
Expired activity
Please go to the PowerPak
homepage and select a course.
Introduction (DOACs vs Warfarin)
Anticoagulants disrupt hemostasis by diminishing vitamin K-dependent coagulation factors or augmenting anticoagulation via antithrombin III (ATIII).1 Despite prescribing patterns signaling an increased use of direct oral anticoagulants (DOACs), warfarin remains the longstanding agent of anticoagulant therapy, accounting for over 40% of anticoagulant prescriptions in the country.2 It is a potent vitamin K antagonist (VKA) that binds vitamin K epoxide reductase, an enzyme responsible for the downstream hepatic formation of active clotting factors (II, VII, IX, and X) in the clotting cascade. Figure 1 demonstrates this clotting cascade and the role of vitamin K-dependent clotting factors in the intrinsic and extrinsic clotting pathways that result in thrombin (factor IIa) conversion of fibrinogen to fibrin, and clot formation and stabilization.3
| Figure 1. Clotting Cascade3 |
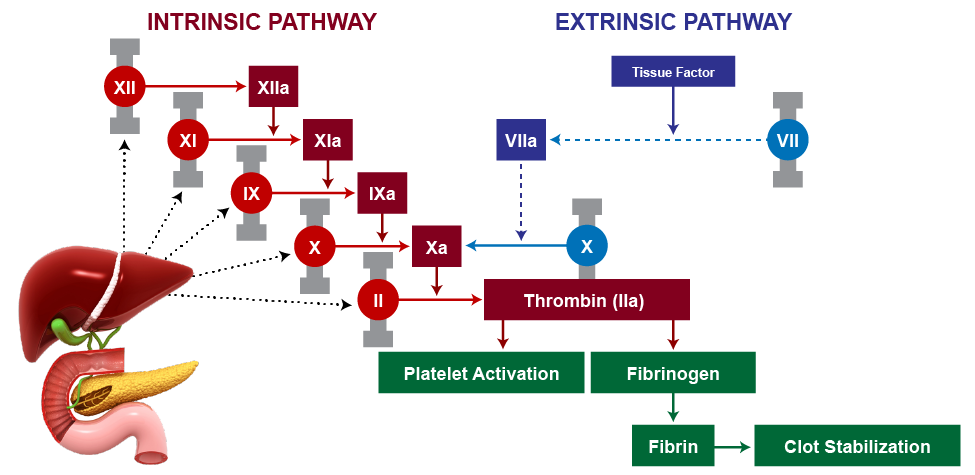 |
However, despite its historic use and inexpensive cost, warfarin possesses several limitations, including a narrow therapeutic range, which necessitates frequent laboratory monitoring as well as dosage adjustment to avoid bleeding, slow onset of action, and the well-known association with several drug-drug and drug-food interactions. The advent of DOACs established practical advantages over VKA therapy due to fewer monitoring requirements, less need for follow-up, and fewer drug and food interactions. The DOACs are grouped into 2 categories: factor IIa inhibitors (dabigatran) and factor Xa (FXa) inhibitors (rivaroxaban, apixaban, edoxaban). Clinical use for patients requiring anticoagulation has increased over the last decade.4
In fact, DOACs have largely supplanted warfarin as the preferred choice for OAC.4-7 Analysis of Medicare claims from a large United States (US) health plan indicate that 79% of patients with nonvalvular atrial fibrillation (AF) starting an OAC in the first quarter (Q1) of 2017 were treated with DOACs. Most patients starting an OAC in Q1 2017 (75%) received an FXa inhibitor, specifically apixaban or rivaroxaban. About 21% received warfarin with the balance (4%) started on the direct thrombin inhibitor dabigatran.7 A similar shift occurred in patients receiving OAC for primary treatment of venous thromboembolism; apixaban and rivaroxaban together accounted for 81% of OAC Medicare claims for this indication in 2017.5
Guidelines recommend DOACs over warfarin for OAC-eligible patients with AF, except those with a mechanical heart valve or moderate to severe mitral stenosis.8,9 DOACs are also recommended over warfarin for patients with a deep vein thrombosis (DVT) and/or pulmonary embolism (PE).10 This recommendation for DOACs is based on evidence of their efficacy in preventing stroke and cardiovascular (CV) death that is similar, and in some cases greater, than the rate of prevention achieved with warfarin.11,12 Incidentally, a number of patients with AF (or other indications requiring anticoagulant therapy) experience bleeding annually due to the increasing frequency of DOAC use as portrayed in Table 1.13
| Table 1. Incidence of DOAC-Related Bleeding13 |
| Registry following 272,315 patients with atrial fibrillation (median follow-up=4 years) |
| |
Major/Life-Threatening Bleeding |
Intracranial Bleeding |
| No anticoagulant |
1.4 (1.3–1.4) |
0.3 (0.3–0.3) |
| DOAC alone |
2.2 (2.1–2.3) |
0.5 (0.4–0.5) |
| DOAC + antiplatelet medication |
4.0 (3.5–4.5) |
0.6 (0.4–0.8) |
| DOAC triple therapy |
8.8 (6.4–11.9) |
0.9 (0.3–2.1) |
Bleeding per 100 person years (95% confidence interval).
