
Expired activity
Please go to the PowerPak
homepage and select a course.
The “Rights” of Medication Therapy and Administration in Obesity Management
» Obesity Pharmacotherapy Quick Reference Guide » Starting Medication for Weight Management
Introduction: The Prevalence of Medication Errors and the “Rights” of Effective Medication Administration
According to the National Coordinating Council for Medication Error Reporting and Prevention, a medication error is "any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health care provider (HCP), patient, or consumer (Figure 1).”1
| Figure 1: Examples of Medication Errors2-5 |
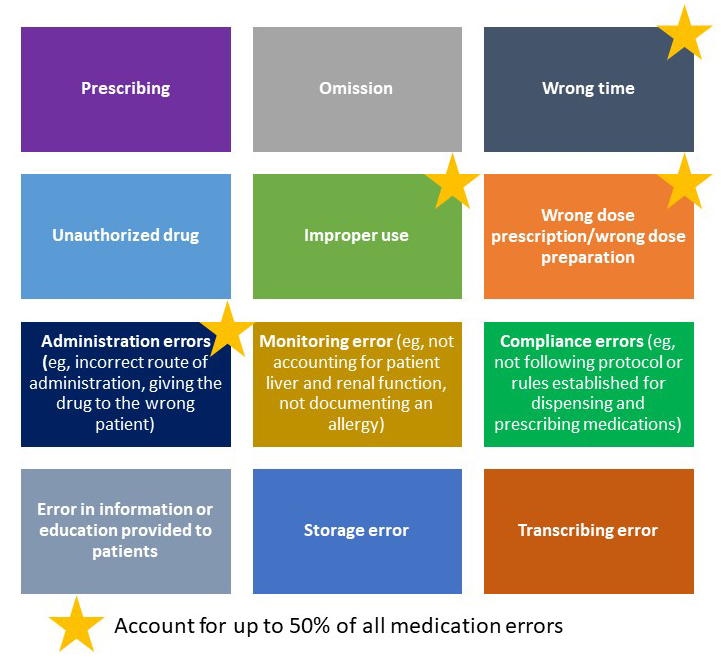
|
| Adapted from International Pharmaceutical Federation (FIP). Patient and medication safety. Pharmacists’ role in minimising preventable harm to patients. The Hague: International Pharmaceutical Federation (FIP); 2019. Available at https://www.fip.org/file/4757. |
Medication errors occur frequently in all care settings and have a substantial impact on both patients and the health care system.6 It has been estimated that in the United States, up to 1.5 million people are harmed and 7000 to 9000 die annually due to medication errors.5,7,8 The costs associated with medication errors are thought to be ≈$42 billion annually worldwide, and ≈$21 billion in the United States.7 Additionally, over 70% of Americans who experienced a medication error said it had a long-term impact on their physical health, emotional health, financial well-being, or family relationships, highlighting the burden on individual patients’ lives.8,9 However, reports suggest that half of all medication errors occurring in the hospital are preventable and around one-third of post-discharge adverse drug events could be prevented.7
Administration Errors with Anti-Obesity Pharmacotherapies
A recent meta-analysis of FDA Adverse Event Reporting System data for obesity medications found a high rate of serious adverse events (SAEs)—including cardiac and renal events—associated with many of the oral and injectable obesity medications.10 Appropriate use of medication according to indications and HCP instructions can minimize the risk of SAEs, and proper administration is a key consideration in appropriate use.10
Administration errors can result in acute or chronic underdosing—leading to nonresponse and resultant inappropriate titration—or overdosing, leading to increased SAEs. The right timing and the right dose of medication is, therefore, crucial. Injectable anti-obesity medications may be particularly worrisome in this regard, with reports in the literature indicating that injectable therapies are associated with a higher incidence of medication errors, including fatal medication errors, versus oral therapy.11-13 Several instances of medication administration errors resulting in overdose have been reported with liraglutide, and there are numerous reports of transcription errors,14,15 including prescribers instructing patients to inject their liraglutide dose in milliliters instead of milligrams, resulting in six-fold overdose.15 There are also case reports of dosing errors wherein 18 mg of liraglutide (ie, the entire pen) has been injected, resulting in hypoglycemia (blood glucose 3.6 mmol/L) and subsequent hospitalization with severe nausea and vomiting.16,17
The Role of the Pharmacist in Reducing Administration Errors
Historically, the medication administration process has focused on the moment a patient takes a medication, but pharmacists are positioned to intervene well before the medication reaches the patient, caregiver, or nurse. Pharmacists provide patient care across many ambulatory and acute health care settings and connect various levels of the medication process (from prescriber to caregiver to patient to monitoring) via a unique “gatekeeper” role, which helps to prevent errors and significant patient harm before the medication comes under the control of the drug administrator.2,18 Involvement of a pharmacist in prescribing and delivery has been shown to reduce errors, improve prescribing practices, and improve patient monitoring.19,20 Pharmacist interventions, including patient education and counseling, drug safety management, and medication review, monitoring and reconciliation, have also been reported to have beneficial effects on various patient outcomes (such as smoking, blood pressure, lipid, and A1C reduction) across a number of chronic conditions, including hypertension or diabetes.21
Following certain “rights” of effective medication administration, from medication selection through medication monitoring, can help with medication error avoidance.
The “Rights” of Effective Medication Administration for Pharmacists2,6,22
- Right patient, right reason: patient is indicated for therapy and free from contraindications
- Right medication: medication class, medication within the class, and dosage form
- Right dose, right route, right timing: developing a medication regimen, including dose titration
- Right response: appropriate monitoring, when to notify prescriber, when to discontinue medication, setting patient expectations for therapy
However, even if the “rights” are adhered to, there is no guarantee that medication administration errors will not occur due to several factors5-8,22:
HCP factors:
- Poor patient-provider communication
- Insufficient patient education
Systemic factors:
- Lack of human resource
- Lack of time
- Poor training
- Poor transition of care
Patient factors
- Low/no health literacy
- Polypharmacy
While some systemic and patient factors may be challenging to resolve, patient-provider communication and insufficient patient education can be addressed through relatively direct, low‑tech approaches that require limited changes to the current workflow and medication administration pathway (Figure 2).
