
Expired activity
Please go to the PowerPak
homepage and select a course.
Antibody Drug Conjugates: Pharmacist Focus on Overcoming the Challenges of Adverse Event Management
THERAPEUTIC APPROACHES WITH ANTIBODY-DRUG CONJUGATES FOR THE TREATMENT OF ONCOLOGY DISEASE STATES
Structure of Antibody-Drug Conjugates
The idea of targeting a drug to a specific site of action has been a vision since at least the early 1900s with Dr. Paul Ehrlich’s “magic bullet” concept.1 That vision particularly resonates in the field of oncology where toxicity to nonmalignant cells has plagued cancer chemotherapy for decades due to the nature of the mechanisms of action of cancer drugs and the inability to selectively target malignant cells. To a large extent, antibody-drug conjugates (ADCs) are modern approximations of such “magic bullets.”2 An ADC consists of 3 distinct components: 1) monoclonal antibody (mAb); 2) linker; and 3) payload (FIGURE 1).3 The mAb acts as the targeting moiety to guide the ADC to the cells that selectively express particular cell-surface proteins. Linkers connect the payload (ie, the chemotherapeutic agent) to the mAb via a variety of chemistries. With the ability to selectively target cancer cells, ADCs are most commonly used in oncology. Excluding the ADC with an immunotoxin payload (moxetumomab pasudotox), there are 11 FDA-approved ADC oncology products with chemotherapeutic payloads.4
| FIGURE 1. Structure of ADCs3 |
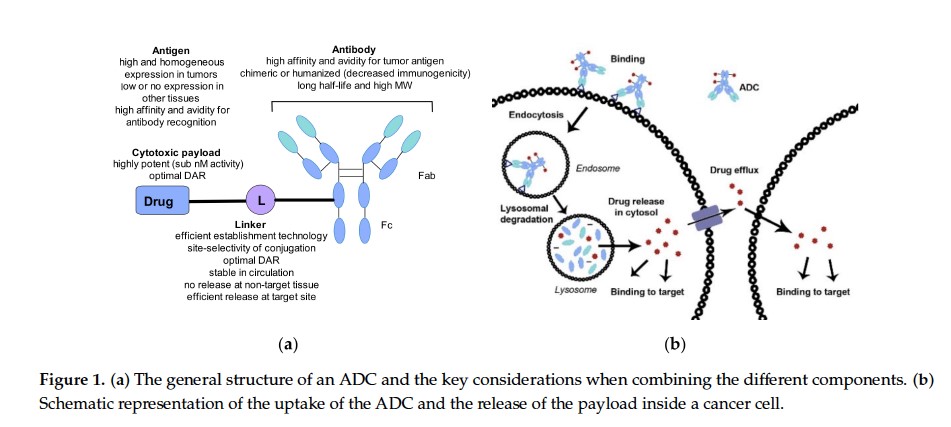 |
Abbreviations: ADC, antibody-drug conjugate; DAR, drug-antibody ratio; Fab, fragment antigen-binding; Fc, fragment crystallizable; L, linker; MW, molecular weight; nM, nanomolar.
Figure adapted (part B of original figure cropped) and reused under Creative Commons Attribution license version 4.0 from: Kostova V, Désos P, Starck JB, Kotschy A. The chemistry behind ADCs. Pharmaceuticals (Basel). 2021;14(5):442. doi:10.3390/ph14050442
Copyright 2021 of authors: Vesela Kostova, Patrice Désos, Jérôme-Benoît Starck, and Andras Kotschy. https://www.mdpi.com/1424-8247/14/5/442 |
Monoclonal Antibody
The ability of a mAb to selectively bind to a protein provides an ADC with its cell-specific targeting capability. The mAbs for ADCs are designed to target cell-surface proteins that cancer cells differentially express compared to nonmalignant cells. The cluster of differentiation (CD) family of cell surface antigens provides a variety of targets for ADCs, including CD19, CD22, CD30, CD33, and CD79b. Other cell surface proteins used as ADC targets include nectin-4, tissue factor, B-cell maturation antigen (BCMA), human epidermal growth factor receptor 2 (HER2), and tumor-associated calcium signal transducer 2 (TROP2).4,5 The ADCs and their corresponding targets are listed in TABLE 1. The mAbs of the immunoglobulin G (IgG) class are typically used in ADCs with IgG1 and IgG4 being the most commonly used IgG subtypes. Of the available ADCs, gemtuzumab ozogamicin and inotuzumab ozogamicin use IgG4, while the remaining ADCs use IgG1 for their mAb components. As biological products, the mAbs of ADCs are produced in cell culture. The mAbs may be fully human, humanized, or chimeric, which can affect immunogenicity and hypersensitivity reactions.4
| TABLE 1. ADCs and Their Components8,9,16,18-25 |
| ADC (Initial US Approval Date) |
Payload
Payload Class/MOA |
mAb/Cell Surface Target |
Linker |
| Gemtuzumab ozogamicin (2000) |
Ozogamicin
Calicheamicin/DNA cleavage |
Humanized IgG4/CD33 |
Hydrolysable/
acid-cleavable |
| Inotuzumab ozogamicin (2017) |
Humanized IgG4/CD22 |
Acid-cleavable |
| Brentuximab vedotin (2011) |
Monomethyl auristatin E
Auristatin/Microtubule inhibitor |
Chimeric IgG1/CD30 |
Protease-cleavable |
| Polatuzumab vedotin (2019) |
Humanized IgG1/CD79b |
Protease-cleavable |
| Enfortumab vedotin (2019) |
Fully human IgG1/nectin-4 |
Protease-cleavable |
| Tisotumab vedotin (2021) |
Human IgG1/ tissue factor |
Protease-cleavable |
| Belantamab mafodotin (2020) |
Monomethyl auristatin F
Auristatin/Microtubule inhibitor |
Humanized IgG1/BCMA |
Non-cleavable |