DOAC triple therapy = DOAC + aspirin + antiplatelet medication. |
While bleeding is the most significant complication of DOACs, the rate of intracranial hemorrhage (ICH) is likely lower with most DOACs.14 In fact, DOACs have shown a reduced risk of major bleeding, and are associated with roughly half the risk of ICH when compared to warfarin.11,12,14 Nevertheless, DOACs may be associated with a higher risk of gastrointestinal (GI) bleeding, compared to warfarin.11,12 Overall the risk of death from major bleeding in patients receiving DOACs is 8% to 15%.14 Certain patient-specific risk factors may also be associated with DOAC-related bleeding (Table 2).15,16
| Table 2. Risk Factors for DOAC-Related Bleeding15,16 |
| Patient Factors |
Medications |
| Older age |
Aspirin |
| Declining kidney function |
NSAIDs |
| History of bleeding |
Concomitant antiplatelet therapy |
| Anemia |
Anemia |
Given the propensity for DOACs to cause major or life-threatening bleeding events, there is often a need to reverse the anticoagulant effect of these agents. Both specific and non-specific reversal agents are available for the DOACs and guidelines for their use have evolved over the years.17-19 This educational activity will review the latest guidelines for DOAC reversal as well as examine the clinical trial evidence supporting these guidelines in order to provide hospital and health-system pharmacists with key clinical insights needed for management of patients requiring DOAC reversal.
Clinical Scenario(s) to Consider the Use of a Reversal Agent
It is important to always consider the site of bleeding, the hemodynamic status, necessity of surgery, and last known ingestion of the anticoagulant. Pharmacologic interventions are likely needed for bleeding that occurs at a critical site (Table 3). Hemodynamic instability in the setting of presumed or confirmed bleeding and critical drops in hemoglobin could impede adequate tissue perfusion and oxygenation and would therefore necessitate DOAC reversal. In the setting of trauma or emergent life-saving surgical intervention (<1 hour), rapid reversal would likely improve survival.20 However, it should be noted that some patients who present with DOAC-related bleeding, may not require active reversal. Consensus guidelines state that patients without significant trauma may not require pharmacologic intervention. Importantly, the anticoagulation effect will dissipate after 4 to 5 half-lives. Therefore, in some cases, interventions may be avoided if presentation is identified as beyond 5 half-lives after drug ingestion or if supportive measures can be implemented in order to stabilize the patient until after 5 half-lives have passed.
Defining Bleed Severity18
If ≥1 of the following factors applies, the bleed is classified as major or life-threatening:
- Bleeding at a critical site (Table 3).
- Hemodynamic instability associated with bleeding
- Clinically overt bleeding with hemoglobin decrease ≥2 g/dL or administration of ≥2 units red blood cells (RBCs)
Non-major bleeding is defined as any bleeding that is not major or life-threatening bleeding as above.
| Table 3. Critical Site Bleeding18 |
| Site |
Type of Bleed |
| Central Nervous System (CNS) |
· Intracranial
· Intraocular
· Spinal |
| Thoracic |
· Abdominal
· Airway
· Cardiac Tamponade
· Hemothorax
· Retroperitoneal |
| Extremities |
· Intra-articular
· Intramuscular |
Reversal for Urgent Surgery or Procedures
Surgery or invasive procedure with significant risk of life-threatening bleeding or bleeding at critical site that cannot be reasonably delayed beyond the length of the anticoagulant’s therapeutic effect (ie, 2 or more half-lives depending on clinical scenario) while empiric medical treatment is delivered.19
Use of Coagulation Tests
The dilute thrombin time, ecarin clotting assay, and ecarin clotting time can be used for quantification of dabigatran. An anti-Xa assay that is specially calibrated for apixaban, edoxaban, or rivaroxaban could be used for quantification of the FXa antagonists. However, these tests for quantitative assessment of DOACs are not always readily available in most clinical settings.18 Tests that are clinically available and may play some role in qualitative assessment include the thrombin time for dabigatran assessment and the anti-Xa assay that is calibrated for heparin for qualitative assessment of the factor FXa antagonists. If the thrombin time is normal, it is unlikely that relevant levels of dabigatran are present. Similarly, if the anti-Xa assay is within normal range, it is unlikely that clinically significant levels of apixaban, edoxaban, or rivaroxaban are present. Interpretation of other commonly available coagulation tests such as prothrombin time (PT) and activated partial thromboplastin time (aPTT) is challenging and often less useful.18
ACC and ACEP Guidance for Anticoagulant Reversal
The American College of Cardiology (ACC) and the American College of Emergency Physicians (ACEP) published expert panel recommendations that address DOAC reversal in 2020.18,19 These guidelines provide guidance on identification of the patient requiring reversal, the optimal pharmacological approach for reversing a DOAC and how to select an appropriate dose, and administer and monitor both the specific (ie, andexanet alfa or idarucizumab) and non-specific (blood factor products) reversal agents.
Who to Reverse?
The first step in identifying which patient to reverse is to assess the severity of bleeding. In general, patients who have major or life-threatening bleeding (described above), should be actively reversed with a pharmacologic agent. Conversely, those with non-major bleeding can be managed with supportive measures and monitored until sufficient DOAC half-lives have passed for elimination of the drug. The other consideration for identifying which patient to reverse is the underlying thrombotic risk of the patient. If thrombotic risk is particularly high, it must be carefully weighed against the risk from the bleeding event. Considerations for determining which patients require reversal are summarized in Table 4.
| Table 4. Characteristics Favoring Reversal vs Conservative Management18,19 |
| Elements That Favor Reversal |
Elements That Favor Watchful Waiting |
· Shock/hemodynamic instability
· Long-acting anticoagulant
· Renal insufficiency with short-acting anticoagulation
· Critical organ or space (eg, intracranial hemorrhage, intraspinal)
· High likelihood of deterioration
· Need for vasopressors
· Massive transfusion requirement |
· Hemodynamically/clinically stable
· High thrombosis risk (eg, mechanical valve, ventricular assist device)
· Short-acting anticoagulant with normal renal function
· Greater than 18 hours since last dose of short-acting anticoagulant |
Periprocedural Management of Anticoagulation21
Temporary interruption (TI), the omission of ≥1 dose of an OAC in preparation for a procedure, is frequently necessary, most often to mitigate bleed risk with surgical or invasive procedures.
