
Expired activity
Please go to the PowerPak
homepage and select a course.
Rett Syndrome:
Optimizing the Management of a Rare Disorder
Overview of Rett Syndrome
Rett syndrome is a rare, progressive neurodevelopmental disorder that predominantly affects girls and in which mutations in a single gene (MECP2) on the X chromosome account for the majority (≈95%) of cases.1 The disease is named after Andreas Rett, who first described the syndrome in 1966.1,2 The predominance of female patients provided ample evidence of a genetic basis for the disease, which was identified in 1999 as MECP2, the gene coding for the methyl-CpG binding protein 2 (MeCP2).1,3,4 While most individuals with Rett syndrome do have MECP2 mutations, some individuals with Rett syndrome do not have such mutations and there are others with MECP2 mutations who do not develop Rett syndrome; therefore, identifying the disease is a clinical diagnosis rather than a purely genetic diagnosis.2,5 As a neurodevelopmental disorder, individuals with Rett syndrome exhibit a range of deficiencies that impact multiple functional domains—behavioral, motor, social, personal, academic, and occupational.6,7 In the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders, Rett syndrome is now classified as a discrete disorder whereas prior editions lumped Rett syndrome into a larger category of developmental disorders, in part due to overlapping symptoms and presentations.8,9
While the disorder has a predominance of neurologic features, the expression of the MeCP2 protein is ubiquitous throughout the body and contributes to the variety of symptoms that people with Rett syndrome experience.10 Pharmacotherapeutic management of Rett syndrome focuses on treating such symptoms as warranted with currently available drugs. However, new drugs to specifically address Rett syndrome are on the horizon, with the first drug approved by the US Food and Drug Administration (FDA) for the disease in March 2023.11 This activity will focus on general treatment of symptoms associated with the disorder and new and emerging therapies that are specifically for Rett syndrome.
Epidemiology
Rett syndrome is considered a rare disorder with prevalence estimates ranging from 5 to 10 in 100,000 females.2,12 A recent systematic review and meta-analysis corroborated that estimated range, with a determined pooled prevalence estimate of 7.1 (95% CI: 4.8-10.5) per 100,000 females.2 Using an estimate of 10 cases per 100,000 females, there are an estimated 15,000 girls in the United States and 350,000 girls worldwide with Rett syndrome.13 Although overwhelmingly found in females, Rett syndrome also affects males, but the extraordinarily low numbers precludes reliable estimates on incidence and prevalence in males.14,15 Males also present with a broader range in the severity of symptoms, often with more subtlety in presentation.16 Given the lop-sided sex distribution of Rett syndrome, the information in this activity will focus on female individuals unless otherwise noted.
Pathophysiology
Genetic aspects
The majority of people with classic Rett syndrome (95%-97%) have a mutation of the MECP2 gene that leads to loss of function in the methyl-CpG binding protein-2 (MeCP2).17 MeCP2 is a transcriptional modulator that interacts with a variety of proteins and protein complexes within the central nervous system (CNS) as well as throughout the body.1 More than 300 gene mutations that cause loss of MeCP2 function and lead to Rett syndrome have been identified.1Most commonly, the MECP2 mutations occur spontaneously in sperm cells and, with the X chromosome location of the gene, lead to the predominance of Rett syndrome in females.12,16Ongoing investigations have determined that particular mutation