| Ado-trastuzumab emtansine (2013) |
Maytansine
Maytansine derivative/Microtubule inhibitor |
Humanized IgG1/HER2 |
Non-cleavable |
| Fam-trastuzumab deruxtecan (2019) |
Deruxtecan
Camptothecin/Topoisomerase I inhibitor |
Humanized IgG1/HER2 |
Protease-cleavable |
| Sacituzumab govitecan (2020) |
Govitecan (SN-38)
Camptothecin/Topoisomerase I inhibitor |
Humanized IgG1/TROP2 |
Hydrolysable/
acid-cleavable |
| Loncastuximab tesirine (2021) |
Tesirine
Pyrrolobenzodiazepine/DNA cleaver
(interstrand cross-linking) |
Humanized IgG1/CD19 |
Protease-cleavable |
| Abbreviations: ADC, antibody-drug conjugate; BCMA, B-cell maturation antigen; CD, cluster of differentiation; IgG, immunoglobulin G; HER2, human epidermal growth factor receptor 2; mAb, monoclonal antibody; MOA, mechanism of action; TROP2, tumor-associated calcium signal transducer 2. |
Linker
The linker is the chemical moiety that attaches the payload to the mAb of the ADC. The choices of the mAb and the linker are the critical components for limiting toxicity and adverse events by reducing off-target release of the payload. Linkers are classified into 2 broad types: cleavable or non-cleavable. Cleavable linkers are designed to degrade either by pH-dependent, glutathione-mediated, or enzymatic mechanisms.3,6 Regardless of the type of linker employed, the payload is typically released in the target cancer cells after endocytosis and degradation in the cytosol or lysosome.6,7
A common pH-dependent linker is the hydrazone group, which is stable at pH 7.5 and hydrolyzes in the acidic environments of the endosome and lysosome.3,6,7 For glutathione-dependent cleavage, disulfide linkers are used to capitalize on higher concentrations of glutathione in tumor cells.3,6,7 Intracellular proteases, such as cathepsin B, in the lysosome cleave enzymatically labile linkers, the most common of which are the dipeptides valine-citrulline or valine-alanine.3,6,7
Non-cleavable linkers remain relatively stable in the physiological milieu until degradation in the lysosome. For ADCs with non-cleavable linkers, the payload is designed to be released after proteolytic degradation of the mAb.7 Belantamab mafodotin and ado-trastuzumab emtansine use the non-cleavable linkers, maleimidocaproyl and 4-(N-maleimidomethyl)cyclohexane-1-carboxylate, respectively.8,9 Since the non-cleavable linker remains attached to the payload, studies indicate that the payload-linker moieties in belantamab mafodotin and ado-trastuzumab emtansine retain the intended chemotherapeutic activity.6
Payload
The payload is the active pharmacological agent of an ADC. In early studies of ADCs, payloads were common anticancer chemotherapy drugs such as methotrexate, doxorubicin, or vinca alkaloids.10-13 Even with mAb targeting, only a small percentage (~0.1%) of the ADC dose reaches the intended target tissue and more potent payloads (eg, IC50 in the picomolar to nanomolar range) were required for efficacy.3,4 The trade off with the increased potency is that off-target release of the payload can lead to increased toxicity. Current payloads of marketed ADCs are ozogamicin, monomethyl auristatin E (MMAE), monomethyl auristatin F (MMAF), maytansine, deruxtecan, govitecan, and tesirine (TABLE 1).
Mechanism(s) of Action
The mechanism of action of an ADC not only encompasses the specific payload mechanism of action but also the overall process by which the ADC interacts with target cells.14 Tong et al summarize that overall process in 8 steps (FIGURE 2)4:
- Circulation of ADC in plasma
- Binding of ADC to target cell-surface antigen
- Receptor-mediated endocytosis
- Transcytosis to extracellular space OR
Endosomal release of payload via cleavable linker
- Endosome-lysosome fusion
- Lysosomal degradation leading to release of payload from cleavable or non-cleavable linker
- Payload exerting its intracellular mechanism of action
- Payload export/efflux out of target cell to act on neighboring tumor cells (bystander effect).4
| Figure 2. Mechanism of Action of ADCs4 |
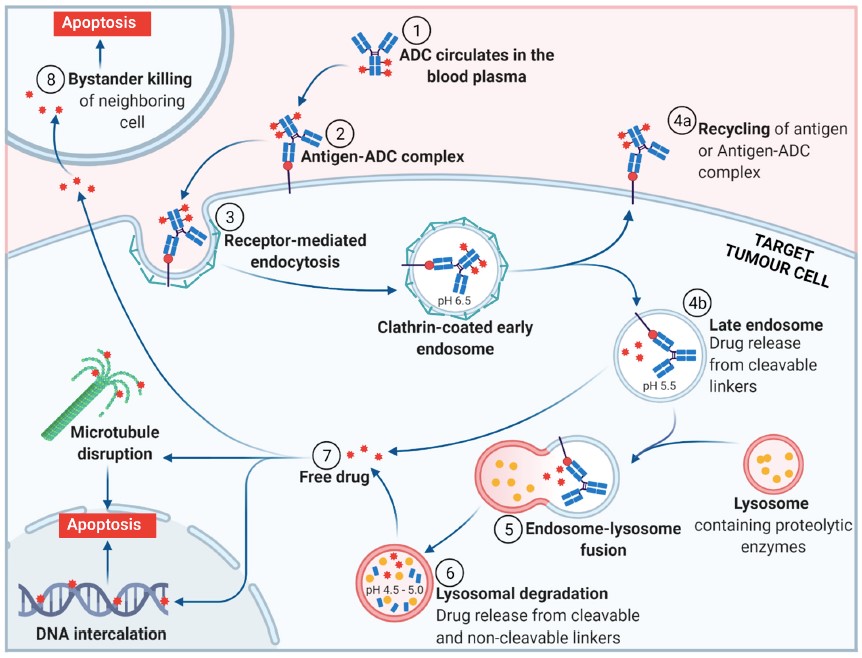 |
Abbreviations: ADC, antibody-drug conjugate.