Guidance Statement for interruption of a DOAC peri-procedurally:
- Interrupt therapy for low bleed-risk procedures in:
- Patients treated with any of the approved DOACs for a duration based on the estimated CrCl (Table 5).
- Interrupt therapy for intermediate, high, or uncertain bleed-risk procedures in:
- Patients treated with any of the approved DOACs for a duration based on the estimated CrCl (Table 5).
| Table 5. Recommended Durations for Withholding DOACs Based on Procedural Bleed Risk and Estimated CrCl When There Are No Increased Patient Bleed Risk Factors21 |
| |
Dabigatran |
Apixaban, Edoxaban, or Rivaroxaban |
| CrCl, mL/min |
≥80 |
50-79 |
30-49 |
15-29 |
<15 |
≥30 |
15-29 |
<15 |
| Estimated drug half-life, h |
13 |
15 |
18 |
27 |
30* |
6-15 |
Apixaban: 17
Edoxaban: 17
Rivaroxaban: 9 |
Apixaban: 17*
Edoxaban: 10-17*
Rivaroxaban: 13* |
| Procedural bleed risk |
| Low |
≥24 h |
≥36 h |
≥48 h |
≥72 h |
No data. Consider measuring dTT and/or withholding ≥96 h. |
≥24 h |
≥36 h |
No data. Consider measuring agent-specific anti-Xa level and/or withholding ≥48 h. |
Uncertain, intermediate,
or high |
≥48 h |
≥72 h |
≥96 h |
≥120 h |
No data. Consider measuring dTT. |
≥48 h |
No data. Consider measuring
agent-specific anti-Xa level and/or withholding ≥72 h. |
*Off dialysis.
CrCl, creatinine clearance; DOAC, direct-acting oral anticoagulant; dTT, dilute thrombin time. |
Specific DOAC Reversal Agents
Two specific agents are available for the reversal of DOACs (Table 6). Idarucizumab is used for the reversal of the direct thrombin inhibitor, dabigatran. Andexanet alfa has been studied for reversal of the anti-FXa agents, apixaban and rivaroxaban, in a large clinical trial.22 Clinical trials to evaluate the impact of andexanet alfa on reversal of edoxaban are not available; however, preliminary data in this area seems promising.23
| Table 6. ACEP Guidance for Administering Reversal Agents19 |
| Class |
Direct Thrombin Inhibitor |
Anti-Xa Inhibitor |
| Agent |
Dabigatran |
Rivaroxaban/Apixaban |
Edoxaban |
| Last dose |
(<8-12 h) |
(<8-12 h) |
(10-14 h) |
| Tier 1 |
Idarucizumab |
Andexanet alfaa |
Andexanet alfab |
| Tier 2c |
PCCd | dialysis |
PCCd |
PCCd |
| aFor bleed only; bNot FDA approved; cIf Tier 1 not available; dIncludes: 4F-PCC (preferred), 3F-PCC, and a PCC. |
Idarucizumab
In 2015, idarucizumab was the first DOAC antidote approved for emergent reversal of a DOAC, specifically dabigatran.24 It binds both bound and unbound dabigatran, effectively neutralizing the anticoagulant effects. Unique to its structure, idarucizumab is a humanized monoclonal antibody fragment (Fab), possessing higher affinity to dabigatran than dabigtran’s affinity for thrombin. Consequently, it is ineffective in binding to FXa inhibitors such as rivaroxaban or apixaban. Idarucizumab is available as single dose, 2.5 g per 50 mL vials and must be stored in the refrigerator (2ºC to 8ºC) protected from light. Once vials are removed, they should be used within 6 hours.25 Idarucizumab is administered as one 5–g (2 × 2.5 g vials) IV bolus injection, with an administration window of <8-12 hours after last dose of dabigatran.25
Idarucizumab was evaluated for reversal of dabigatran in the REVERSE-AD trial.26 REVERSE-AD included approximately 300 patients with DOAC-associated bleeding and 200 patients who required reversal for urgent surgery or procedure. The primary endpoint was laboratory-based reversal of the anticoagulant effect of dabigatran, but there was also a clinical endpoint of time to cessation of bleeding (for bleeding patients) and periprocedural hemostasis (surgery/procedure patients). Bleeding cessation occurred in 68% of bleeding patients and the vast majority of surgery/procedure patients had normal periprocedural hemostasis. It is notable that 4.8% of patients experienced a thrombotic event within 30 days of anticoagulation reversal.
Andexanet Alfa
Much like idarucizumab, andexanet alfa is also a specific reversal agent and acts as a factor Xa decoy protein.27 It Is catalytically inactive, thus neutralizing any anticoagulation effects once bound and binds to direct FXa inhibitors such as apixaban, edoxaban, and rivaroxaban. Despite its ability to bind to most FXa inhibitors, there is limited clinical evidence to support reversal of edoxaban and therefore is only approved by the US Food and Drug Administration (FDA) for reversal of apixaban and rivaroxaban.
The ANNEXA-4 trial was a prospective, multicenter, open-label, single group study of andexanet alfa for reversal of FXa inhibitors (primarily apixaban and rivaroxaban) in patients presenting with major/life-threatening bleeding.22 The co-primary endpoints of ANNEXA-4 were laboratory-based reversal of the DOAC and hemostatic efficacy within 12 hours of anticoagulant reversal. Over 250 patients were included in the efficacy analysis and hemostatic efficacy was noted to be excellent or good in over 80% of patients. The rate of thrombosis within 30 days of DOAC reversal in ANNEXA-4 was 10% and most thrombotic events occurred before anticoagulation had been reinitiated.