patterns correlate with severity of symptoms. Increased symptom severity is associated with early truncating mutations, large deletion mutations, and certain point mutations (e.g., R168X, R106W, R255X, and R270X). Less severe symptoms are associated with C-terminal truncations and other point mutations (e.g., R133C, R294X, and R306X).10,12,18 In addition to correlating with the highest clinical severity score used as a marker for severity of the disease, the R168X mutation is also associated with increased seizure frequency whereas the R133C mutation correlates with the lowest clinical severity score.18
Diagnosis
Presentation and clinical stages
The diagnostic criteria for Rett syndrome were revised in 2010 and remain the basis for clinical diagnosis.17 One common feature of people with Rett syndrome is the timeline of symptoms, which typically has 4 stages (Figure).10,19 After a normal period of development, stage I (the early onset or stagnation stage) may occur between 6 to 18 months of age. It presents as a period in which development stalls and can last for several weeks to several months. During stage I, microcephaly may begin, along with gross motor postural delays, and changes in interactions with others can be seen. During stage II, the regression of developmental milestones, such as sitting upright or crawling, and the loss of the use of hands and communication skills occur. The onset of stage II (a rapid regression or destructive stage) may occur between 1 to 4 years of age and can last several weeks to several months. Stage II is also characterized by the appearance of repetitive hand movements and breathing irregularities, progression of microcephaly, and the onset of seizures. Stage III (the pseudo-stationary or plateau stage) has a typical onset of age 4 to 8 years and can last several years to decades. Stage III starts when the regression is over. Characteristics include stabilization of or slow gross motor progression, significant hand apraxia/dyspraxia, worsening of breathing dysfunction, musculoskeletal progressive changes, and frequent seizures. Also in stage III, improved eye contact typically develops and is used for communication along with the return of some alertness and interaction with loved ones. Stage IV (the late motor deterioration stage) is not experienced by all individuals but can last decades. During Stage IV, patients will exhibit decreased mobility (possibly leading to wheelchair dependence), progression of spasticity, rigidity, dystonia, and lessening of hand stereotypies, with preservation of eye communication.10,19
| Figure. Stages and Timeline of Rett Syndrome Symptoms |
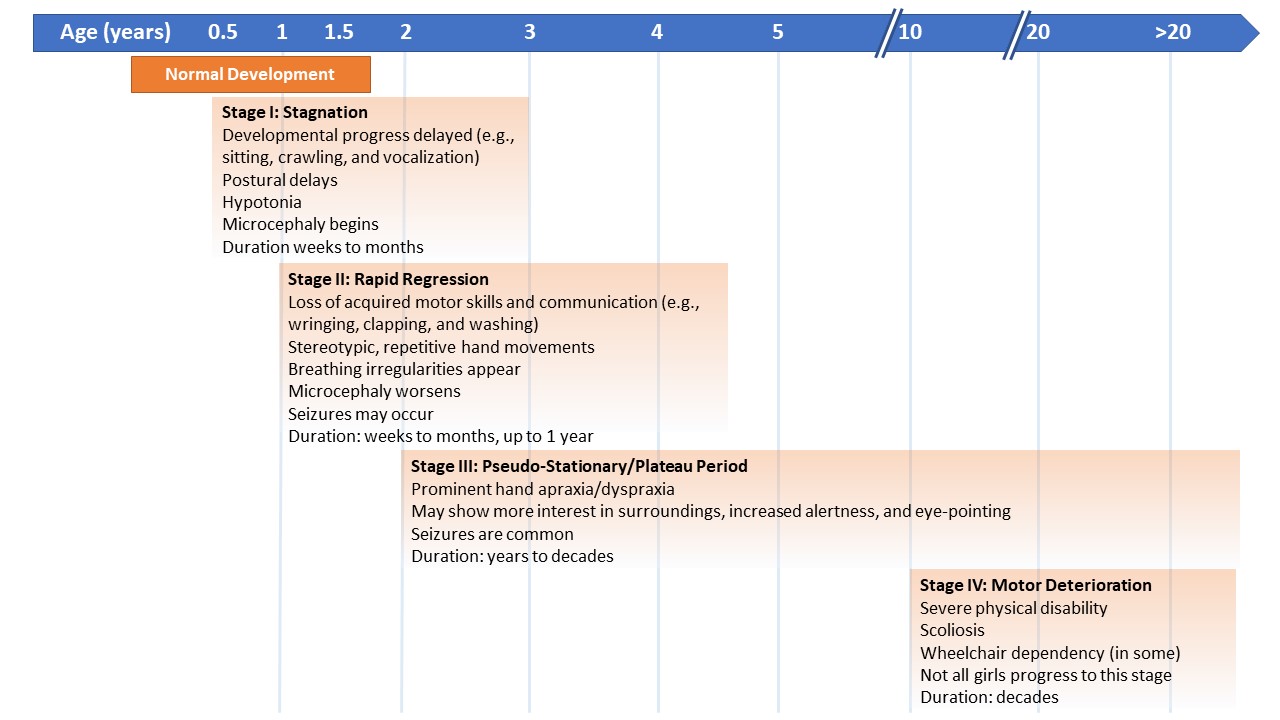 |
| Adapted from Kyle et al.10 |
Diagnostic criteria and classifications: classical versus atypical
The diagnostic criteria for Rett syndrome can be characterized as (1) 4 main inclusion criteria, (2) 2 exclusion criteria, and (3) 11 supportive criteria (Table 1).17,19 For a diagnosis of classic Rett syndrome, all 4 main inclusion criteria are met; no exclusion criteria are present; and, while supportive criteria are not required, they are often present. For a diagnosis of atypical Rett syndrome, 2 of 4 main criteria must be met, no exclusion criteria are present, and at least 5 out of 11 supportive criteria are met. Whether classic or atypical, breathing and swallowing difficulties are common as well as seizures. Other clinical features include anxiety, depression, abrupt mood changes, aggression, self-abuse, and impulsivity.20 Clinicians will often observe that individuals with Rett syndrome (classic and atypical types) will have a range of presentations and, although symptoms may be intermittent, they may continue to contribute to a diagnosis.