| Figure 2: Practical Tips to Improve Patient-Provider and Patient-Caregiver Communication and Education at Each Consultation4,23-25 |
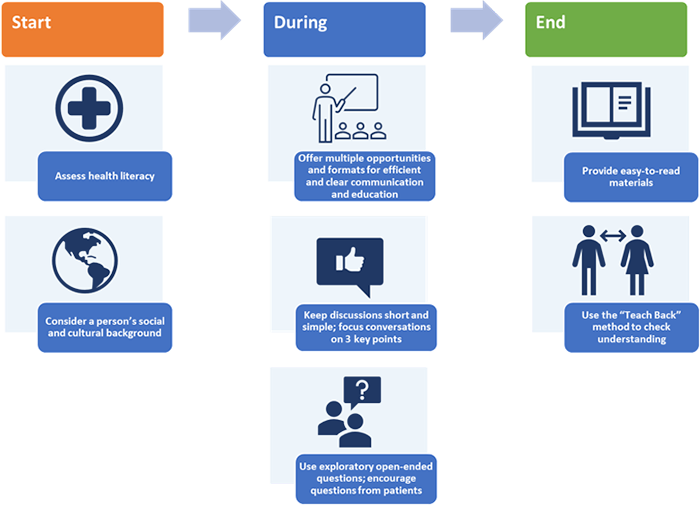
|
Right Patient, Right Reason: Ensuring the Patient is Indicated for Anti-Obesity Therapy and is Free From Contraindications
Defining Obesity
Obesity is a complex chronic, relapsing, medical condition characterized by an abnormal or excessive amount of body fat, that impairs health.26,27 For adults, obesity has traditionally been defined using body mass index (BMI) cut points, whereas for children and adolescents BMI is defined by percentiles (Table 1).26,28-30 While BMI is a simple, widely accessible, and noninvasive surrogate measure of body fat, clinical utility is limited.28 For example, BMI does not distinguish between excess fat, muscle or bone mass, and cannot account for sex-related differences in fat distribution or age-related muscle mass decline.28,29 Despite the established drawbacks of BMI as a measure, it is still widely used as an indication for certain therapies to manage obesity (eg, pharmacotherapy and surgery).26
| Table 1: BMI Categories |
| Weight status |
BMI (adults) |
Percentile ranking
(children and adolescents) |
| Underweight |
< 18.5 |
< 5th |
| Normal/healthy weight |
18.5 to 24.9 |
5th to < 85th |
| Overweight |
25.0 to 29.9 |
85th to < 95th |
| Class I obesity,a obesityb |
30 to 34.9 |
≥ 95th |
| Class II obesitya |
35 to 39.9 |
N/A |
| Class III obesitya |
≥ 40 |
N/A |
| a Adults; b Children and adolescents. N/A, not applicable. |
| Materials developed by CDC. |
Managing Obesity
The etiology of obesity is multifactorial, arising from complex interactions between genetic, pathophysiological, and environmental factors.26,27 Thus, obesity management has moved away from historical recommendations of “eat-less-move-more” (reducing calories consumed and increasing calories expended), toward multicomponent care; regular, long-term follow-up; and the ultimate goal of improving overall health and quality of life.26,27 As part of a comprehensive approach to obesity treatment, it is also important to review patients’ co-medications for obesity-related comorbidities, which can be weight gain promoting (eg, some antidepressants, steroids, glucose-lowering therapies).31,32 Identification of such agents and use of weight neutral or weight loss promoting alternatives is recommended where possible.27,31
Individualized lifestyle interventions (eg, medical nutrition therapy [diet], physical activity, and behavioral strategies) form the foundation of all obesity management plans.26 These strategies alone are associated, on average, with up to 5% reduction in total body weight and subsequent meaningful improvements in several obesity-related comorbidities.31,33 Amount of weight loss differs substantially between people with obesity, and although some people can achieve greater than 5% weight loss with lifestyle and behavioral interventions, it is often not sustainable over the long-term due to the underlying pathophysiology of obesity and psychosocial factors.27 Weight loss goals differ for each individual based on a range of patient- and disease-specific factors, such as starting BMI, “ideal weight”, and obesity-related comorbidities. For some individuals, it is important to achieve and maintain a greater proportion of weight loss than can typically be achieved with lifestyle and behavioral interventions alone; for example, some obesity-related comorbidities require > 10% to 15% weight loss for clinically meaningful benefit. Combining pharmacotherapy and/or surgery with lifestyle/behavioral approaches is associated with greater average percentage weight loss than lifestyle interventions alone and can support long-term weight loss maintenance. These approaches should, therefore, be considered where necessary to support attainment of individualized weight loss goals.26,27,31
Whichever goals and treatments are agreed between the patient and the provider, it is important to manage patients’ weight loss expectations and counsel individuals that management of obesity is lifelong, so chosen strategies must be sustainable over the long term.
Check Yourself
At what BMI are adults considered to have obesity?
Answer
A BMI of 30 or higher is considered obesity.
|
Who is the “Right” Candidate for Anti-Obesity Pharmacotherapy?