A post hoc analysis of data from ANNEXA-4 evaluated the relationships between reinitiation of anticoagulation and the risk of thrombosis, rebleeding, and mortality.22,28 In this analysis, 28% of patients (100/352) restarted therapeutic anticoagulation within 30 days. Those who were reinitiated on anticoagulation were younger and less likely to have presented with ICH. In an adjusted analysis at 14 days post-reversal, reinitiation was associated with a reduction in thrombotic events (hazard ratio, 0.112, 95% CI 0.001–0.944; P=0.043) and an increase in bleeding events (hazard ratio, 8.39, 95% CI 1.13–62.29; P=0.037). A time-dependent Cox model showed that reinitiating anticoagulation was associated with a reduction in a composite of thrombotic events, bleeding, and death (hazard ratio, 0.384, 95% CI 0.161-0.915; P=0.031).28
Dosing
The andexanet alfa package insert classifies dosing strategies into low and high andexanet alfa doses, each given as an initial intravenous bolus followed by an intravenous infusion. Dosing depends upon timing and amount of the last known DOAC dose (Table 7). If the strength and timing of the last dose of the DOAC are unknown, then the high dose should be used.29
- Low dose: 400 mg (30 mg/min) IV boslus + 4 mg/min for up to 120 minutes
- High dose: 800 mg (30 mg/min) IV bolus + 8 mg/min for up to 120 minutes
| Table 7. Andexanet Alfa Dosing Strategies for FXa Inhibitors29 |
| FXa Inhibitor |
FXa Inhibitor
Last Dose |
<8 Hours or Unknown From Last Dose |
≥8 Hours From
Last Dose |
| Rivaroxaban |
≤10 mg |
Low dose |
Low dose |
| >10 mg/unknown |
High dose |
| Apixaban |
≤5 mg |
Low dose |
| >5 mg/unknown |
High dose |
As noted previously, andexanet alfa is not approved for use in reversal of edoxaban; however, if it were to be used in a patient with bleeding associated with edoxaban, the higher dose of andexanet alfa has been recommended.18,22
Non-Specific DOAC Reversal Agents
Prior to the availability of specific reversal agents, non-specific DOAC reversal agents were employed. Limited data exists to support the use of either 4-factor prothrombin complex concentrates (PCCs) or FEIBA (factor eight inhibitor bypassing agent). There are no prospective randomized trials of the non-specific DOAC reversal agents for either dabigatran or the FXa antagonists. Data are extremely limited to support dabigatran reversal with non-specific agents and includes healthy volunteer studies, animal data, and case reports. Most data regarding reversal of the FXa antagonists with non-specific agents are limited to observational studies. Studies of 4-factor PCCs have generally focused on patients with DOAC-associated bleeding, rather than patients requiring urgent surgery or procedures. Hemostatic efficacy after reversal of apixaban or rivaroxaban was achieved in approximately 70% to 80% of patients and thrombosis occurred in about 4% to 8% of patients in these studies.18,30 Study of the use of FEIBA for DOAC reversal is largely limited to case reports and case series. Both 4-factor PCCs and FEIBA have been studied in doses ranging from 10 to 25 units/kg.18,19
Specific Reversal Agents or PCCS?
The design of pivotal clinical trials of the 2 specific DOAC reversal agents available in the US did not include a placebo or active comparator arm. Therefore, it is unclear whether the specific DOAC reversal agents offer advantages over non-specific options. A prospective, randomized, open-label study comparing andexanet alfa to usual care in patients with an acute ICH associated with an oral FXa inhibitor is in progress.31
While prospective randomized data comparing andexanet alfa with a 4-factor PCC are not available, comparison of the 2 reversal approaches has been attempted with observational data.32-35 Table 8 summarizes these findings below.
| Table 8. Comparing Andexanet Alfa and PCC32-25 |
| Study |
Design |
Patients |
Results |
| Ammar 202132 |
Single-center retrospective |
FXa inhibitor-associated ICH; n=28, AA; n=16, 4F-PCC
|
· AA≈4F-PCC on all outcomes*
· ICH stability on CT scan at 6, 10, 24 hrs
· mRS at discharge
· Thromboembolic events
|
| Barra 202033 |
Single-center retrospective |
Apixaban- or rivaroxaban-associated ICH;
n=18, AA; n=11, 4F-PCC
|
· Hemostasis: AA 89% vs
4F-PCC 60%†
· Thrombosis at 30 days:
AA 17%, 4F-PCC 9%
|
| Stevens 202134 |
Retrospective cohort study |
Apixaban or rivaroxaban-associated ICH; n=16, AA; n=16, 4F-PCC
|
· Hemostasis: AA 75% vs
4F-PCC 62% (P =0.70)
· Thrombotic event: AA 25% (n=4) vs 4F-PCC 18.8 (n=3); (P=0.99)
· Mortality incidence:
AA 12.5% vs 4F-PCC 31.3% (P=0.39)
|
| Coleman 202135 |
Retrospective database analysis, 45 US hospitals, major bleeding hospitalizations associated with FXa inhibitors |
n=3030
|
· In-hospital death: 4% AA, 10% 4F-PCC, 11% FFP; 8% other agents/no agents†
|
*P>0.05; †No statistical comparison performed.