| Table 1. Diagnostic Criteria for Rett Syndrome17,19 |
|
Main Inclusion Criteria
|
Supportive Criteria
|
Exclusion Criteria
|
-
Partial or complete loss of acquired purposeful hand skills
-
Partial or complete loss of acquired spoken language
-
Gait abnormalities: impaired (dyspraxic) or absence of ability
-
Stereotypic hand movements (e.g., hand wringing/squeezing, clapping/tapping, mouthing, and washing/rubbing automatisms)
|
-
Breathing disturbances when awake
-
Bruxism when awake
-
Impaired sleep pattern
-
Abnormal muscle tone
-
Peripheral vasomotor disturbances
-
Scoliosis/kyphosis
-
Growth retardation
-
Small, cold hands and feet
-
Inappropriate laughing/screaming spells
-
Diminished response to pain
-
Intense eye communication—“eye pointing”
|
-
Peri- or postnatal brain injury secondary to trauma, neurometabolic disease, or severe infection that causes neurologic problems
-
Grossly abnormal psychomotor development in first 6 months of life
|
| For both classic and atypical presentations of Rett syndrome, patients exhibit a period of regression followed by stabilization or recovery. In classic Rett syndrome, all 4 main criteria are met, no exclusion criteria are present, and supportive criteria are not required but are often present. In atypical Rett syndrome, 2 of the 4 main criteria must be met, no exclusion criteria are present, and 5 out of 11 supportive criteria are met. |
Common Systemic Complications and Suggested Approaches for Management
Current Standard of Care
People with Rett syndrome may experience a range of comorbidities that can require medical, pharmacologic, or physical interventions. Rett syndrome comorbidities are categorized as neurologic, neuropsychiatric, cardiac, gastrointestinal/nutritional, and orthopedic.21 The prevalence rates of specific comorbidities range from a low of 4% for gall bladder dysfunction to ≥90% for dysphagia, epilepsy, sleep dysfunction, irregular breathing pattern, and behavioral disturbances. A list of common morbidities (i.e., prevalence ≥50%) is listed in Table 2.21
| Table 2. Common Comorbidities in Rett Syndrome (Prevalence ≥50%) |
|
Comorbidity
|
Prevalence (%)
|
|
Irregular breathing pattern
|
95
|
|
Epilepsy
|
90
|
|
Dysphagia
|
90
|
|
Sleep dysfunction
|
80-93
|
|
Movement disorders
|
63-84
|
|
Behavioral disturbance
|
97
|
|
Constipation
|
80
|
|
Low bone mineral mass
|
59
|
|
Scoliosis
|
80
|
|
Hip displacement
|
50
|
| Adapted from Fu et al.21 |
Because there is no curative treatment for Rett syndrome, management of the disorder and corresponding comorbidities involves treating the particular symptoms as they present. Although treatment options may not be strictly evidence-based (i.e., tested rigorously in clinical trials of individuals with Rett syndrome), consensus guidelines provide a framework for symptom management.12 Management of Rett syndrome requires individualized therapy and knowledge of a person’s particular comorbidities, which will inform the appropriateness of therapy. An overview of the more common signs, symptoms, and treatment approaches follows with a focus on the pharmacologic therapies.
Comorbidities and Treatment Options
Neurologic
Neurologic comorbidities dominate the presentation of Rett syndrome. Breathing irregularities related to autonomic dysregulation affect 95% or more of people with the disease and include elevated breathing rates, breath-holding, and air-swallowing. Typically evident by 4 years of age, the breathing symptoms can remain throughout the patient’s life, though in many individuals the symptoms lessen with age.21 Epilepsy/seizures are one of the most common comorbidities in Rett syndrome and affect approximately 90% of individuals.21 No single antiseizure medication is indicated specifically for Rett syndrome; it depends on the type of seizure or seizures and changes exhibited on electroencephalogram, along with any other comorbidities that are present and concomitant medications. Although all antiseizure medications are an option, in certain patients topiramate may be preferred because of its potential beneficial effects on breathing irregularities, which are common in Rett syndrome.22,23
Neuropsychiatric
Anxiety is a relatively common symptom in patients with Rett syndrome. In a natural history study of individuals with Rett syndrome, 16.6% received drug treatment for anxiety with approximately 72% of those individuals reporting satisfactory control of symptoms.24 Older individuals and those with mild MECP2 variants were more apt to use anxiolytics and selective serotonin reuptake inhibitors (SSRIs).24 It may be preferrable to use SSRIs with a reduced risk of causing prolonged QTc interval (described below) such as escitalopram.12
Cardiologic
The primary cardiology-related symptom/comorbidity in Rett syndrome is prolonged QTc interval affecting 10% to 18% of patients.21 An annual electrocardiogram is recommended to screen for prolonged QTc with referral to a cardiologist as needed based on the results.12 Medications that can precipitate prolonged QTc interval should be avoided in patients with Rett syndrome given the elevated risk. Numerous drugs may increase QTc interval and any prescribed medicine should be screened prior to dispensing to a person with Rett syndrome.25,26 A few commonly prescribed drugs with this potential adverse event (AE) include quetiapine, risperidone, amiodarone, macrolide antibiotics, fluoroquinolone antibiotics, and citalopram.25
Gastroenterologic/nutritional
Constipation and reflux are 2 of the more common gastroenterologic symptoms in patients with Rett syndrome. To treat constipation, osmotic laxatives (e.g., magnesium hydroxide, polyethylene glycol, or glycerin suppository) and/or stimulant laxatives (e.g., bisacodyl suppository) may be used with the goal of achieving a daily bowel movement.12 Proton pump inhibitors (e.g., omeprazole, esomeprazole, or lansoprazole), calcium carbonate, or histamine-2 receptor blockers (e.g., nizatidine) may be used to treat reflux.12 Individuals with Rett syndrome often develop difficulties with feeding to the point where a gastric tube is necessary to provide nutrition. This symptom should be considered when evaluating formulation options for prescribed drugs for which liquid/suspension formulations may be preferred.