Pharmacotherapy is a key component of obesity care.26,27,31 It can be used to increase the amount of weight loss or maintain weight loss that has been achieved through lifestyle interventions.26,27,31 It can also support the prevention of weight regain.26,27,31 There are several approved agents available in the United States to support the long-term management of obesity, with specific eligibility criteria for their use (Table 2). For most medications, patients must meet age, BMI, and comorbidity conditions.34
| Table 2: Summary of Indications and Contraindications for Available Anti-Obesity Pharmacotherapies Approved for Long-Term Weight Management34 |
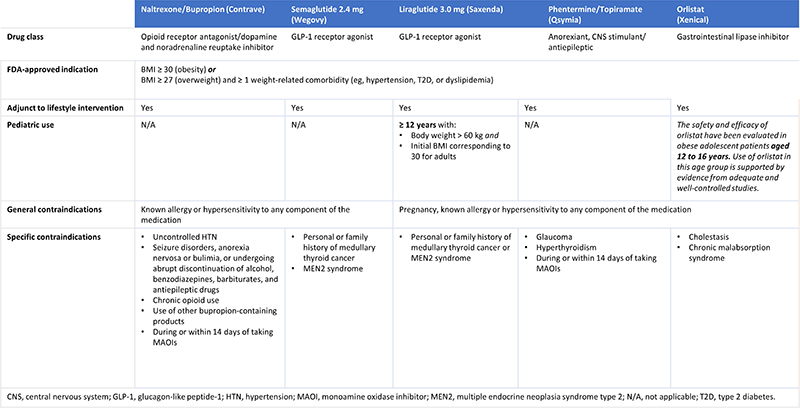
|
General Indications
Generally, people need to be ≥ 18 years of age, have a BMI ≥ 30 (obesity) or BMI ≥ 27 (overweight) with ≥ 1 weight-related comorbidity (eg, hypertension, T2D, or dyslipidemia) for anti-obesity pharmacotherapy to be indicated.34 It is important to ensure that anti-obesity medications are used alongside lifestyle interventions.34 Semaglutide 2.4 mg (Wegovy®), liraglutide 3.0 mg (Saxenda®), phentermine/topiramate (Qsymia®), naltrexone/bupropion (Contrave®), and orlistat 120 mg (Xenical®) are all approved by the FDA for chronic weight management.35
Orlistat 60 mg (alli®) is also available as an over-the-counter (OTC) product for adults with BMI ≥ 25 for use alongside a reduced-calorie, low-fat diet.34 Setmelanotide (Imcivree™) is approved by the FDA for extremely rare genetic disorders associated with severe obesity; patients must be ≥ 6 years of age and meet specific genetic criteria to be eligible for treatment.34
Several anorectic agents are also approved by the FDA for short-term use (a few weeks), including phentermine (Adipex-P®, Lomaira™), diethylpropion (controlled-release and extended-release), and phendimetrazine (conventional and extended-release; Adipost®, Anorex-SR®, Bontril PDM®, Bontril Slow Release®, Melfiat®, Obezine®, Phendiet®, Prelu-2®).34,36,37 Long-term use of phentermine has not been studied in the clinical trial setting, although some data support its use beyond 12 weeks as acknowledged in the Obesity Algorithm.35 For this reason, some prescribers may choose to continue phentermine beyond the FDA-approved duration of use. However, agents of this class have addictive potential and tachyphylaxis may emerge, limiting their usefulness when also considering the possible risks inherent in their use.34,38 Additionally, the lack of evidence to support long-term health benefits with short-term treatment regimens means they are generally not recommended for obesity management; use of agents indicated for long-term therapy is preferred.26,34 This paper will focus on agents that are FDA-indicated for long-term management of non-genetic forms of obesity.
Pediatric Indications
Of the anti-obesity agents approved for long-term use, only liraglutide 3.0 mg and orlistat 120 mg are indicated in pediatric patients over the age of 12.34
General Contraindications
Generally, most obesity medications are contraindicated in pregnancy and those with hypersensitivity or allergies to any of the components of the medication.34 Pregnancy was recently removed as a contraindication for naltrexone/bupropion, and semaglutide is not contraindicated in pregnancy, but either agent should be discontinued when a pregnancy is recognized.34
Specific Key Contraindications
For people with personal or family history of medullary thyroid cancer or multiple endocrine neoplasia syndrome type 2 (MEN2), liraglutide and semaglutide are contraindicated.34 Both agents have an FDA black box warning for thyroid C-cell tumors based on studies in rodents; human relevance has not been determined.34 Orlistat is contraindicated for people with cholestasis or chronic malabsorption syndrome.34 In people with glaucoma, hyperthyroidism, or those who are currently taking or have taken monoamine oxidase inhibitors (MAOIs) up to 14 days previously, phentermine/topiramate is contraindicated.34 Naltrexone/bupropion has multiple contraindications including concomitant or recent (up to 14 days prior) use of MAOIs, uncontrolled hypertension, opioid use, use of other bupropion-containing drugs, seizure disorders, anorexia nervosa, bulimia or patients undergoing abrupt discontinuation of alcohol, benzodiazepines, barbiturates, or antiepileptic drugs. Additionally, naltrexone/bupropion shouldn’t be administered with a high fat meal, as this significantly increases systemic exposure.34
Right Medication: Selecting an Appropriate Medication Within the Preferred Medication Class
Individualizing Pharmacotherapy Selection for People Living With Obesity
Shared patient-provider decision making is fundamental to selecting a medication.26,31 Choice of agent should be based on the following factors26,31:
- Mechanism of action
- Side effects
- Safety and tolerability
- Contraindications
- Existing comorbidities
- Current medications
- Individual values and preferences
Obesity pharmacotherapies that also have indications for diabetes or are known to lower blood glucose may be preferred if the patient has concomitant diabetes.31,34 It should be noted, however, that 2 agents of the same class should not be prescribed together (eg, liraglutide and semaglutide; both GLP-1 receptor agonists), and existing medications may need to be adjusted with the addition of an anti-obesity pharmacotherapy (Table 3).34