4F-PCC,4-factor prothrombin complex concentrate; AA, andexanet alfa; CT, computed tomography; FFP, fresh frozen plasma; FXa, factor Xa; hrs, hours; ICH, intracranial hemorrhage; mRS, modified Rankin Score. |
The aforementioned retrospective analyses demonstrate that improvements in measures of bleeding control were associated with both specific and non-specific reversal agents; thrombotic adverse events were also associated with both types of reversal approaches in several of the analyses. Comparative hemostatic control data should be interpreted with caution, since these reports included a low number of patients and were not randomized, increasing the potential for confounding to have an impact on the results. The largest study included above (n=3030) compared andexanet alfa to multiple different non-specific reversal strategies and it is unclear whether patients who received the different treatments were similar in risk for death, or if selection bias could have influenced the mortality findings. The prospective, randomized, open-label study comparing andexanet alfa to usual care in patients with an acute ICH taking an oral FXa inhibitor noted previously should help to clarify whether non-specific or specific reversal strategies represent the optimal reversal approach.31
A recent systematic review and meta-analysis has been completed, which included 3 of the studies in Table 8 above. The meta-analysis revealed that andexanet alfa was associated with lower mortality compared to 4F-PCC (OR, 0.37; 95% CI, 0.20-0.71), among patients with ICH. No difference in thrombotic events was noted between patients receiving AA and 4F-PCC (OR, 2.40; 95% CI, 0.36-15.84). Length of hospital stay, and intensive care unit (ICU) stay were also similar between groups.36
Guideline Recommendations for DOAC Reversal
The ACC and ACEP guidelines recommend idarucizumab as a first-line agent for reversing dabigatran. Andexanet alfa is recommended for reversal of the FXa inhibitors, apixaban and rivaroxaban. Both guidelines also recommend that andexanet alfa be used to reverse edoxaban, despite the lack of clinical trial data and FDA approval for this use.18,19 If idarucizumab is not available, the ACC guidelines recommend that 4-factor PCC be used and the ACEP guidelines suggest that 4-factor PCC or FEIBA could be used. If andexanet alfa is unavailable, both the ACC and ACEP guidelines recommend that either 4-factor PCC or FEIBA could be used, although 4-factor PCC is preferred (Table 6).18,19 Hence, the decision to administer reversal agents is multifactorial. (See Figure 2.)
| Figure 2. ACC Expert Consensus Decision Pathway*18 |
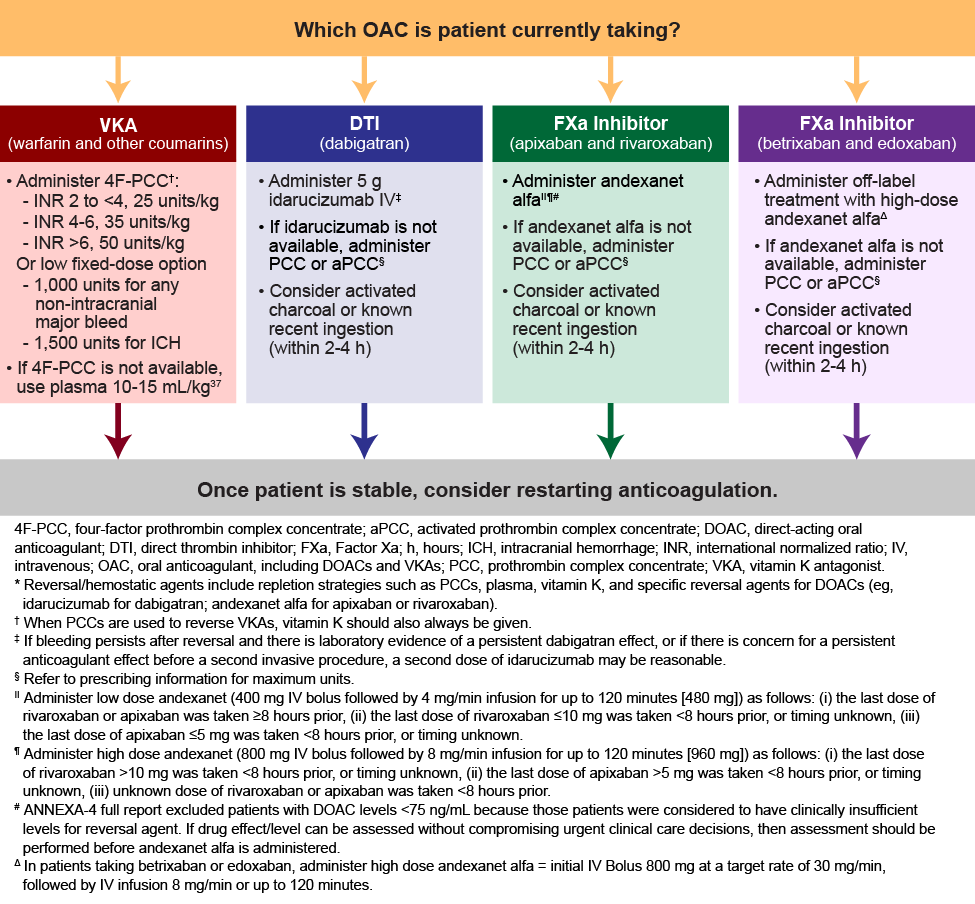 |
Minimizing Thrombosis Risk After DOAC Reversal
While management of the major or life-threatening bleeding event is usually the primary focus when caring for patients with DOAC-associated bleeding, it is important to remember that the patient was taking an oral anticoagulant for a reason. Often, patients who present with significant bleeding associated with a DOAC, are also at high risk for thrombosis. The relatively high rate of thrombosis in the REVERSE-AD, ANNEXA-4 trials and most of the 4-factor PCC studies highlights this high thrombotic risk that likely results from not only removing an anticoagulant, but also actively reversing the effects of that anticoagulant.
Prompt reinitiation of anticoagulation has been shown to prevent thrombosis but may promote risk of rebleeding. The ACC expert panel recommendations for DOAC reversal addresses this topic.18 The ACC guidelines recommend that a stepwise approach be undertaken in determining whether it is appropriate to restart anticoagulation therapy.