Orthopedic
As people with Rett syndrome age, the risk of orthopedic maladies increases. Common orthopedic comorbidities (percent prevalence) include scoliosis (80%), hip dysplasias (50%), and fractures (28%-32%).21 Physical, occupational, and exercise therapies are important tools for prevention to delay worsening dysfunction, for recovery after surgeries, and for managing contractures that patients also may experience. Drugs used for orthopedic complications primarily focus on pain management and reducing spasticity and/or rigidity. Pain medications that reduce respiratory rate (e.g., opioids) should be used with caution given the breathing difficulties that patients with Rett syndrome often experience.
Novel Drugs in Development: Mechanisms of Action and Safety and Efficacy Data
To date, the FDA has approved only 1 agent, trofinetide, for the treatment of Rett syndrome.11 Pipeline drugs that are in various stages of clinical development include blarcamesine and gene therapy.
Trofinetide
Trofinetide is an analogue of glycine-proline-glutamate, which is the N-terminal tripeptide of insulin-like growth factor-1 (IGF-1).27 IGF-1 has multiple roles in the CNS related to nerve growth and development.28 Preclinical and early clinical evidence suggested that recombinant IGF-1 could be beneficial in the treatment of Rett syndrome; however, subsequent clinical trials did not demonstrate efficacy.29,30 The results led to testing and development of trofinetide. Although the particular mechanism of action is not clear, studies suggest that trofinetide improves neuronal and glial functions via anti-inflammatory and antioxidant activity.31,32 In March 2023, trofinetide became the first drug approved by the FDA specifically for the treatment of Rett syndrome and is approved for adults and children aged 2 years and older.11,33
Early-phase clinical studies in patients with Rett syndrome or fragile X syndrome suggest that trofinetide, administered as an oral liquid, is well-tolerated at doses ranging from 35 mg/kg to 200 mg/kg twice daily.31,34,35 The most common AEs (typically mild or moderate) were diarrhea, vomiting, upper respiratory infections, headache, pyrexia, and fatigue.27,31,34,35 With a half-life of 1.4 hours, trofinetide is dosed twice daily orally or via a gastrostomy tube.11,27,36 There appear to be inconclusive data on the effect of food on bioavailability, with 1 study showing an apparent decrease from 63% to 50%36 and another showing a negligible effect between fed and fasted states.27 Therefore, the prescribing information for trofinetide states that the drug can be taken without regard to food.11 Trofinetide dosing is based on body weight and ranges from 5000 mg (25 mL) twice daily for patients who weigh 9 kg to 12 kg and 12,000 mg (60 mL) twice daily for patients who weigh 50 kg or more. In the prescribing information, warnings and precautions for trofinetide focus on diarrhea and weight loss. Precautions include discontinuing laxatives prior to initiating trofinetide therapy and adjusting trofinetide therapy (i.e., pausing therapy, reducing the dose, or discontinuing treatment) in the event of severe diarrhea, dehydration, or significant weight loss. Potential drug interactions may be observed with CYP3A4, OATP1B1, and OATP1B3 substrates.11
Two phase 2 clinical trials and a phase 3 clinical trial provided the efficacy data on trofinetide.31,35,37,38 The first phase 2 trial (double-blind, randomized, placebo-controlled) studied the safety and tolerability of trofinetide in adolescent and adult female patients with Rett syndrome at doses of 35 mg/kg or 70 mg/kg while evaluating efficacy measures that would be used in subsequent trials.35 In the second phase 2 trial (also double-blind, randomized, placebo-controlled), pediatric participants aged 5 to 15 years received placebo or 1 of 3 trofinetide doses (50 mg/kg, 100 mg/kg, or 200 mg/kg twice daily).31 In this second phase 2 trial, participants in the highest trofinetide dose exhibited statistically significant improvements in 3 out of 5 efficacy outcomes—the Rett Syndrome Behaviour Questionnaire (RSBQ), the Rett syndrome–Clinician Domain Specific Concerns–Visual Analog Scale (RTT-DSC-VAS), and the Clinical Global Impression Scale–Improvement (CGI-I). Compared with placebo, the 200-mg/kg trofinetide group exhibited a decrease in the mean RSBQ of -6.7 (±1.46 standard error; P = .042), a decrease in median RTT-DSC-VAS of -76.00 (P = .025), and an increase in mean CGI-I of 3.0 (±0.13; P = .029).31 AEs of both phase 2 trials were summarized above.31,35
The safety, tolerability, and efficacy results from those trials led to the phase 3 LAVENDER study (double-blind, randomized, placebo-controlled). In the LAVENDER trial, study participants were females with Rett syndrome ranging in age from 5 to 20 years.38,39 Participants received either placebo or trofinetide (50 mg/kg, 100 mg/kg, or 200 mg/kg twice daily) over 12 weeks. Primary endpoints were RSBQ and CGI-I scores.39 While top-line results were announced in December 2021 and presented at the 2022 American Academy of Neurology Virtual Annual Meeting,40,41 full study results were not published at the time of this activity. The top-line results suggest that trofinetide treatment produced statistically significant improvements in the 2 primary endpoints (P = .0175 for RSBQ; P = .0030 for CGI-I) while also leading to increased study discontinuation rates (17.2% for trofinetide; 2.1% for placebo).40,41 Diarrhea was the most common AE affecting 80.6% of participants receiving trofinetide compared with 19.1% receiving placebo.40 Study participants were invited to continue trofinetide in a 40-week open-label extension study (NCT04279314).42