In patients with cardiovascular disease, pharmacotherapy-induced weight loss can improve cardiovascular risk factors, including lipid parameters and hypertension. Naltrexone/bupropion attenuates the blood pressure reduction associated with weight loss and is contraindicated in patients with uncontrolled hypertension; caution should also be taken in those with controlled hypertension.31,34 Cardiovascular outcome trials (CVOTs) of GLP-1 receptor agonists in type 2 diabetes have demonstrated that these agents may reduce the risk of major cardiovascular events independent of weight loss. A CVOT of semaglutide in patients with obesity and without type 2 diabetes is currently underway.39
| Table 3: Comorbidities and Conditions that may Warrant Caution or Avoidance When Using Specific Long-Term Anti-Obesity Pharmacotherapies26,31,34 |
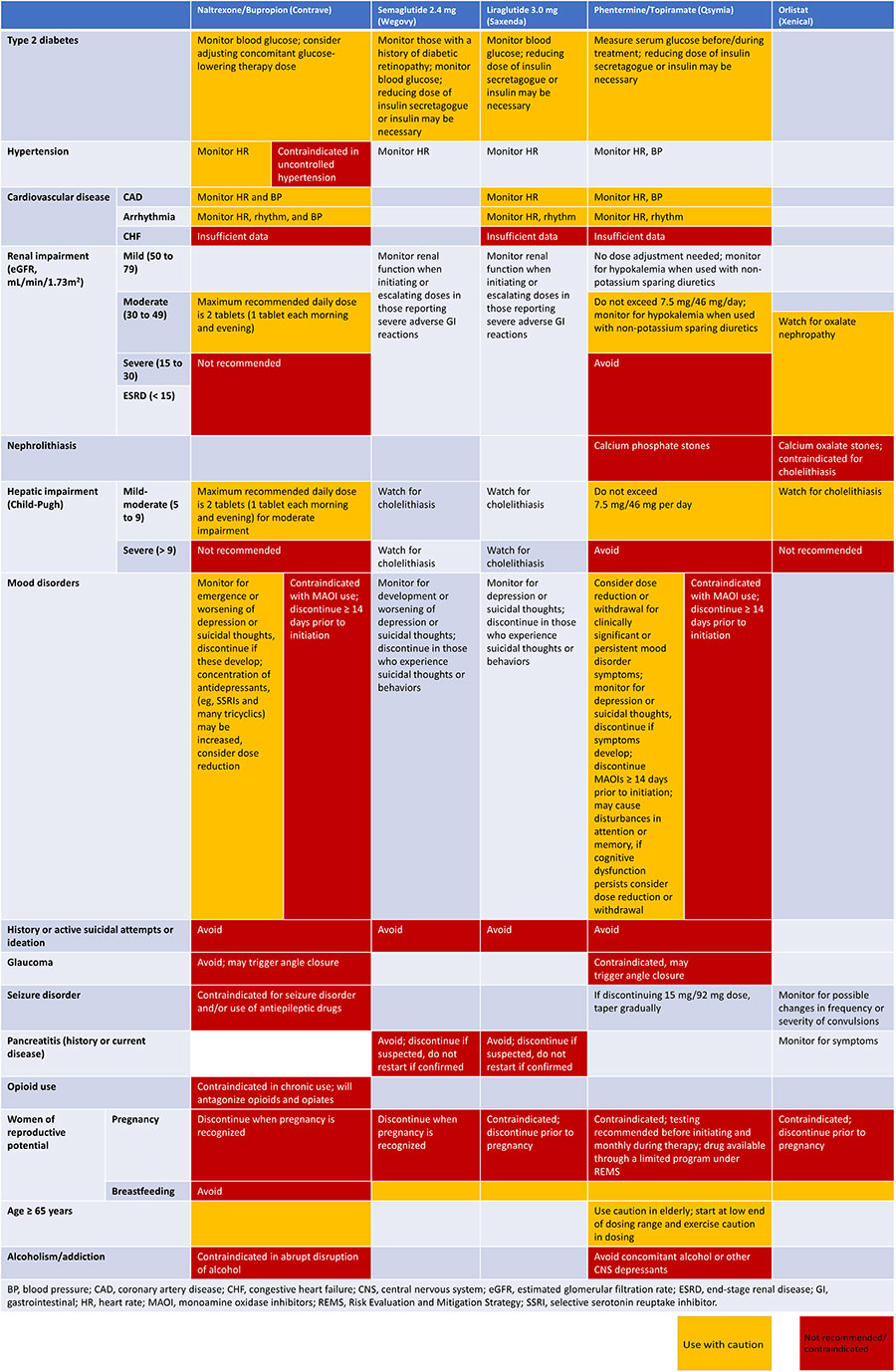
|
| Adapted from Garvey WT, et al. Endocr Pract. 2016;22(Suppl 3):1-203, Copyright 2016, with permission from Elsevier. |
Current medications should also be checked to prevent potential drug–drug interactions (DDIs) with an obesity pharmacotherapy. Importantly, semaglutide 2.4 mg and liraglutide 3.0 mg may affect absorption of some medications due to slowing of gastric emptying.34 Orlistat can disrupt absorption of fat-soluble vitamins (A, D, E, and K); therefore, patients are required to take a multivitamin supplement at least 2 hours before or after taking orlistat, to ensure adequate nutrition.34 Due to the effect of orlistat on vitamin K absorption, those receiving warfarin and orlistat should be monitored closely for changes in coagulation parameters. Orlistat can also reduce cyclosporine (Gengraf®, Neoral®, Sandimmune®) and amiodarone (Pacerone®) exposure when coadministered with either of these drugs; more frequent monitoring is recommended. Those treated concomitantly with orlistat and levothyroxine (Euthyrox®, Levoxyl®, Levo-T®, Levothroid®, Levoxine®, Synthroid®, Thyro-Tabs®, Thyquidity™, Tirosint®, Tirosint®-SOL, Unithroid®) should be monitored for changes in thyroid function as orlistat can affect levothyroxine absorption, and patients taking orlistat alongside antiepileptic drugs should be monitored for possible changes in frequency or severity of convulsions as orlistat my affect absorption of these drugs.34,40 While coadministration of phentermine/topiramate with oral contraceptives may cause irregular bleeding due to altered exposure, it does not increase risk of pregnancy—patients should be advised not to discontinue oral contraceptives if spotting occurs. Phentermine/topiramate is known to potentiate central nervous system (CNS) depressant effects; thus, concomitant use of alcohol should be avoided. Use of phentermine/topiramate with non-potassium sparing diuretics may potentiate hypokalemia, so potassium should be measured before and during treatment.34 Finally, naltrexone/bupropion is associated with numerous DDIs, which stem from bupropion inhibition of the CYP2D6 enzyme. In patients taking medications metabolized by CYP2D6 (eg, selective serotonin reuptake inhibitors, beta blockers, antipsychotic agents, type 1C antiarrhythmic agents, and tricyclic antidepressants), dose reduction should be considered when starting naltrexone/bupropion. CNS toxicity can occur when naltrexone/bupropion is used concomitantly with dopaminergic drugs (eg, levodopa [Sinemet®], amantadine [Gocovri®]) and coadministration of naltrexone/bupropion with MAOIs is associated with increased risk of hypertensive reactions. Naltrexone/bupropion may decrease plasma digoxin levels; thus, monitoring of digoxin levels is required. When administered concomitantly with drugs that lower seizure threshold, naltrexone/bupropion should be dosed with caution. Bupropion is primarily metabolized by the CYP2B6 enzyme system. Thus, concomitant treatment with CYP2B6 inhibitors (eg, ticlopidine [Ticlid®] or clopidogrel [Plavix®]) can increase bupropion exposure, and naltrexone/bupropion dosing should not exceed 1 tablet twice daily when taken with CYP2B6 inhibitors. Conversely, CYP2B6 inducers and medications that contain them (eg, ritonavir [Norvir®], lopinavir/ritonavir [Kaletra®], efavirenz [Sustiva®], efavirenz/emtricitabine/tenofovir [Atripla™], carbamazepine [Tegretol®, Tegretol® XR, Epitol®], phenobarbital [Luminal®], and phenytoin [Dilantin®]) may reduce efficacy by reducing bupropion exposure; therefore, concomitant use of these agents with naltrexone/bupropion should be avoided.34
Weight Loss With Obesity Pharmacotherapies
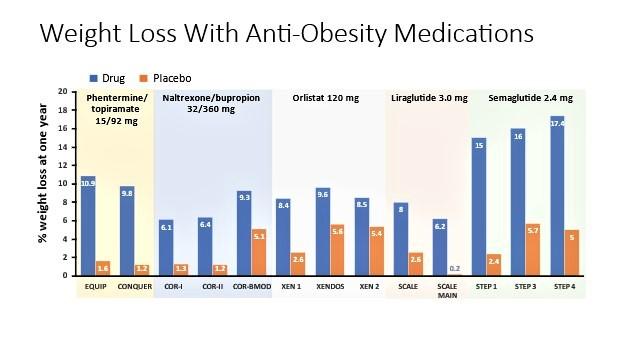
In clinical trials, obesity management pharmacotherapies have been associated with clinically significant weight loss at 1 year compared with placebo (Figure 3).26,31,34,38,41-53 It should be noted that while weight loss efficacy differs between agents, direct comparisons between data cannot be made due to differences in trial designs and patient populations.34 Additionally, orlistat may have different results based on dose, as both 60 mg (OTC) and 120 mg (prescription-only) are available.34,54 Factors other than average percent of weight loss associated with each drug, such as ease of administration, effects on comorbidities, and cost, should also be included in shared provider-patient treatment decisions as discussed above.31
| Figure 3: Average Percentage Weight Loss of Long-Term Anti-Obesity Medications in Clinical Trials at 1 Year41-46,48-53,55 |
|
Check Yourself
When is obesity pharmacotherapy indicated for chronic weight management in adults?