Figure reused from under Creative Commons Attribution license version 4.0 from: Tong JTW, Harris PWR, Brimble MA, Kavianinia I. An insight into FDA approved antibody-drug conjugates for cancer therapy. Molecules. 2021;26(19), which was adapted from “Antibody-Drug Conjugate Release,” by BioRender.com.
Regarding the payload mechanism of action, the drugs used in current ADCs consist of compounds that a) inhibit tubulin or b) damage DNA (TABLE 1).4,15 The microtubule inhibitors in marketed ADCs include MMAE, MMAF, and maytansine. The ADC payloads that damage DNA include ozogamicin, deruxtecan, govitecan, and tesirine. |
Treatment Indications
For the purposes of this activity, treatment indications will be discussed from a broad perspective. The cell-surface protein target is the major factor determining the indication for an ADC, which can be classified into 2 categories: 1) solid tumors and 2) hematology. Solid tumor indications include urothelial cancer, cervical cancer, breast cancer, and gastric cancer. Many of the solid tumor indications focus on locally advanced or metastatic cancers. Hematology indications include acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), classical Hodgkin lymphoma, anaplastic large cell lymphoma (ALCL), peripheral T-cell lymphomas (PTCLs), mycosis fungoides, and diffuse large B-cell lymphoma (DLBCL). Specific indications, places in therapy, and related dosing regimens can be found in the prescribing information for the ADCs and in guidelines such as those from the National Comprehensive Cancer Network (NCCN).8,9,16-25
Dosing
The ADCs all require intravenous (IV) infusion for administration with recommended infusion times ranging from 30 to 120 minutes. ADCs are dosed on a per kilogram body weight or per square meter basis and are dependent on multiple factors including the payload, the indication, and the chemotherapy regimen cycle, among others.8,9,16,18-25 Dose modifications based on adverse reactions are also common. As with the mechanisms of action and treatment indications, clinicians should consult the package inserts for specifics on dosing and administration.
Mono- and/or Combination Therapy
While many oncology protocols call for the use of multiple agents, only 3 of the currently available ADCs have combination therapy indications. With no monotherapy indication, polatuzumab vedotin has a combination indication for relapsed or refractory DLBCL when administered with bandmaster and a rituximab product.21 Brentuximab vedotin can be used in combination with doxorubicin, vinblastine, and dacarbazine for stage III or IV classical Hodgkin lymphoma (previously untreated). Brentuximab vedotin can also be used in combination with cyclophosphamide, doxorubicin, and prednisone for previously untreated PTCLs that express CD30 or previously untreated systemic ALCL. Brentuximab vedotin also has monotherapy indications for classical Hodgkin lymphoma, systemic ALCL, primary cutaneous ALCL, and CD30-expressing mycosis fungoides.20 The combination therapy indication for gemtuzumab ozogamicin (combined with daunorubicin and cytarabine) is in newly diagnosed patients with de novo AML (CD33 positive).18 The remaining ADCs (inotuzumab ozogamicin, enfortumab vedotin, tisotumab vedotin, belantamab mafodotin, ado-trastuzumab emtansine, fam-trastuzumab deruxtecan, sacituzumab govitecan, and loncastuximab tesirine) have monotherapy indications. TABLE 2 summarizes the indications of the ADCs.8,9,16,18-25
| TABLE 2. Indications and Major Adverse Events of ADCs8,9,16,18-25 |
| ADC |
Indicationsa |
Black Box Warnings |
Major Adverse Events |
Suggested Premedicationsa |
| Gemtuzumab ozogamicin |
· Newly-diagnosed CD33-positive AML in adults and pediatric patients ≥1 month
· Relapsed or refractory CD33-positive AML in adults and pediatric patients ≥2 years |
Hepatotoxicity leading to veno-occlusive disease |
Myelosuppression (including prolonged thrombocytopenia), hemorrhage, infusion reactions |
Acetaminophen, diphenhydramine, and methylprednisolone |
| Inotuzumab ozogamicin |
· Adults with relapsed or refractory B-cell precursor ALL |
Hepatotoxicity leading to veno-occlusive disease and higher post-HSCT nonrelapse mortality |
Thrombocytopenia, neutropenia, infection, hemorrhage |
Corticosteroid, antihistamine, and antipyretic |
| Brentuximab vedotin |
Adult patients for:
· Combination therapy with doxorubicin, vinblastine, and dacarbazine for stage III or IV classical Hodgkin lymphoma (previously untreated)
· Classical Hodgkin lymphoma at high risk of relapse or progression as post-autologous HSCT consolidation
· Classical Hodgkin lymphoma after failure of auto-HSCT or after failure of at least 2 multi-agent chemotherapy regimens in patients who are not auto-HSCT candidates
· Combination therapy with cyclophosphamide, doxorubicin, and prednisone for previously untreated systemic ALCL or other CD30-expressing PTCLs (including angioimmunoblastic T-cell lymphoma and PTCL not otherwise specified)
· Systemic ALCL after failure of at least 1 multi-agent chemotherapy regimen
· Primary cutaneous ALCL or CD30-expressing mycosis fungoides who have received prior systemic therapy |
JCV infection leading to progressive multifocal leukoencephalopathy |
Peripheral neuropathy, myelosuppression (especially thrombocytopenia), infections |
Acetaminophen, antihistamine, and corticosteroid if prior infusion-related reaction |
| Polatuzumab vedotin |
· Combination therapy with bendamustine and a rituximab product for the treatment of adult patients with relapsed or refractory DLBCL, not otherwise specified, after at least 2 prior therapies |
None |
Neutropenia, thrombocytopenia, anemia, peripheral neuropathy, infusion reactions, infections |
Antihistamine and antipyretic |
| Enfortumab vedotin |
Adult patients with locally advanced or metastatic urothelial cancer who have previously received:
· A) a PD-1 or PD-L1 inhibitor and platinum-containing chemotherapy OR