Blarcamesine
In the search for biological targets to treat neurologic disorders, the sigma-1 receptor has been postulated to be involved in diseases such as Alzheimer disease, Parkinson disease, and Rett syndrome.43-45 The sigma-1 receptor is involved in cellular responses to stress and activation of the receptor appears to exhibit neuroprotective effects.43,45 Blarcamesine, one such sigma-1 receptor agonist, is currently being investigated for Alzheimer disease, fragile-X syndrome, and Rett syndrome.46-48 The ongoing EXCELLENCE study (NCT04304482) is a phase 2/3 study in pediatric patients with Rett syndrome aged 5 to 17 years,49 and the ongoing AVATAR (NCT03941444) phase 3 study focuses on adult patients with Rett syndrome aged 18 years and older.50 Dosing of blarcamesine is up to 30 mg once daily as an oral liquid. While results of the trials have not been published yet, the manufacturer posted top-line results for the AVATAR study with 33 adult patients.51 According to that press release, the drug appears to show improvements in primary and secondary outcomes with similar rates of AEs compared with placebo.51
Investigational Gene Therapies
As a disease primarily caused by single gene mutations, Rett syndrome may be a ripe target for gene therapy treatment and gene therapy approaches continue to be studied in preclinical models.1,20,52,53 One of the challenges of using gene therapy for Rett syndrome is avoiding overexpression of the target protein, MeCP2. As discussed herein, Rett syndrome is typically characterized by low or mutated expression of MECP2. Overexpression of MECP2, however, can lead to MeCP2 duplication syndrome.52 One such therapy, TSHA-102, is currently in phase 1/2 clinical trials. TSHA-102 uses a recombinant, non-replicating adenovirus vector and is administered via the intrathecal route.54 Although the manufacturer expected preliminary data by the end of 2022,54 no results have been presented to date and recruitment appears to be ongoing.55 Another gene therapy, NGN-401, recently received investigational new drug clearance to begin an open-label, single-arm phase 1/2 clinical trial in female pediatric patients with Rett syndrome. NGN-401 will be administered as a 1-time intracerebroventricular injection using a proprietary gene delivery technology designed to avoid overexpression of MECP2.56
Pharmacist Considerations
With a single new FDA-approved treatment for Rett syndrome, the potential considerations for pharmacists are based on comparisons with orphan drugs for other rare diseases and focus on newly-approved trofinetide and blarcamesine, which is the next furthest along in clinical development. Because the manufacturers of trofinetide and blarcamesine are using orphan drug status for their respective compounds, drug costs could be substantial, which is common for even small-molecule orphan drugs.57
Formulary Development
Cost-effectiveness of a drug is one of the major factors considered in formulary decisions.58 With trofinetide approved just in March 2023 and blarcamesine not yet on the market, there is no price/cost information available for the drugs at the time of publication. Information on formulary coverage of orphan drugs in general may be helpful to prepare for their potential FDA approvals. In the United States, there are over 1,000 health insurance companies as of 2020.59 As could be surmised, coverage of orphan drugs can range widely across the various US payers, including the Medicare Part D program.57,60 Chambers et al analyzed coverage decisions for orphan and non-orphan specialty drugs.57 The researchers found that orphan drugs are almost universally covered by health insurance plans, that the frequency of applied restrictions on access vary widely (11%-65%), and that orphan drugs had fewer restrictions compared with non-orphan drugs.57 On the other hand, Cohen and Felix determined that orphan drugs had more restrictions than non-orphan drugs in Medicare Part D coverage.61 Yehia et al also analyzed orphan drug coverage in Medicare Part D and determined there were coverage restrictions for 92% of orphan drugs and 85% of orphan drugs were categorized at the highest cost-sharing level.60 For patients and their caregivers, therefore, it is important to note that health insurance coverage for a drug does not necessarily translate to affordability. Medicaid patients may experience different access issues to specialty drugs. Although the Medicaid Drug Rebate Program requires coverage of all approved drugs of participating drug manufacturers, states may employ prior authorization (PA) and formulary restrictions to manage access.62-64
Prior Authorizations
Prior authorization is one of the most common restrictions used to control drug costs to the payer. In one analysis from 2020, up to 76% of orphan drugs required PA in Medicare Part D plans.60 There is a dearth of specific biomarkers that can be used to track improvements in patients with Rett syndrome. The clinical study outcomes for trofinetide and blarcamesine focused on caregiver and physician scoring of symptoms. Hence, a potential question that may affect PAs and payer coverage of Rett syndrome drugs is, “What does treatment success look like for Rett syndrome?” How payers answer that question for Rett syndrome drugs remains to be seen.