A post hoc analysis of data from the pivotal clinical trial of andexanet alfa evaluated the relationships between restarting anticoagulation and the risk of thrombosis, rebleeding, and mortality.22,28 A time-dependent Cox model showed that restarting anticoagulation was associated with a reduction in a composite of thrombotic events, bleeding, and death (hazard ratio, 0.384, 95% CI 0.161-0.915; P=0.031).28 In this trial, 28% of patients (100/352) restarted therapeutic OAC within 30 days. Nearly three-quarters (74%) of those not restarted had an ICH. Younger age also was associated with restarting. Among those who restarted, none had a thrombotic event, 3 patients had a bleeding event, and 3 patients died. In those who did not restart, 12 patients had thrombotic events, 2 had a bleeding event, and 17 died. One possible explanation for these findings could be that lower-risk patients are more likely to be restarted on OAC.28
A number of systematic reviews and meta-analyses demonstrate that restarting anticoagulant therapy following an OAC-related bleeding event may result in lower risk of thromboembolic complications, and is not associated with an increase in mortality or ICH recurrence. Nevertheless, it is noted that evidence from randomized controlled studies is needed to further clarify the clinical benefit of restarting OAC in indicated patients.38-40
After a patient has a bleeding event related to an oral anticoagulant, the indication for anticoagulation should be reassessed to determine whether continued therapy is warranted.18 Figure 3 includes guidance for considering when and whether a patient should resume anticoagulation therapy.
| Figure 3. Considerations for Restarting Anticoagulation18 |
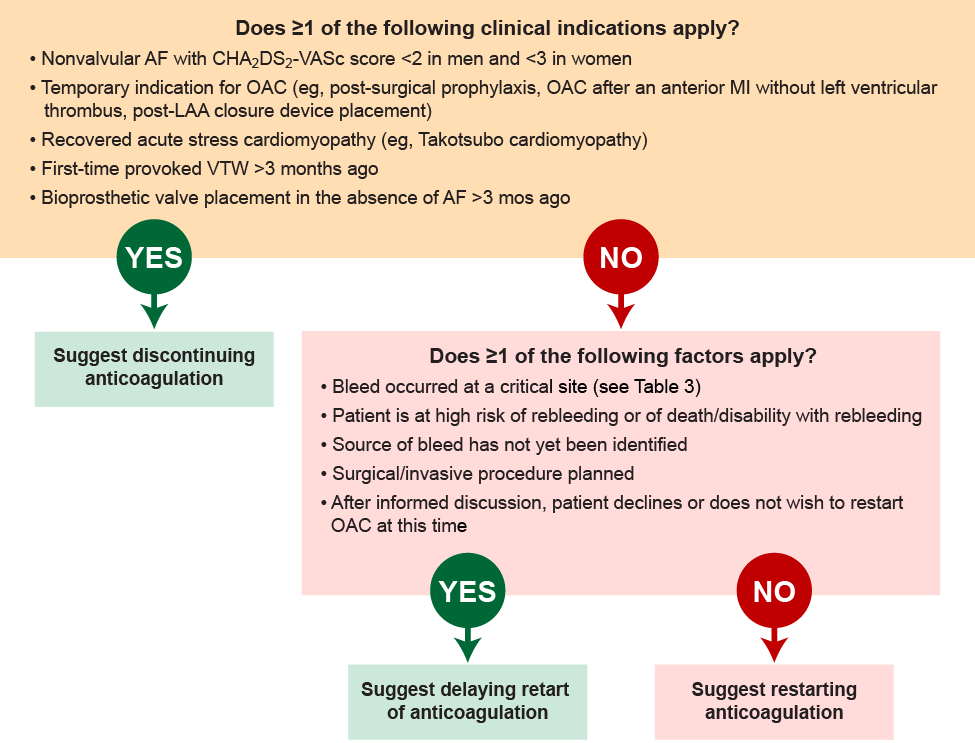 |
Once it has been determined that the patient continues to have an indication for anticoagulation, the next step is to determine whether reinitiation should occur. The decision on timing is based on assessment of the risk for thrombosis balanced with the risk for a recurrent bleeding event.18
Patients who have a known bleeding site and who did not experience bleeding at a critical site, who aren’t planning to undergo an invasive surgery or procedure, and who are not at high risk of experiencing another bleeding event or a poor outcome should a bleeding event occur, may be reinitiated earlier. When reinitiation is to occur, it is important to limit future bleeding risk by discontinuing unnecessary antiplatelet agents, accounting for potential drug interactions, and adjusting drug doses as appropriate for organ dysfunction.18
Reinitiation of anticoagulation should be delayed if the patient has any of the high-risk features noted above. The amount of time required before reinitiation is an area of debate and depends on the specific patient scenario. For example, the ACC guidance suggests that anticoagulation reinitiation should be delayed at least 4 weeks in a patient who had experienced intracranial hemorrhage. If there is considerable risk for a recurrent bleeding event, an assessment of thrombotic risk should occur. When thrombotic risk is low or moderate, anticoagulation reinitiation can be delayed, although it may be reasonable to consider venous thromboembolism prophylaxis for these patients. When both thrombotic and recurrent bleeding risks are high, temporary anticoagulation should be considered with intravenous heparin, venous thromboembolism prophylaxis agents, or non-pharmacologic approaches, as appropriate.18
Reversal Stewardship/Pharmacist Role
Cost of Reversal
While the cost of reversal with idarucizumab is fairly similar to reversal with a 4-factor PCC, reversal with andexanet alfa is considerably more expensive. By far, the biggest barrier to access to the specific reversal agents centers around cost and the ability to justify use. This may be particularly true in hospitals that do not have policies, guidelines, or processes in place for ensuring appropriate use.