Answer
Obesity pharmacotherapy is indicated in adults with BMI ≥ 30 (obesity)
or BMI ≥ 27 (overweight) with ≥ 1 weight-related comorbidity
(eg, hypertension, T2D, or dyslipidemia). Liraglutide and orlistat are
also indicated for chronic obesity management in pediatric populations.
|
Right Dose, Right Route, Right Timing: Developing a Medication Regimen and Verifying that the Medication Regimen is Appropriate
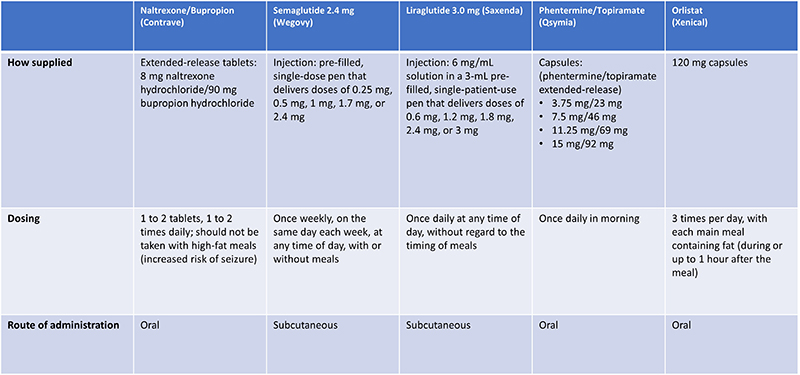
How a medication is supplied, the dosing and the administration options, differ between agents (Table 4).34 Notably, liraglutide 3.0 mg and semaglutide 2.4 mg are both injectable agents, while phentermine/topiramate, naltrexone/bupropion, and orlistat are taken orally.34 Timing of administration also differs; semaglutide 2.4 mg is currently the only obesity pharmacotherapy dosed weekly, whereas liraglutide 3.0 mg, and phentermine/topiramate are dosed daily, naltrexone/bupropion is dosed up to 2 times daily, and orlistat is dosed 3 times daily.34 Importantly, phentermine/topiramate, naltrexone/bupropion, and orlistat must be taken at certain times of the day.34
| Table 4: Characteristics of Available Long-Term Obesity Pharmacotherapies26,34 |
|
Initiation and Titration of Obesity Medications
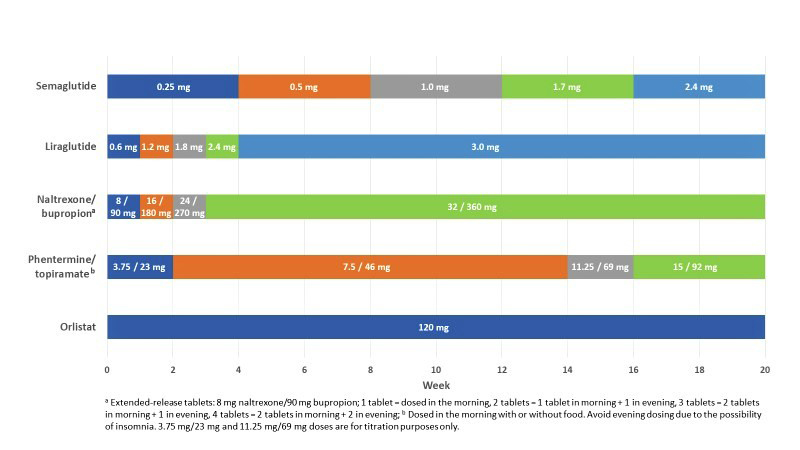
Most obesity medications have specific titration profiles that are indicated to minimize side effects and can last from a few weeks to several months (Figure 4), the only exception being orlistat, which is initiated at the maintenance dose (applicable to both the 60 mg and 120 mg forms). Administration errors, including uptitrating too rapidly, are of particular concern with injectable anti-obesity medications (eg, liraglutide 3.0 mg and semaglutide 2.4 mg), due to the potential risk of severe hypoglycemia and increased frequency and severity of gastrointestinal (GI) side effects.34 Importantly, titration may take longer in pediatric versus adult patients, and maximum tolerated doses also differ.34
| Figure 4: Recommended Titration Schedules for Long-Term Obesity Medications up to 20 Weeks34 |
|
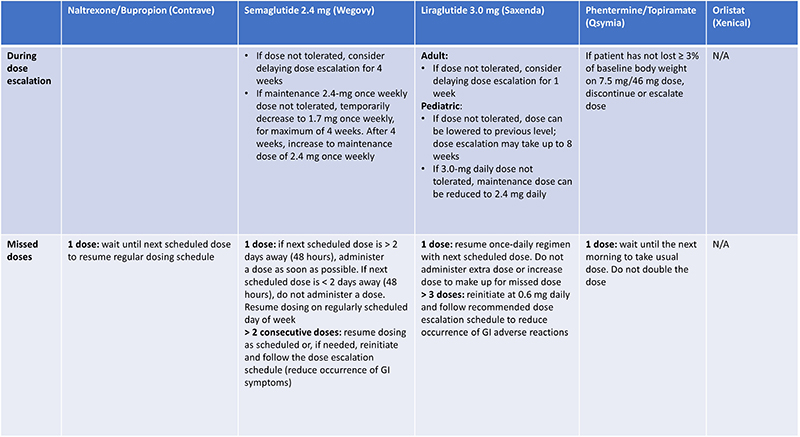
Medications should be initiated at a lower dose and uptitrated as long as the current dose is adequately tolerated.34 Where an increased dose isn’t tolerated during titration, dose escalation can be delayed for semaglutide 2.4 mg and liraglutide 3.0 mg.34 Additionally, if someone does not take their medication for a period of several days, it may be necessary to reinitiate treatment at a lower dose and titrate up to the maximum tolerated dose again (Table 5).34
| Table 5: Recommendations for Titration and Missed Doses with Long-Term Obesity Medications34 |
|
Check Yourself
Which agents approved for chronic weight management are injectable agents?