As a comparison, we can look at the payer criteria applied for PA of edaravone, a specialty drug for amyotrophic lateral sclerosis. Criteria included using clinical trial inclusion criteria for determining patient eligibility, limiting approval time period to the length of the clinical trials, and requiring evidence of clinical stabilization or benefit for reauthorization.65 The patient perspective on PAs should not be lost in the discussion. Pasquini et al conducted a qualitative study of parents of children with rare diseases to learn about their perspectives on the US health insurance system.66 The researcher’s suggestions from parent interviews include limiting the time for insurers to make coverage decision, increasing transparency in the insurance processes (including PAs), and expanding authorizations for continuation of therapy.66
Adherence Monitoring: Dosing and Storage
Based on clinical trial formulations, both trofinetide and blarcamesine are expected to be orally dosed liquids. For trofinetide, the volume of solution administered at recommended dosages varies by body weight and ranges from 25 mL to 60 mL.11 Blarcamesine was dosed once daily in a liquid formulation (unspecified volume) in the phase 2/3 and phase 3 studies.49,50 As described earlier, individuals with Rett syndrome often have complications with swallowing. Pharmacists and other clinicians should be aware of the potential for reduced adherence in individuals who struggle with taking food by mouth.
Summary
Rett syndrome is a rare neurodevelopmental disorder that primarily affects females, most often due to mutations on the MECP2 gene on the X chromosome. Individuals with Rett syndrome present with certain developmental issues (e.g., delays in developmental milestones and lack of communication skills) that may overlap with other neurodevelopmental disorders (e.g., autism spectrum disorder). Specific criteria for Rett syndrome are important for diagnosis with or without genetic analysis. Individuals with Rett syndrome typically follow a particular timeline of symptomology with 4 major stages—stagnation, rapid regression, plateau, and motor deterioration. Common comorbidities associated with Rett syndrome include breathing irregularities, seizures, hand apraxia/dyspraxia, and complications with swallowing. Care for individuals with Rett syndrome focuses on management of symptoms and comorbidities. When considering pharmacotherapy options, clinicians should focus on minimizing polypharmacy to limit the potential to exacerbate certain symptoms (e.g., elongated QTc interval). The recent approval of trofinetide, an analogue of the N-terminal tripeptide of IGF-1, provides a new option for the pharmacologic treatment of individuals with Rett syndrome. An investigational drug on the horizon is the sigma-1 receptor agonist, blarcamesine. In addition, given the genetic nature of the disease, gene therapy options are being actively explored at the phase 1/2 stages. Although the disease is very rare, clinicians should be aware of current management approaches as well as new agents—approved and in the pipeline—designed specifically to treat Rett syndrome.
REFERENCES
1. Vashi N, Justice MJ. Treating Rett syndrome: from mouse models to human therapies. Mamm Genome. 2019;30(5-6):90-110. doi:10.1007/s00335-019-09793-5
2. Petriti U, Dudman DC, Scosyrev E, Lopez-Leon S. Global prevalence of Rett syndrome: systematic review and meta-analysis. Syst Rev. 2023;12(1):5. doi:10.1186/s13643-023-02169-6
3. Amir RE, Van den Veyver IB, Wan M, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23(2):185-188. doi:10.1038/13810
4. Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases. Ann Neurol. 1983;14(4):471-479. doi:10.1002/ana.410140412
5. Cianfaglione R, Clarke A, Kerr M, et al. A national survey of Rett syndrome: age, clinical characteristics, current abilities, and health. Am J Med Genet A. 2015;167(7):1493-1500. doi:10.1002/ajmg.a.37027
6. Mullin AP, Gokhale A, Moreno-De-Luca A, et al. Neurodevelopmental disorders: mechanisms and boundary definitions from genomes, interactomes and proteomes. Transl Psychiatry. 2013;3(12):e329. doi:10.1038/tp.2013.108