The US Centers for Medicare & Medicaid Services (CMS) has offered a New Technology Add-On Payment (NTAP) for andexanet alfa since October 2018 to expand access to the agent for eligible beneficiaries (Figure 4). The NTAP is an added payment, in addition to the payment for the hospitalization, that is specifically available when andexanet alfa is used to treat a patient with standard Medicare. The NTAP covers about 65% of the wholesale acquisition cost of the standard dose and can be provided in addition to the payment for the hospitalization.41
| Figure 4. Wholesale Costs of DOAC Reversal Agents42-43 |
Idarucizumab42
Andexanet43
- Low dose ~ $25,000
- High dose ~ $49,500
|
Medicare New Technology Add-On Payments (NTAP)
CMS has granted ANDEXXA additional NTAP payment up to
$18,281.25
Effective October 1, 2019
Hospital Inpatient Setting
Medicare Inpatient Coding and Payment (Part A)
This information details our general understanding of the application of certain codes to Andexxa. It is the provider’s sole responsibility to determine the appropriate codes for any action taken in billing. This information is not intended to be definitive or exhaustive, and Portola makes no warranties or guarantees as to the accuracy or appropriateness of this information. Before filing any claim, providers should verify these requirements with specific payers.
Only one MS-DRG is assigned to a patient for a particular hospital admission, and determined by ICD-10-CM diagnoses and procedure codes.
Patients who receive Andexxa during their hospital stay may be assigned to different MS-DRGs based on these variables.
It is important to use one of the two unique ICD-10-PCS procedure codes that were created effective October 1, 2016, for the introduction of Andexxa.
|
The pharmacist can play a key role in ensuring that DOAC reversal agents are used in a safe and cost-effective manner. Pharmacists can influence the appropriate selection and monitoring of a reversal agent for the bleeding patient, as an interdisciplinary team member at the bedside, especially in the emergency department or ICU setting. The pharmacist at the bedside can also assist the team in identifying when reinitiation of anticoagulation may be appropriate and in identifying the reason for the bleeding event in the first place (ie, drug interaction or inappropriate DOAC dose).
From an organizational standpoint, pharmacy staff can be instrumental in ensuring that reimbursement is maximized by helping to establish processes for submitting for the NTAP when appropriate. The pharmacist can be that leader that develops and maintains anticoagulation reversal guidelines that define optimal reversal approaches for the organization. Once these institutional guidelines have been developed, the pharmacist can evaluate compliance with these guidelines through medication use evaluations.
References
- Alquwaizani M, Buckley L, Adams C, et al. Anticoagulants: A Review of the Pharmacology, Dosing, and Complications. Curr Emerg Hosp Med Rep. 2013;1(2):83-97.
- Wheelock KM, Ross JS, Murugiah K, et al. Clinician Trends in Prescribing Direct Oral Anticoagulants for US Medicare Beneficiaries. JAMA Netw Open. 2021;4(12):e2137288.
- Kalus JS. Pharmacologic interventions for reversing the effects of oral anticoagulants. Am J Health Syst Pharm. 2013;70(10 Suppl 1):S12-S21.
- Colacci M, Tseng EK, Sacks CA, Fralick M. Oral anticoagulant utilization in the United States and United Kingdom. J Gen Intern Med. 2020;35(8):2505-2507.
- Lutsey PL, Walker RF, Maclehose RF, Alonso A, Adam TJ, Zakai NA. Direct oral anticoagulants and warfarin for venous thromboembolism treatment: Trends from 2012 to 2017. Res Pract Thromb Haemost. 2019;3(4):668-673.
- Perreault S, de Denus S, White-Guay B, et al. Oral anticoagulant prescription trends, profile use, and determinants of adherence in patients with atrial fibrillation. 2020;40(1):40-54. Pharmacotherapy. 2020;40(1):40-54.
- Zhu J, Alexander GC, Nazarian S, Segal JB, Wu AW. Trends and variation in oral anticoagulant choice in patients with atrial fibrillation, 2010-2017. 2018;38(9):907-920. Pharmacotherapy. 2018;38(9):907-920.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104-132.
- Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chest. 2018;154(5):1121-1201.
- Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Advances. 2020;4(19):4693-4738.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomized trials. 2014;383(9921):955-962. Lancet. 2014;383(9921):955-962.
- López-López JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. 2017;28;359:j5058. BMJ. 2017;28;359:j5058.
- van Rein N, Heide-Jørgensen U, Lijfering WM, et al. Major bleeding rates in atrial fibrillation patients on single, dual, or triple antithrombotic therapy: results from a nationwide danish cohort study. Circulation. 2019;139:775-786.
- Siegal DM. What we have learned about direct oral anticoagulant reversal. 2019;2019(1):198-203. Circulation. 2019;139:775-786.
- Hylek EM, Held C, Alexander JH, et al. Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin: The ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation): Predictors, Characteristics, and Clinical Outcomes. J Am Coll Cardiol 2014;63(20):2141-2147.
- Sherwood MW, Nessel CC, Hellkamp AS, et al. Gastrointestinal Bleeding in Patients With Atrial Fibrillation Treated With Rivaroxaban or Warfarin: ROCKET AF Trial. J Am Coll Cardiol. 2015;66(21):2271-2281.
- Cuker A, Burnett A, Triller D, et al. Reversal of direct oral anticoagulants: Guidance from the Anticoagulation Forum. Am J Hematol. 2019;94(6):697-709.
- Tomaselli GF, Mahaffey KW, Cuker A, et al. 2020 ACC Expert Consensus Decision Pathway on management of bleeding in patients on oral anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(5):594-622.