· B) are ineligible for cisplatin-containing chemotherapy and have previously received 1 or more prior lines of therapy |
Severe, even fatal skin reactions including SJS and TEN |
Hepatotoxicity, peripheral neuropathy, diabetic ketoacidosis, pneumonitis, ocular toxicities, rash |
None |
| Tisotumab vedotin |
· Adult patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy |
Ocular toxicities to corneal epithelium and conjunctiva resulting in vision changes including severe vision loss and corneal ulceration. Requires ophthalmic exams at baseline and prior to each dose |
Peripheral neuropathy, hemorrhage/bleeding, pneumonitis |
Requires ophthalmic premedication before, during, and after infusion |
| Belantamab mafodotin |
· Adult patients with relapsed or refractory multiple myeloma who have received at least 4 prior therapies including an anti-CD38 monoclonal antibody, a proteasome inhibitor, and an immunomodulatory agent |
Drug is available through REMS program. Ocular toxicities to corneal epithelium and conjunctiva resulting in vision changes including severe vision loss, corneal ulceration, blurred vision, and dry eye. Requires ophthalmic exams at baseline and prior to each dose |
Thrombocytopenia, keratopathy (corneal epithelium change on eye exam), decreased visual acuity, nausea, blurred vision |
Premedicate if patient had prior infusion-related reaction (PI does not specify medications) |
| Ado-trastuzumab emtansine |
Adult patients as a single agent for:
· A) the treatment of HER2-positive, metastatic breast cancer previously treated with trastuzumab and a taxane (separately or in combination)
· B) as adjuvant treatment of HER2-positive early breast cancer with residual invasive disease after neoadjuvant taxane and trastuzumab-based treatment |
Hepatotoxicity, cardiac toxicity (reduced ejection fraction), and embryo-fetal toxicity. Assess ejection fraction prior to initiation and at regular intervals (eg, every 3 months) during treatment. Refer to PI for recommendations on hold (these are different depending on early breast cancer indication or metastatic disease) |
Neuropathy, thrombocytopenia, bleeding, hepatotoxicity, fatigue, nausea, musculoskeletal pain, hemorrhage |
None |
| Fam-trastuzumab deruxtecan |
Adult patients with:
· A) unresectable or metastatic HER2-positive breast cancer who have received 2 or more prior anti-HER2-based regimens in the metastatic setting OR
· B) locally advanced or metastatic HER2-positive gastric or gastroesophageal junction adenocarcinoma who have received a prior trastuzumab-based regimen |
Interstitial lung disease and pneumonitis (monitor for cough, dyspnea, fever, and other new or worsening respiratory symptoms); embryo-fetal toxicity |
Reduced ejection fraction, neutropenia, nausea |
None |
| Sacituzumab govitecan |
Adult patients with:
· Unresectable locally advanced or metastatic triple-negative breast cancer after ≥2 prior systemic therapies (at least 1 for metastatic disease) OR
· Locally advanced or metastatic urothelial cancer who have previously received a platinum-containing chemotherapy and either PD-1 or PD-L1 inhibitor |
Severe or life-threatening neutropenia (monitor blood counts periodically during treatment); severe diarrhea (monitor patients with diarrhea and administer fluid and electrolytes as needed; treat based on early onset versus late with atropine vs loperamide, respectively) |
Nausea; patients with reduced UGT1A1 are at risk for severe life-threatening neutropenia |
Antipyretic and H1 or H2 blocker; add corticosteroid for prior infusion-related reaction |
| Loncastuximab tesirine |
· Adult patients with relapsed or refractory large B-cell lymphoma after 2 or more lines of systemic therapy, including DLBCL not otherwise specified, DLBCL arising from low-grade lymphoma, and high-grade B-cell lymphoma |
None |
Fluid retention, effusions and edema, myelosuppression, infections, cutaneous reactions (wear sunscreen), elevated GGT |
Dexamethasone 4 mg PO or IV daily for 3 days prior to therapy |
Abbreviations: ADC, antibody-drug conjugate; ALCL, anaplastic large cell lymphoma; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; DLBCL, diffuse large B-cell lymphoma; GGT, gama-glutamyl transferase; H2, histamine-2; human epidermal growth factor receptor 2; HSCT, hematopoietic stem cell transplantation; JCV, John Cunningham virus; PD-1, programmed death receptor-1; PD-L1, programmed death-ligand 1; PI, prescribing information; PTCL, peripheral T-cell lymphoma; REMS, Risk Evaluation and Mitigation Strategy; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis.
a See full PI for comprehensive list of potential adverse events. |
Compounding/Preparation
As complex drug products, the ADCs require careful and specific preparation procedures. Since recommended procedures can change and in lieu of listing those procedures for 11 products, clinicians should consult the package inserts.8,9,16,18-25 There are several shared compounding/preparation highlights between the ADCs. All of the ADCs are supplied as lyophilized powders that require reconstitution. With the cytotoxic nature of the payloads, special handling and disposal procedures are required for all ADCs.26 Being biological products, ADCs are sensitive to handling and temperature and 3 warnings are included in all of the ADC package inserts: 1) do not shake the product vial to reconstitute the solution, 2) do not freeze the prepared product, and 3) avoid mixing with other drugs in the IV bag and/or in the IV infusion line. After reconstitution, dilution instructions often specify a concentration range for the final IV bag, and many ADCs require an in-line or add-on filter for IV administration. Avoiding exposure to direct sunlight is a common recommendation. As with many reconstituted IV products, ADCs may require a specific diluent and IV solution, which are indicated in the package inserts.