Answer
Liraglutide 3.0 mg and semaglutide 2.4 mg are injectable GLP-1 receptor agonists. There is an oral version of semaglutide that is not indicated for obesity management.
|
Educating on Appropriate Administration and Use of Anti-Obesity Medications
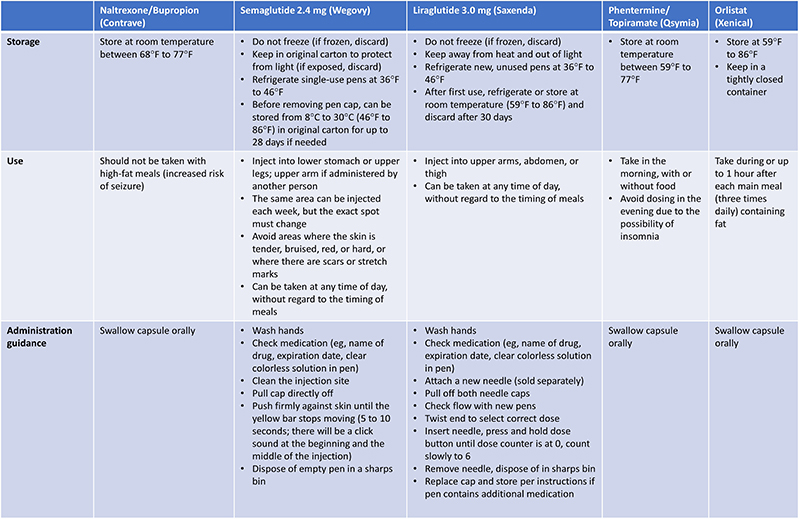
Providing education on appropriate storage, use (including titration), and administration of obesity medications is essential to ensure safety and optimal outcomes for patients. This is of particular importance for injectable agents, which can be considered more complex than oral tablets (Table 6), where use of the “teach-back” is valuable for checking that patients are comfortable and confident handling injections independently (see Figure 2). For example, asking patients to demonstrate their first injection in the pharmacy (or HCP’s office) can go a long way towards empowering patients and reassuring them that they are taking the medication correctly. Directing individuals to reliable patient education resources covering injection technique, such as those provided by professional societies (eg, AACE) or pharmaceutical companies can also help to empower patients by providing a free reference point whenever needed.
| Table 6: Key Storage, Use, and Administration Guidance for Patients34 |
|
>
Check Yourself
Which is the only anti-obesity medication that does not require dose titration?
Answer
Orlistat is initiated at the maintenance dose. All other medications should be initiated at a lower dose and uptitrated according to the schedule in the prescribing information. Titration may take longer in pediatric patients and in those who are not tolerating the higher dose.
|
Right Response: Monitoring of Medication Therapy, When to Notify Prescriber, When to Discontinue Medication
When a person begins obesity pharmacotherapy, monitoring is crucial to ensure patient safety and well-being. Specifically, efficacy (weight loss response), tolerability, and any safety issues should be monitored at least every month for the first 3 months of therapy, then every 3 months if therapy is continued.32
Monitoring Efficacy (Weight Loss Response)
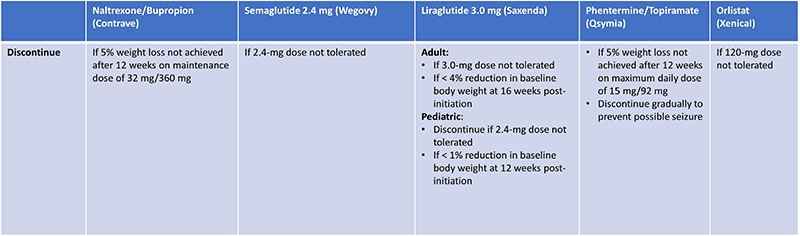
Weight loss response differs from patient-to-patient, and some will be nonresponders.31 If, after a specified period, no treatment benefit or a suboptimal response is seen, even at the maximum tolerated dose, liraglutide 3.0 mg, phentermine/topiramate and naltrexone/bupropion should be discontinued (Table 7). Importantly, phentermine/topiramate should be gradually down-titrated upon discontinuation to avoid potential seizures.34 As mechanisms of action differ between agents, lack of response to one medication does not confer lack of response to all, and trying another agent can be discussed with the patient as appropriate.31,34
| Table 7: Recommendations for Discontinuation of Long-Term Anti-Obesity Medications34 |
|
Monitoring Tolerability
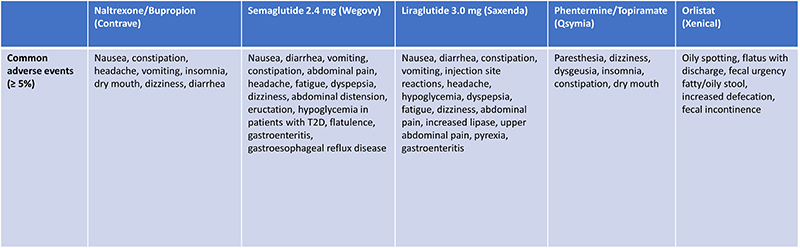
Each anti-obesity pharmacotherapy is associated with an adverse event (AE) profile that patients taking these drugs should be made aware of. The most common AEs associated with orlistat are loose oily stools, fecal urgency, and flatus. Phentermine/topiramate and naltrexone/bupropion are both associated with dizziness, insomnia, constipation, and dry mouth.