- Baugh CW, Levine M, Cornutt D, et al. Anticoagulant reversal strategies in the emergency department setting: Recommendations of a multidisciplinary expert panel. Ann Emerg Med. 2020;76(4):470-485.
- Nutescu EA, Dager WE, Kalus JS, et al. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm. 2013;70(21):1914-1929.
- Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC Expert Consensus Decision Pathway for Periprocedural Management of Anticoagulation in Patients With Nonvalvular Atrial Fibrillation: A Report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol. 2017;69(7):871-898.
- Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326-1335.
- Lu G, Conley PB, Leeds JM, et al. A Phase 2 PK/PD Study of Andexanet Alfa for Reversal of Rivaroxaban and Edoxaban Anticoagulation in Health Volunteers. Blood Adv. 2020;4:728-739.
- Eikelboom JW, Quinlan DJ, van Ryn J, Weitz JI. Idarucizumab: The Antidote for Reversal of Dabigatran. Circulation. 2015;132(25):2412-2422.
- Praxbind (idarucizumab) prescribing information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2018 Apr.
- Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for Dabigatran Reversal - Full Cohort Analysis. N Engl J Med. 2017;377(5):431-441.
- Holzmacher JL, Sarani B. Indications and Methods of Anticoagulation Reversal. Surg Clin North Am. 2017;97(6):1291-1305.
- Milling TJ, King B, Yue P, et al. Restart of anticoagulant therapy and risk of thrombosis, rebleeding and death after factor Xa inhibitor reversal in major bleeding patients. Thromb Haemost. 2021 Feb 25.
- Andexxa (coagulation factor Xa [recombinant], inactivated-zhzo) prescribing information. Boston, MA: Alexion Pharmaceuticals, Inc; 2021 Feb. Accessed January 24, 2022. https://alexion.com/Documents/andexxa_uspi.pdf.
- Panos NG, Cook AM, Sayona J, et al. Factor Xa inhibitor-related intracranial hemorrhage: Results from a multicenter, observational cohort receiving prothrombin complex concentrates. Circulation. 2020;141:1681-1689.
- Trial of andexanet in ICH patients receiving an oral FXa inhibitor. gov Identifier: NCT03661528. Updated July 28, 2020. Accessed January 24, 2022. https://clinicaltrials.gov/ct2/show/NCT03661528
- Ammar AA, Ammar MA, Owusu KA, et al. Andexanet alfa versus 4-factor prothrombin complex concentrate for reversal of factor Xa inhibitors in intracranial hemorrhage. [published online ahead of print, 2021 Jan 6]. Neurocrit Care. 2021;35(1):255-261.
- Barra ME, Das AS, Hayes BD, et al. Evaluation of andexanet alfa and four-factor prothrombin complex concentrate (4F-PCC) for reversal of rivaroxaban- and apixaban-associated intracranial hemorrhages. J Thromb Haemost. 2020;18(7):1637-1647.
- Stevens VM, Trujillo TC, Kiser TH, et al. Retrospective Comparison of Andexanet Alfa and 4-Factor Prothrombin Complex for Reversal of Factor Xa-Inhibitor Related Bleeding. Clin Appl Thromb Hemost. 2021;27:10760296211039020.
- Coleman CI, Dobesh PP, Danese S, Ulloa J, Lovelace B. Real-world management of oral factor Xa inhibitor-related bleeds with reversal or replacement agents including andexanet alfa and four-factor prothrombin complex concentrate: a multicenter study. Future Cardiol. 2021;17(1):127-135.
- Shrestha DB, Budhathoki P, Adhikari A, et al. Efficacy and Safety of Andexanet Alfa for Bleeding Caused by Factor Xa Inhibitors: A Systematic Review and Meta-Analysis. Cureus. 2021;13(12):e20632.
- Sarode R, Milling TJ Jr, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: A randomized, plasma controlled, phase IIIb study. Circulation. 2013;128:1234-1243.
- Murthy SB, Gupta A, Merkler AE, et al. Restarting Anticoagulant Therapy After Intracranial Hemorrhage: A Systematic Review and Meta-Analysis. Stroke. 2017;48(6):1594-1600.
- Newman TV, Chen N, He M, Saba S, Hernandez I. Effectiveness and Safety of Restarting Oral Anticoagulation in Patients with Atrial Fibrillation after an Intracranial Hemorrhage: Analysis of Medicare Part D Claims Data from 2010-2016. Am J Cardiovasc Drugs. 2020;20(5):471-479.
- Zhou Z, Yu J, Carcel C, et al. Resuming anticoagulants after anticoagulation-associated intracranial hemorrhage: systematic review and meta-analysis. BMJ Open. 2018;8(5):e019672.
- Portola Pharmaceuticals. US Centers for Medicare & Medicaid Services (CMS) proposes extending new technology add-on payment (NTAP) reimbursement for Portola Pharmaceuticals’ Andexxa® for third year. May 14, 2020. Accessed January 24, 2022. https://www.prnewswire.com/news-releases/us-centers-for-medicare--medicaid-services-cms-proposes-extending-new-technology-add-on-payment-ntap-reimbursement-for-portola-pharmaceuticals-andexxa-for-third-year-301059044.html
- Buchheit J, Reddy P, Connor JM. Idarucizumab (Praxbind) Formulary Review. Crit Pathw Cardiol. 2016;15(3):77-81. https://journals.lww.com/critpathcardio/toc/2016/09000
- Beik N, Reddy P, Sylvester K, et al. Andexanet Alfa (Andexxa) Formulary Review. Crit Pathw Cardiol. 2019;18(2):66-71. https://journals.lww.com/critpathcardio/toc/2019/06000
Back to Top