7. Posar A, Visconti P. Neurodevelopmental disorders between past and future. J Pediatr Neurosci. 2017;12(3):301-302. doi:10.4103/jpn.JPN_95_17
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013. doi:10.1176/appi.books.9780890425596
9. Hodges H, Fealko C, Soares N. Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Transl Pediatr. 2020;9(suppl 1):S55-S65. doi:10.21037/tp.2019.09.09
10. Kyle SM, Vashi N, Justice MJ. Rett syndrome: a neurological disorder with metabolic components. Open Biol. 2018;8(2):170216. doi:10.1098/rsob.170216
11. Daybue [package insert]. San Diego, CA: Acadia Pharmaceuticals, Inc; 2023. Accessed March 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217026s000lbl.pdf
12. Fu C, Armstrong D, Marsh E, et al. Consensus guidelines on managing Rett syndrome across the lifespan. BMJ Paediatr Open. 2020;4(1):e000717. doi:10.1136/bmjpo-2020-000717
13. Rett Syndrome Research Trust. About Rett syndrome. 2023. Accessed February 1, 2023. https://reverserett.org/about-rett-syndrome
14. Shah J, Patel H, Jain D, et al. A rare case of a male child with post-zygotic de novo mosaic variant c.538C > T in MECP2 gene: a case report of Rett syndrome. BMC Neurol. 2021;21(1):469. doi:10.1186/s12883-021-02500-5
15. Schönewolf-Greulich B, Bisgaard AM, Dunø M, et al. Mosaic MECP2 variants in males with classical Rett syndrome features, including stereotypical hand movements. Clin Genet. 2019;95(3):403-408. doi:10.1111/cge.13473
16. Neul JL, Benke TA, Marsh ED, et al. The array of clinical phenotypes of males with mutations in Methyl-CpG binding protein 2. Am J Med Genet B Neuropsychiatr Genet. 2019;180(1):55-67. doi:10.1002/ajmg.b.32707
17. Neul JL, Kaufmann WE, Glaze DG, et al. Rett syndrome: revised diagnostic criteria and nomenclature. Ann Neurol. 2010;68(6):944-950. doi:10.1002/ana.22124
18. Rodriguez LM, Percy AK, Cutter GR. Rett syndrome: novel correlations linking >96% genotype, disease severity, and seizures. Transl Sci Rare Dis. 2020;5(3-4):131-141. doi:10.3233/TRD-200047
19. Kong Y, Li Q-B, Yuan Z-H, et al. Multimodal neuroimaging in Rett syndrome with MECP2 mutation. Front Neurol. 2022;13:838206. doi:10.3389/fneur.2022.838206
20. Ozlu C, Bailey RM, Sinnett S, Goodspeed KD. Gene transfer therapy for neurodevelopmental disorders. Dev Neurosci. 2021;43(3-4):230-240. doi:10.1159/000515434
21. Fu C, Armstrong D, Marsh E, et al. Multisystem comorbidities in classic Rett syndrome: a scoping review. BMJ Paediatr Open. 2020;4(1):e000731. doi:10.1136/bmjpo-2020-000731
22. Goyal M, O’Riordan MA, Wiznitzer M. Effect of topiramate on seizures and respiratory dysrhythmia in Rett syndrome. J Child Neurol. 2004;19(8):588-591. doi:10.1177/088307380401900804
23. Krajnc N. Severe respiratory dysrhythmia in Rett syndrome treated with topiramate. J Child Neurol. 2014;29(10):NP118-NP121. doi:10.1177/0883073813508313
24. Buchanan CB, Stallworth JL, Joy AE, et al. Anxiety-like behavior and anxiolytic treatment in the Rett syndrome natural history study. J Neurodev Disord. 2022;14(1):31. doi:10.1186/s11689-022-09432-2
25. Farzam K, Tivakaran VS. QT prolonging drugs. In: StatPearls. StatPearls Publishing; 2022. Accessed February 1, 2023. http://www.ncbi.nlm.nih.gov/books/NBK534864/
26. Tisdale JE. Drug-induced QT interval prolongation and torsades de pointes: role of the pharmacist in risk assessment, prevention and management. Can Pharm J (Ott). 2016;149(3):139-152. doi:10.1177/1715163516641136
27. Darwish M, Youakim JM, Harlick J, et al. A phase 1, open-label study to evaluate the effects of food and evening dosing on the pharmacokinetics of oral trofinetide in healthy adult subjects. Clin Drug Investig. 2022;42(6):513-524. doi:10.1007/s40261-022-01156-4
28. Vahdatpour C, Dyer AH, Tropea D. Insulin-like growth factor 1 and related compounds in the treatment of childhood-onset neurodevelopmental disorders. Front Neurosci. 2016;10:450. doi:10.3389/fnins.2016.00450
29. O’Leary HM, Kaufmann WE, Barnes KV, et al. Placebo-controlled crossover assessment of mecasermin for the treatment of Rett syndrome. Ann Clin Transl Neurol. 2018;5(3):323-332. doi:10.1002/acn3.533
30. Khwaja OS, Ho E, Barnes KV, et al. Safety, pharmacokinetics, and preliminary assessment of efficacy of mecasermin (recombinant human IGF-1) for the treatment of Rett syndrome. Proc Natl Acad Sci U S A. 2014;111(12):4596-4601. doi:10.1073/pnas.1311141111