The most common AEs associated with liraglutide 3.0 mg and semaglutide 2.4 mg are GI in nature, including nausea, constipation, diarrhea, and/or vomiting (Table 8).34 GI AEs occur most frequently when initiating therapy or escalating
the dose, although for most people the effects are short, self-limiting
episodes that ease with time and treatment persistence.56,57 AEs associated with orlistat can be managed to some extent through the
adoption of a reduced fat diet (< 30% total daily calories from fat).34 There are also a few simple steps that can be taken to reduce the risk
of GI AEs associated with liraglutide and semaglutide, and support
people in managing them56-58:
- Titrate the dose slowly when possible
- Educate people to decrease their food intake and stop eating when full
- Advise people around limiting certain foods (eg, spicy meals or those with high fat content)
- Reassure people that events are transient and should decrease over time
| Table 8: Common Adverse Events Associated With Long-Term Obesity Pharmacotherapies34 |
|
Check Yourself
Which agents should be discontinued if there is <4% reduction in body weight at 16 weeks after initiation?
Answer
Liraglutide 3.0 mg should be discontinued if < 4% reduction in baseline body weight at 16 weeks post-initiation. Naltrexone/bupropion and phentermine/topiramate should be discontinued if <5% weight loss is achieved at the maintenance dose at 12 weeks. All medications should be discontinued if not tolerated.
|
Monitoring Safety
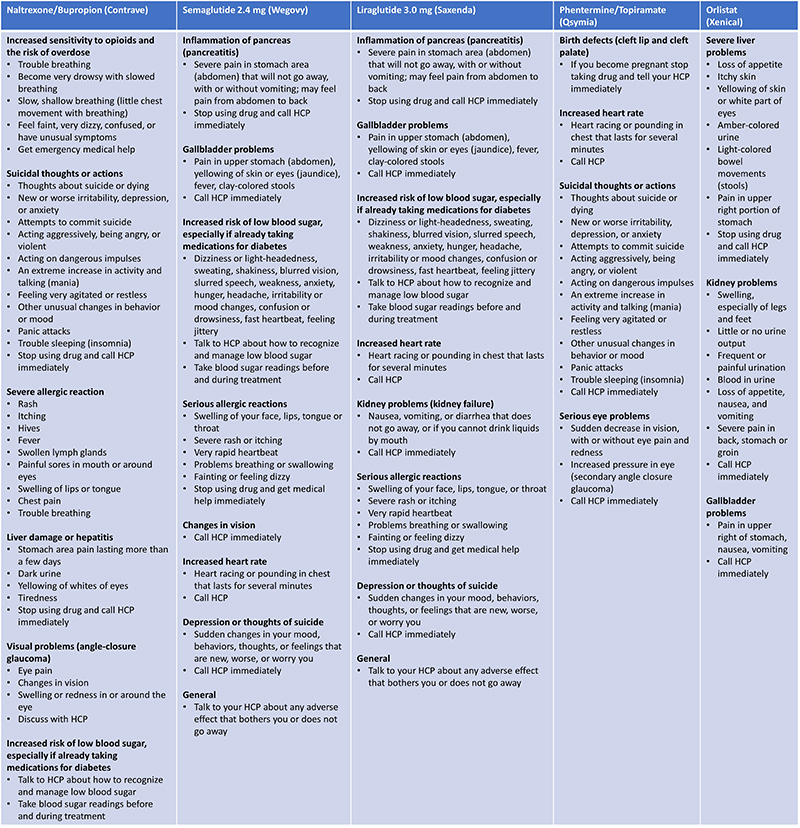
Certain signs and symptoms that patients may experience when using an anti-obesity medication can indicate an SAE (Table 9).34 If SAEs occur, obesity pharmacotherapy should be discontinued. It is important to inform patients of these so that they are recognized promptly, and ensure patients understand if/when to stop taking their medication and/or contact their HCP. Pharmacists should be prepared to counsel patients concerning these signs and symptoms.
| Table 9: Signs and Symptoms Requiring Urgent HCP Contact34 |
|
Conclusion
Obesity medications are an integral part of current obesity management and can provide effective support for weight loss, maintenance of weight loss, and prevention of weight regain when administered alongside lifestyle modifications.34 Available anti-obesity pharmacotherapies differ in their indications, dosing, administration and titration schedules, and key safety considerations, and their proper use is essential to avoid occurrence of SAEs throughout the medication administration process.34 As “gatekeepers” of this process, pharmacists have a central role in evaluating medication according to the “rights” of effective medication administration, and educating patients, caregivers, and other HCPs about appropriate use of therapy to optimize patient safety and outcomes.2,4,6,22-25
References
- National Coordinating Council for Medication Error Reporting and Prevention. About medication errors. Accessed March 1, 2022. https://www.nccmerp.org/about-medication-errors
- International Pharmaceutical Federation (FIP). Patient and medication safety. Pharmacists’ role in "medication without harm". The Hague: International Pharmaceutical Federation (FIP); 2020. https://www.fip.org/file/4757
- Single Care. Medication errors statistics 2022. Accessed March 1, 2022. https://www.singlecare.com/blog/news/medication-errors-statistics/#:~:text=The%20FDA%20receives%20more%20than,Learn%20how%20to%20prevent%20them
- Agency for Healthcare Research and Quality. The impact of communication on medication errors. Accessed February 18, 2021. https://psnet.ahrq.gov/web-mm/impact-communication-medication-errors
- Tariq RA, Vashisht R, Sinha A, Scherbak Y. Medication dispensing errors and prevention. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Elliot M, Liu Y. The nine rights of medication administration: an overview. Br J Nurs. 2010;19(5).
- da Silva BA, Krishnamurthy M. The alarming reality of medication error: a patient case and review of Pennsylvania and National data. J Community Hosp Intern Med Perspect. 2016;6(4):31758.
