non-small cell lung cancer. Cancer Discov. 2021;11:1672-1687.
- Han H, Li S, Chen T, et al. Targeting HER2 exon 20 insertion-mutant lung adenocarcinoma with a novel tyrosine kinase inhibitor mobocertinib. Cancer Res. 2021;81(20):5311-5324.
- Riely GJ, Neal JW, Camidge DR, et al. Activity and safety of mobocertinib (TAK-788) in previously treated non-small cell lung cancer with EGFR exon 20 insertion mutations from a phase I/II trial. Cancer Discov. 2021;11(7):1688-1699.
- Exkivity (mobocertinib) prescribing information. Takeda Pharmaceuticals USA, Inc; September 2021.
- Zhou C, Ramalingam SS, Kim TM, et al. Treatment outcomes and safety of mobocertinib in platinum-pretreated patients with EGFR exon 20 insertion-positive metastatic non-small cell lung cancer: a phase 1/2 open-label nonrandomized clinical trial. JAMA Oncol. 2021 Oct 14;e214761.
- Lumakras (sotorasib) prescribing information. Amgen, Inc; May 2021.
- Tabrecta (capmatinib) prescribing information. Novartis Pharmaceuticals; May 2020.
- Tepmetko (Tepotinib) prescribing information. EMD Serono, Inc; February 2021.
- Ikesue H, Kusuda K, Satsuma Y, et al. Evaluation of the usefulness of protocol-based pharmacist-facilitated laboratory monitoring to ensure the safety of immune checkpoint inhibitors in patients with lung cancer. J Clin Pharm Ther. 2020;45(6):1288-1294.
- Mackler E, Segal EM, Muluneh B, et al. 2018 Hematology/Oncology Pharmacist Association best practices for the management of oral oncolytic therapy: pharmacy practice standard. J Oncol Pract. 2019;15(4):e346-e355.
- McLouth LE, Nightingale CL, Levine BJ, et al. Unmet care needs and financial hardship in patients with metastatic non-small-cell lung cancer on immunotherapy or chemoimmunotherapy in clinical practice. JCO Oncol Pract. 2021;17(8):e1110-e1119.
- Fajardo S, Zook F, Dotson E. Specialty pharmacy for hematologic malignancies. Am J Health Syst Pharm. 2016;73(11):797-809.
- Joret R, Matti N, Beck M, Michel B. Medication adherence and persistence among patients with non-small cell lung cancer receiving tyrosine kinase inhibitors and estimation of the economic burden associated with the unused medicines. J Oncol Pharm Pract. 2021 Apr 24;10781552211012452.
- Hess LM, Louder A, Winfree K, et al. Factors associated with adherence to and treatment duration of erlotinib among patients with non-small cell lung cancer. J Manag Care Spec Pharm. 2017;23(6):643-652.
- QOPI Certification Program Standards. Institute for Quality: American Society of Clinical Oncology (ASCO). Updated January 1, 2020. Accessed November 25, 2021. https://practice.asco.org/sites/default/files/drupalfiles/2019-12/QOPI%20Certification%20Standards%20January%202020.pdf.
- Ahmed A, Bharali A, Dutta A, et al. The role of pharmacists in optimizing molecular testing with evolving biomarkers and treatment for non-small cell lung cancer. Eur J Mol Clin Med. 2021;8:2240-2259. Accessed November 25, 2021. https://ejmcm.com/article_10425.html
- Rosenberg SM, Petrie KJ, Stanton AL, et al. Interventions to enhance adherence to oral anti-neoplastic agents: a scoping review. J Natl Cancer Inst. 2020;112(5):443-465.
- Walter C, Mellor JD, Rice C, et al. Impact of a specialist clinical cancer pharmacist at a multidisciplinary lung cancer clinic. Asia Pac J Clin Oncol. 2016;12(3):e367-374.
- Ignoffo RJ, Knapp KK, Seung A, et al. Trends in the delivery of care to oncology patients in the United States: emphasis on the role pharmacists on the healthcare team. J Oncol Pharm Pract. 2021;27(1):5-13.
- Khrystolubova N, Shieh M, Patel AJ, Bailey R. Pharmacist-led patient education and adverse event management in patients with non-small cell lung cancer receiving afatinib in a community-based, real-world clinical setting. J Oncol Pharm Pract. 2020;26(1):13-22.
- Cheema PK, Thawer A, Leake J, et al. Multi-disciplinary proactive follow-up algorithm for patients with advanced NSCLC receiving afatinib. Support Care Cancer. 2019;27(3):1029-1039.
- Nishibe-Toyosato S, Ando Y, Goto Y, et al. The influence of intervening on the pharmaceutical consultation targeting outpatients with advanced non-small cell lung cancer receiving erlotinib treatment. Biol Pharm Bull. 2021;44(9):1280-1285.
- Yeoh TT, Si P, Chew L. The impact of medication therapy management in older oncology patients. Support Care Cancer. 2013;21(5):1287-1293.
Back to Top