MANAGEMENT OF ADVERSE EVENTS ASSOCIATED WITH ADC AGENTS
Review of Adverse Events
Adverse events associated with ADCs are typically due to the biological nature of the mAb component and the payload. Infusion of mAbs can cause 2 common adverse events—hypersensitivity/allergic reactions and infusion reactions, which are distinct phenomena that can have overlapping symptoms (eg, dyspnea, urticaria).27 Infusion reactions typically occur 30 to 120 minutes after the initiation of ADC infusion and can range from mild/moderate symptoms (eg, headache, chills, dyspnea, fever, rash) to severe (eg, anaphylactic or cardiac reactions).28,29
Non-infusion-related adverse events vary based on the payload. Some of these adverse events can be severe, even deadly, and the majority of ADCs carry black box warnings (BBWs), the exceptions being polatuzumab vedotin and loncastuximab tesirine.16,21 For sacituzumab govitecan, the BBW cautions for neutropenia and diarrhea.25 Belantamab mafodotin and tisotumab vedotin carry BBWs for ophthalmic toxicities—corneal epithelium damage that can result in change in vision, severe vision loss, and/or corneal ulcer.8,23 For belantamab mafodotin, the risk is sufficiently severe that the FDA requires a Risk Evaluation and Mitigation Strategy (REMS) program.8,30 The black box for enfortumab vedotin focuses on severe and potentially fatal dermatological toxicities—toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS).22 Brentuximab vedotin carries a BBW for progressive multifocal leukoencephalopathy and death caused by John Cunningham virus (JCV) infection.20
The black boxes for gemtuzumab ozogamicin and inotuzumab ozogamicin warn for hepatoxicity that can lead to veno-occlusive disease or sinusoidal obstruction syndrome, while the inotuzumab ozogamicin BBW also includes an increased risk of nonrelapse mortality in patients who are post-hematopoietic stem cell transplant.18,19 Hepatoxicity, cardiac toxicity, and embryo-fetal toxicity are highlighted in the BBW for ado-trastuzumab emtansine.9 In addition, the black box for fam-trastuzumab deruxtecan warns for interstitial lung disease and embryo-fetal toxicity.24 Clinicians should consult the full prescribing information for details on BBWs and potential adverse events. 8,9,16,18-25,30
Recognition and Monitoring
For those ADCs with the potential for serious toxicities, clinicians should be prepared to recognize, monitor, and prevent the adverse reactions.31 With belantamab mafodotin, which has a REMS program, patients will need to have eye exams before they begin therapy and before each dose of the drug. In addition, patients should use preservative-free lubricating eye drops 4 or more times per day during treatment and should avoid wearing contact lenses.30 Treatment for ophthalmic toxicities may include dose adjustments or discontinuation of the drug.8 Precautions with tisotumab vedotin are similar in that a baseline eye exam is required prior to therapy with follow-up exams before each dose. Treatment with tisotumab vedotin, however, also requires use of corticosteroid eye drops before and 72 hours after an infusion, the use of vasoconstrictor drops prior to infusion, and application of lubricating eye drops throughout therapy and for 30 days after the last dose of ADC.23
The potential to develop veno-occlusive disease with gemtuzumab ozogamicin or inotuzumab ozogamicin can be difficult to manage as effective remedies are limited. Patients should be screened with hepatic function tests prior to each dose of gemtuzumab ozogamicin or inotuzumab ozogamicin. If elevations in hepatic function tests or other symptoms (eg, hepatomegaly, ascites, rapid weight gain) are observed, the ADC therapy may need to be withheld, dose reduced, or therapy discontinued.18,19 Management of pulmonary toxicity with trastuzumab deruxtecan is based on severity and generally consists of pausing therapy until symptoms resolve with the administration of corticosteroids, if needed, or discontinuation of therapy if symptoms are sufficiently severe.24 There is an increased risk of fluid retention with loncastuximab tesirine therapy and premedication with dexamethasone is recommended.16,32 The severe diarrhea that may occur with sacituzumab govitecan can be managed with fluids and electrolytes. For early onset diarrhea (onset during infusion or within several hours of infusion), an anticholinergic such as atropine may be administered. For late onset diarrhea not caused by an infectious agent, high-dose loperamide is recommended.25
Management Strategies: Infusion Reactions
Step-by-Step Process to Practice Incorporation of Available Guidelines Into Decision-Making for Preventing and Managing Toxicities
Many of the ADCs require premedication in their prescribing information with the goal to prevent infusion-related reactions. Even if premedication is not required per the package insert, institutions may set premedication protocols for ADCs. The most common drugs used in premedication regimens are acetaminophen, an antihistamine, and a corticosteroid. Dexamethasone (4 mg PO or IV daily for 3 days prior to therapy) is recommended for loncastuximab tesirine to help prevent edema.16,32 Premedication for preventing infusion-related reactions is specified in the prescribing information for polatuzumab vedotin (nonspecific antihistamine and antipyretic at least 30 minutes before infusion); brentuximab vedotin (suggest acetaminophen, antihistamine, and corticosteroid if the patient had a prior infusion-related reaction); gemtuzumab ozogamicin (acetaminophen, diphenhydramine, and methylprednisolone); inotuzumab ozogamicin (nonspecific corticosteroid, antihistamine, and antipyretic prior to infusion); belantamab mafodotin (nonspecific premedication if patient had a prior infusion-related reaction); and sacituzumab govitecan (nonspecific antipyretics and histamine [H1 and H2] blockers prior to infusion; corticosteroid also if prior infusion-related reaction).8,18-21,25
CONSIDERATIONS FOR ONCOLOGY PHARMACISTS
Patient-Centered Therapy Selection
Patient-centered oncology care (PCOC) models are based on the patient-centered medical home (PCMH), a framework of coordinating health care with a solid patient-physician relationship at its core.33 Key oncology-specific components of a PCOC model include a triage process to address symptom management, employing evidence- and standard-based protocols for symptom management, providing patient navigators, incorporating patient preferences in treatment decisions, and coordinating care between the myriad clinicians on the health care team.33,34 Implementing a PCOC model may improve coordination of care, patient satisfaction, and health outcomes.33,34
Patient Education
Education may be considered a foundational building block for PCOC.33,34 The oncology pharmacist is well positioned to provide that education, which would not be limited to oncology treatments. Pharmacist-led patient education can cover a wide range of topics including overall drug management (eg, oncology treatments, prescription drugs, over-the-counter [OTC] drugs, herbal medications), drug interactions, potential adverse effects, and medication errors. Providing such education will help empower patients to take ownership of their care.35 Pharmacists should ideally be present to assist patients with treatment decisions and therapy selection based on their oncologic history, comorbidities, preferences, and goals. They can educate the patient, nurse, physician, and other team members on how to monitor adverse events associated with ADCs during the course of treatment. Additionally, as toxicities occur or patients have questions about their therapy, pharmacists should be available to answer these types of questions throughout the course of the patient's treatment.