- World Health Organization. WHO launches global effort to halve medication-related errors in 5 years. Accessed February 18, 2022. https://www.who.int/news/item/29-03-2017-who-launches-global-effort-to-halve-medication-related-errors-in-5-years
- NORC at the University of Chicago and IHI/NPSF Lucian Leape Institute. Americans’ experiences with medical errors and views on patient safety: final report. Accessed March 1, 2022. https://psnet.ahrq.gov/issue/americans-experiences-medical-errors-and-views-patient-safety
- Alsuhibani A, Alrasheed M, Gari M, Hincapie AL, Guo JJ. Descriptive analysis of reported adverse events associated with anti-obesity medications using FDA Adverse Event Reporting System (FAERS) databases 2013-2020. Int J Clin Pharm. 2022;44(1):172-179.
- Cheragi MA, Manoocheri H, Mohammadnejad E, Ehsani SR. Types and causes of medication errors from nurse's viewpoint. Iran J Nurs Midwifery Res. 2013;18(3):228-231.
- Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285(16):2114-2120.
- Phillips J, Beam S, Brinker A, et al. Retrospective analysis of mortalities associated with medication errors. Am J Health Syst Pharm. 2001;58(19):1835-1841.
- Bode SF, Egg M, Wallesch C, Hermanns-Clausen M. 10-fold liraglutide overdose over 7 months resulted only in minor side-effects. J Clin Pharmacol. 2013;53(7):785-786.
- Cohen MR. Medication errors. Nursing. 2018;48(2):72.
- Elmehdawi RR, Elbarsha AM. An accidental liraglutide overdose: case report. Libyan J Med. 2014;9:23055.
- Nafisah SB, Almatrafi D, Al-Mulhim K. Liraglutide overdose: A case report and an updated review. Turk J Emerg Med. 2020;20(1):46-49.
- Mansur JM. Medication safety systems and the important role of pharmacists. Drugs Aging. 2016;33(3):213-221.
- Agency for Healthcare Research and Quality. Pharmacist role in patient safety. Accessed February 18, 2022. https://psnet.ahrq.gov/perspective/pharmacist-role-patient-safety
- Gillani SW, Gulam SM, Thomas D, et al. Role and services of a pharmacist in the prevention of medication errors: A systematic review. Curr Drug Saf. 2020;16(3):322-328.
- Omboni S, Caserini M. Effectiveness of pharmacist's intervention in the management of cardiovascular diseases. Open Heart. 2018;5(1):e000687.
- Agency for Healthcare Research and Quality. Medication administration errors. Accessed February 18, 2022. https://psnet.ahrq.gov/primer/medication-administration-errors
- Sassoli M, Day G. Understanding pharmacist communication and medication errors: A systematic literature review. Asia Pac J Health Manag. 2017;12:47-61.
- Digital Pharmacist. Patient communication for pharmacy: 5 powerful tips to improve patient engagement. Accessed February 18, 2022. https://www.digitalpharmacist.com/blog/patient-communication-for-pharmacy/
- Agency for Healthcare Research and Quality. Approach to improving patient safety: Communication. Accessed February 18, 2022. https://psnet.ahrq.gov/perspective/approach-improving-patient-safety-communication
- Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22 Suppl 3:S1-S203.
- Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192(31):e875-e891.
- Centers for Disease Control and Prevention. Body mass index - considerations for practitioners. Accessed March 1, 2022. https://stacks.cdc.gov/view/cdc/25368
- Gurunathan U, Myles PS. Limitations of body mass index as an obesity measure of perioperative risk. Br J Anaesth. 2016;116(3):319-321.
- Centers for Disease Control and Prevention. About child & teen BMI. Accessed January 22, 2022. https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html
- Pedersen SD, Manjoo P, Wharton S. Pharmacotherapy in obesity management. Canadian adult obesity clinical practice guidelines. Accessed February 18, 2022. https://obesitycanada.ca/guidelines/pharmacotherapy/
- Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362.
- The Look Ahead Research Group. Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring). 2014;22(1):5-13.
- US Food & Drug Administration. Drugs@FDA: FDA-Approved Drugs. Accessed February 18, 2022. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm
- Bays H, McCarthy W, Burridge S, et al. Adult obesity algorithm eBook: Detailed overview of obesity medicine. Accessed January 24, 2022. www.obesityalgorithm.org
- Drugs.com. Phendimetrazine. Accessed February 18, 2022. https://www.drugs.com/ingredient/phendimetrazine.html
- Mayo Clinic. Phendimetrazine (oral route). Accessed February 18, 2022. https://www.mayoclinic.org/drugs-supplements/phendimetrazine-oral-route/description/drg-20075140
- Muller TD, Bluher M, Tschop MH, DiMarchi RD. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov. 2021:1-23.
- Wilding JPH, Jacob S. Cardiovascular outcome trials in obesity: A review. Obes Rev. 2021;22(1):e13112.
- Drugs.com. Levothyroxine. Accessed February 18, 2022. https://www.drugs.com/levothyroxine.html
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330-342.
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341-1352.
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605.
- Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: The STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414-1425.
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22.
- Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21(5):935-943.
- Wadden TA, Volger S, Tsai AG, et al. Managing obesity in primary care practice: an overview with perspective from the POWER-UP study. Int J Obes (Lond). 2013;37 Suppl 1:S3-11.
- Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19(1):110-120.
- Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989.
- Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: The STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403-1413.
- Finer N, James WP, Kopelman PG, Lean ME, Williams G. One-year treatment of obesity: a randomized, double-blind, placebo-controlled, multicentre study of orlistat, a gastrointestinal lipase inhibitor. Int J Obes Relat Metab Disord. 2000;24(3):306-313.
- Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. Xenical in the prevention of diabetes in obese subjects (XENDOS) study. Diabetes Care. 2004;27:155–161.
- James WP, Avenell A, Broom J, Whitehead J. A one-year trial to assess the value of orlistat in the management of obesity. Int J Obes Relat Metab Disord. 1997;21:S24-S30.
- Rossner S, Noack R, Meinders AE, Noseda G. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. European Orlistat Obesity Group. Obes Res. 2000;8(1):49-61.
- Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37(11):1443-1451.
- Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30(3):202-210.
- Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46:101102.
- Romera I, Cebrian-Cuenca A, Alvarez-Guisasola F, Gomez-Peralta F, Reviriego J. A review of practical issues on the use of glucagon-like peptide-1 receptor agonists for the management of type 2 diabetes. Diabetes Ther. 2019;10(1):5-19.
Back to Top