Drug-Drug Interactions
Overall, the payload drives the drug-drug interactions (DDIs) that may be observed with a particular ADC. Clinicians may consider that the overall dose of payload may be a limiting factor in the potential for DDIs. Several ADCs have apparent risk of DDIs such that changes in pharmacotherapy may be warranted. Interactions with CYP3A4 inhibitors or inducers and p-glycoprotein inhibitors are possible with the vedotin payload ADCs (tisotumab vedotin, polatuzumab vedotin, enfortumab vedotin, and brentuximab vedotin).20-23 CYP3A4 interactions are also possible with ado-trastuzumab emtansine.9 Drugs that are known to prolong QT interval should be avoided in patients receiving inotuzumab ozogamicin since concomitant use “may increase the risk of a clinically significant QTc interval prolongation.”19 For sacituzumab govitecan, potential interactions may occur with inhibitors or inducers of UGT1A1.25
Provider Consultations
With the complexities of oncology treatments and protocols, pharmacists can be valuable resources on the oncology team.35 Pharmacists will often provide knowledge of potential adverse events and how to mitigate them; potential DDIs; and proper preparation, handling, and administration.35,36 Whereby permitted by state law, pharmacists may enter in collaborative practice agreements under a physician’s oversight to provide enhanced care to oncology patients.35
Managing Financial Toxicities
As with most other antibody-based therapeutics, ADCs come with hefty price tags. For example, with the cost of inotuzumab ozogamicin estimated to be approximately $67,000 to $90,000 per cycle of treatment and 2 to 6 treatment cycles possible for patients, cost of treatment can reach close to $450,000.37 Estimates (from 2013) of other ADCs include brentuximab vedotin with average annual treatment costs of >$100,000 and ado-trastuzumab emtansine with an average monthly cost of $9,800.38 Even with insurance coverage, the out-of-pocket cost of an ADC can be beyond the reach of many patients. The inability to pay for pharmacological treatments leads to a condition termed financial toxicity.39 Financial toxicity related to cancer treatment can negatively impact a range of patient outcomes from health-related quality of life to overall survival.40 Pharmacists may help patients navigate the complexities of health insurance coverage as well as patient-assistance programs that may be available from manufacturers of ADCs.
SUMMARY
ADCs are complex and potent biological/drug products that use the specific protein-binding ability of mAbs to selectively target cancer cells. Coupled with highly potent payloads, modern ADCs represent significant advances in medical oncology. While researcher have made substantial progress in ADC technologies (ie, mAbs, linkers, payloads), toxicity and adverse events, due in part to off-target release of payload, can still limit application of ADCs. Clinicians should be aware of the structure-function of ADCs and their components. As the list of available ADCs grows, clinicians should stay up-to-date on new approvals and indications. Staying knowledgeable in the advances of ADCs allows clinicians the best opportunity to provide patients with quality information so that patients can make informed decisions on therapy. Finally, with the exorbitant costs of drugs such as ADCs, pharmacists may want to be prepared to assist patients in avoiding financial toxicities.
| UPDATE |
-
No new drug approvals or black box warnings
-
New indications
-
On August 5, 2022, the FDA granted approval to fam-trastuzumab deruxtecan-nxki foradults with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH -) breast cancer who have received a prior chemotherapy in the metastatic setting or have developed disease recurrence during or within 6 months of completing adjuvant chemotherapy.
-
On August 11, 2022, the FDA granted approval to fam-trastuzumab deruxtecan-nxki foradults with unresectable or metastatic non-small cell lungcancer with activating human epidermal growth factor receptor 2 (HER2 [ERBB2]) mutations, detected by an FDA test, and who have received a prior systemic therapy.
-
New safety labeling changes
-
On August 5, 2022, drug-related safety changes were added to the labeling of fam-trastuzumab deruxtecan-nxki such that toxicities could occur in other HER2-mutant solid tumors in addition to metastatic breast cancer.
-
On October 12, 2022, drug-related safety changes were added to the labeling of loncastuximab tesirine-lpyl
-
Contraception should be used during and for 10 months after last dose in women and during and for 7 months after last dose in males with female partners
-
Skin reactions including telangiectasia, blister, vesicular rash have been reported
-
New clinical trial data
-
On July 28, the 6-year follow-up results of the ECHELON-1 trial were published in the New England Journal of Medicine. In this study, patients with classic Hodgkin’s lymphoma were treated with 6 cycles of brentuximab vedotin (A) and doxorubicin, vinblastine, and dacarbazine (A+AVD) or doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD).
-
Overall survival was higher in the A+AVD group (94% vs 89%) and progression-free survival was also longer (hazard ratio for progression/death, 0.68, 95% confidence interval, 0.53-0.86).
-
Fewer patients in the A+AVD group received subsequent therapy, such as transplantation, and fewer secondary cancers were reported
-
Primary prophylaxis with granulocyte colony-stimulating factor is recommended with A+AVD because of increased incidence of febrile neutropenia
-
Peripheral neuropathy was also higher with A+AVD but in most patients resolution or amelioration was noted at last follow-up visit
-
On August 29, 2022, a safety analysis from the ASCENT trial evaluating sacituzumab govitecan in the treatment of metastatic breast cancer was published in NPJ Breast Cancer.
-
This results of this study demonstrate manageable safety with this drug, including in those who are 65 years and older and who have neutropenia or diarrhea.
-
Patients with UGT1A1 *28/*28 genotype have higher rates of grade 3 (or higher) neutropenia, febrile neutropenia, anemia and diarrhea. Therefore these individuals should be closely monitored.
- No new drug approvals or black box warnings
- On May 5, 2022, the FDA granted regular approval to fam-trastuzumab deruxtecan-nxki for adults with unresectable or metastatic HER2-positive breast cancer who have received a prior anti-HER2-based regimen in the metastatic setting or in the neoadjuvant or adjuvant setting and have developed disease recurrence during or within 6 months of completing therapy. It previously had received accelerated approval for adults with unresectable or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2-based regimens in the metastatic setting
- On May 4, 2022, drug-related safety changes were added to the labeling of fam-trastuzumab deruxtecan-nxki based on the updated results of the DESTINY-Breast03 study (n=492).
- 13% of patients developed interstitial lung disease (rather than 9% previously reported); median onset of toxicity was 5.5 months
- A higher incidence of left ventricular dysfunction noted 2.6% vs 0.9% previously reported
- A higher incidence of grade 3-4 adverse reactions have been observed in patients 65 years and older (60% vs 49%).
- Treatment-emergent antibodies developed in 2.1%; neutralizing antibodies in 0.1%. However, due to small number of patients, no conclusions can be drawn regarding immunogenicity’s effect on safety or efficacy.
- On June 3, 2022, drug-related safety changes were added to the labeling of sacituzumab govitecan-hziy
- No starting dose adjustment is needed for patients with mild hepatic impairment
- Safety has not been determined for patients with moderate hepatic impairment (total bilirubin > 1.5-3 x upper limit of normal [ULN] or severe hepatic impairment (total bilirubin > 3 x ULN) or those with AST or ALT > 3 ULN without liver metastases or AST or ALT < 5 ULN with metastases. No recommendations are provided for starting dose in these patients.
- In May 2022, enfortumab vedotin-ejfv’s package insert was revised, but no major changes to safety, efficacy or black box warnings occurred.
|
-
New drug approvals or black box warnings
-
On November 14, 2022, the FDA granted accelerated approval for mirvetuximab soravtansine-gynx for adults with folate receptor alpha–positive platinum-resistant epithelial ovarian, fallopian tube or primary peritoneal cancer in those who have received 1-3 systemic treatments. This new ADC is a folate receptor alpha directed antibody combined with an microtubule inhibitor conjugate
-
New indications
-
On November 10, 2022, the FDA granted approval to brentuximab vedotin when combined with doxorubicin, vincristine, etoposide, prednisone and cyclophosphamide in pediatric patients with classical Hodgkin’s lymphoma
-
New safety labeling changes
-
On November 10, 2022, drug-related safety changes were added to the labeling of brentuximab vedotin related to use in pediatric patients.
-
Pediatric patients had peripheral neuropathy (20%), which is lower than that in adult studies
-
Recommendation for primary G-CSF prophylaxis with cycle 1 when combined with combination chemotherapy for first-line treatment of classical Hodgkin lymphoma
-
On November 28, 2022 drug-related safety changes were added to the labeling of enfortumab vedotin regarding holding therapy for persistent or recurrent grade 2 skin reactions
-
New clinical trial data
-
On November 3, 2022, the results of the EuroNET-PHL-C1 trial were published in the New England Journal of Medicine. In this study, pediatric patients with newly diagnosed high risk classic Hodgkin’s lymphoma were randomized and treated with 5 cycles of brentuximab vedotin and doxorubicin, vinblastine, etoposide, prednisone and cyclophosphamide or standard bleomycin-containing therapy alone
- Event-free survival higher with brentuximab vedotin + chemotherapy (HR, 0.41; 95% CI, 0.25-0.67; p<0.001)
- Similar adverse events between 2 groups
- Overall survival was 99.3% at 3 years (compared with 98.5%)
-
On January 24, 2023, updated results of the DESTINY-Breast03 trial were published in The Lancet. The updated results confirm that trastuzumab deruxtecan is the standard of care in the 2nd-line setting for HER2-positive metastatic breast cancer
-
Overall survival and progression-free survival were both significantly improved compared with trastuzumab emtansine in patients with previously treated. Overall survival is not yet reached with trastuzumab deruxtecan after median follow-up of 28 months.
|
REFERENCES
- Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8(6):473-480. doi:10.1038/nrc2394
- Nawrat A. Timeline: charting the choppy history of ‘magic bullet’ antibody-drug conjugates. Pharmaceutical Technology. Published February 10, 2021. Accessed March 6, 2022. https://www.pharmaceutical-technology.com/features/antibody-drug-conjugates-timeline/
- Kostova V, Désos P, Starck JB, Kotschy A. The chemistry behind ADCs. Pharmaceuticals (Basel). 2021;14(5):442. doi:10.3390/ph14050442
- Tong JTW, Harris PWR, Brimble MA, Kavianinia I. An insight into FDA approved antibody-drug conjugates for cancer therapy. Molecules. 2021;26(19):5847. doi:10.3390/MOLECULES26195847
- Liu D. Cancer biomarkers for targeted therapy. Biomark Res. 2019;7(1):25. doi:10.1186/s40364-019-0178-7
- Dan N, Setua S, Kashyap VK, et al. Antibody-drug conjugates for cancer therapy: chemistry to clinical implications. Pharmaceuticals (Basel). 2018;11(2):32. doi:10.3390/PH11020032
- Salomon PL, Reid EE, Archer KE, et al. Optimizing lysosomal activation of antibody-drug conjugates (ADCs) by incorporation of novel cleavable dipeptide linkers. Mol Pharm. 2019;16(12):4817-4825. doi:10.1021/acs.molpharmaceut.9b00696
- Blenrep (belantamab mafodotin-blmf) prescribing information. GlaxoSmithKline; February 2022.
- Kadcyla (ado-trastuzumab emtansine) prescribing information. Genentech, Inc; February 2022.
- Shen W, Ballou B, Ryser H, Hakala T. Targeting, internalization, and cytotoxicity of methotrexate-monoclonal anti-stage-specific embryonic antigen-1 antibody conjugates in cultured F-9 teratocarcinoma cells. Cancer Res. 1986;46(8):3912-3916.
- Johnson D, Laguzza B. Antitumor xenograft activity with a conjugate of a Vinca derivative and the squamous carcinoma-reactive monoclonal antibody PF1/D. Cancer Res. 1987;47(12):3118-3122.
- Dillman R, Johnson D, Shawler D, Koziol J. Superiority of an acid-labile daunorubicin-monoclonal antibody immunoconjugate compared to free drug. Cancer Res. 1988;48(21):6097-6102.
- Tolcher AW, Sugarman S, Gelmon KA, et al. Randomized phase II study of BR96-doxorubicin conjugate in patients with metastatic breast cancer. J Clin Oncol. 1999;17(2):478-484. doi:10.1200/jco.1999.17.2.478
- Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol. 2021;18(6):327-344. doi:10.1038/S41571-021-00470-8
- Masters JC, Nickens DJ, Xuan D, et al. Clinical toxicity of antibody drug conjugates: a meta-analysis of payloads. Invest New Drugs. 2018;36(1):121-135. doi:10.1007/S10637-017-0520-6
- Zynlonta (loncastuximab tesirine-lpyl) prescribing information. ADC Therapeutics, SA; September 2021.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Accessed March 6, 2022. https://www.nccn.org/guidelines/category_1
- Mylotarg (gemtuzumab ozogamicin) prescribing information. Wyeth Pharmaceuticals, Inc (division of Pfizer); August 2021.
- Besponsa (inotuzumab ozogamicin) prescribing information. Wyeth Pharmaceuticals, Inc (division of Pfizer); March 2018.
- Adcetris (brentuximab vedotin) prescribing information. Seagen, Inc; February 2022.
- Polivy (polatuzumab vedotin-piiq) prescribing information. Genentech, Inc; September 2020.
- Padcev (enfortumab vedotin-egfv) prescribing information. Astellas Pharma, Inc; November 2021.
- Tivdak (tisotumab vedotin-tftv) prescribing information. Seagen, Inc; January 2022.
- Enhertu (fam-trastuzumab deruxtecan-nxki) prescribing information. DaichiiSankyo, Inc; January 2021.
- Trodelvy (sacituzumab govitecan-hziy) prescribing information. Gilead Sciences, Inc; October 2021.
- Power LA, Coyne JW. ASHP guidelines on handling hazardous drugs. Am J Health Syst Pharm. 2018;75(24):1996-2031. doi:10.2146/ajhp180564
- Cáceres MC, Guerrero-Martín J, Pérez-Civantos D, et al. The importance of early identification of infusion-related reactions to monoclonal antibodies. Ther Clin Risk Manag. 2019;15:965-977. doi:10.2147/TCRM.S204909
- Khongorzul P, Ling CJ, Khan FU, et al. Antibody-drug conjugates: a comprehensive review. Mol Cancer Res. 2020;18(1):3-19. doi:10.1158/1541-7786.MCR-19-0582
- Rombouts MD, Swart EL, Van Den Eertwegh AJM, Crul M. Systematic review on infusion reactions to and infusion rate of monoclonal antibodies used in cancer treatment. Anticancer Res. 2020;40(3):1201-1218. doi:10.21873/anticanres.14062
- GlaxoSmithKline. Blenrep REMS (Risk Evaulation and Administration Strategy). Published August 2020. Accessed March 7, 2022. https://www.blenreprems.com/#Main
- Lievano FA, Scarazzini LJ, Tyczynski JE, et al. Risk minimization of antibody-drug conjugates in oncology: a review. Drug Saf. 2021;44(7):733-742. doi:10.1007/s40264-021-01069-9
- Hamadani M, Radford J, Carlo-Stella C, et al. Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma. Blood. 2021;137(19):2634-2645. doi:10.1182/blood.2020007512
- Liang H, Tao L, Ford EW, et al. The patient-centered oncology care on health care utilization and cost: a systematic review and meta-analysis. Health Care Manage Rev. 2020;45(4):364-376. doi:10.1097/HMR.0000000000000226
- Tirodkar MA, Roth L, Fuld Nasso S, et al. Facilitators and barriers to implementing a patient-centered oncology care model. JCO Oncol Pract. 2020;16(12):e1441-e1450. doi:10.1200/op.20.00231
- Ma C. Role of pharmacists in optimizing the use of anticancer drugs in the clinical setting. Integr Pharm Res Pract. 2014;3:11-24. https://doi.org/10.2147/IPRP.S40428
- Mirkov S, Rosman J. Chapter 16. A checklist for pharmacists on biologics and biosimilars. In: Razman I, ed. Biologics, Biosimilars, and Biobetters: An Introduction for Pharmacists, Physicians, and Other Health Practitioners. Wiley Online Library; 267-293. Published December 30, 2020. https://doi.org/10.1002/9781119564690.ch16
- Jain T, Litzow MR. No free rides: management of toxicities of novel immunotherapies in ALL, including financial. Blood Adv. 2018;2(22):3393-3403. doi:10.1182/bloodadvances.2018020198
- Zolot RS, Basu S, Million RP. Antibody-drug conjugates. Nat Rev Drug Discov. 2013;12(4):259-260. doi:10.1038/nrd3980
- Zafar SY, Abernethy AP. Financial toxicity, part I: a new name for a growing problem. Oncology (Williston Park). 2013;27(2):80-81, 149.
- de Souza JA, Yap BJ, Wroblewski K, et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: the validation of the COmprehensive Score for financial Toxicity (COST). Cancer. 2017;123(3):476-484. doi:10.1002/cncr.30369
Back to Top