31. Glaze DG, Neul JL, Kaufmann WE, et al. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric Rett syndrome. Neurology. 2019;92(16):e1912-e1925. doi:10.1212/WNL.0000000000007316
32. Lu X-CM, Si Y, Williams AJ, et al. NNZ-2566, a glypromate analog, attenuates brain ischemia-induced non-convulsive seizures in rats. J Cereb Blood Flow Metab. 2009;29(12):1924-1932. doi:10.1038/jcbfm.2009.109
33. US Food and Drug Administration. FDA approves first treatment for Rett syndrome. March 13, 2023. Accessed March 13, 2023. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-first-treatment-rett-syndrome
34. Berry-Kravis E, Horrigan JP, Tartaglia N, et al. A double-blind, randomized, placebo-controlled clinical study of trofinetide in the treatment of fragile X syndrome. Pediatr Neurol. 2020;110:30-41. doi:10.1016/j.pediatrneurol.2020.04.019
35. Glaze DG, Neul JL, Percy A, et al. A double-blind, randomized, placebo-controlled clinical study of trofinetide in the treatment of Rett syndrome. Pediatr Neurol. 2017;76:37-46. doi:10.1016/j.pediatrneurol.2017.07.002
36. Oosterholt SP, Horrigan J, Jones N, et al. Population pharmacokinetics of NNZ-2566 in healthy subjects. Eur J Pharm Sci. 2017;109S:S98-S107. doi:10.1016/j.ejps.2017.05.032
37. A safety study of NNZ-2566 in pediatric Rett syndrome. ClinicalTrials.gov identifier: NCT02715115. Updated August 14, 2020. Accessed January 30, 2023. https://clinicaltrials.gov/ct2/show/NCT02715115
38. Study of trofinetide for the treatment of girls and women with Rett syndrome (LAVENDERTM). ClinicalTrials.gov identifier: NCT04181723. Updated November 2, 2022. Accessed January 24, 2023. https://clinicaltrials.gov/ct2/show/NCT04181723
39. Neul JL, Percy AK, Benke TA, et al. Design and outcome measures of LAVENDER, a phase 3 study of trofinetide for Rett syndrome. Contemp Clin Trials. 2022;114:106704. doi:10.1016/j.cct.2022.106704
40. Neul JL, Percy, Alan K, et al. Efficacy and safety of trofinetide for the treatment of Rett syndrome: results from the pivotal phase 3 LAVENDER study. Abstract presented at: AAN 2022 Annual Meeting; April 2-7, 2022; Seattle, WA. Accessed March 29, 2023. https://www.aan.com/siteassets/home-page/conferences-and-community/annual-meeting/abstracts-and-awards/abstracts/2022-emerging-science-abstracts.pdf
41. Acadia Pharmaceuticals. Acadia Pharmaceuticals announces positive top-line results from the pivotal phase 3 Lavender trial of trofinetide in Rett syndrome. December 6, 2021. Accessed January 25, 2023. https://acadia.com/media/news-releases/acadia-pharmaceuticals-announces-positive-top-line-results-from-the-pivotal-phase-3-lavender-trial-of-trofinetide-in-rett-syndrome/
42. Open-label extension study of trofinetide for the treatment of girls and women with Rett syndrome (LILAC™). ClinicalTrials.gov identifier: NCT04279314. Updated September 23, 2022. Accessed January 30, 2023. https://clinicaltrials.gov/ct2/show/NCT04279314
43. Penke B, Fulop L, Szucs M, Frecska E. The role of sigma-1 receptor, an intracellular chaperone in neurodegenerative diseases. Curr Neuropharmacol. 2018;16(1):97-116. doi:10.2174/1570159X15666170529104323
44. Wu N-H, Ye Y, Wan B-B, et al. Emerging benefits: pathophysiological functions and target drugs of the sigma-1 receptor in neurodegenerative diseases. Mol Neurobiol. 2021;58(11):5649-5666. doi:10.1007/s12035-021-02524-5
45. Bogár F, Fülöp L, Penke B. Novel therapeutic target for prevention of neurodegenerative diseases: modulation of neuroinflammation with Sig-1R ligands. Biomolecules. 2022;12(3):363. doi:10.3390/biom12030363
46. Kaufmann WE, Sprouse J, Rebowe N, et al. ANAVEX®2-73 (blarcamesine), a sigma-1 receptor agonist, ameliorates neurologic impairments in a mouse model of Rett syndrome. Pharmacol Biochem Behav. 2019;187:172796. doi:10.1016/j.pbb.2019.172796
47. Hampel H, Williams C, Etcheto A, et al. A precision medicine framework using artificial intelligence for the identification and confirmation of genomic biomarkers of response to an Alzheimer’s disease therapy: analysis of the blarcamesine (ANAVEX2-73) phase 2a clinical study. Alzheimers Dement (N Y). 2020;6(1):e12013. doi:10.1002/trc2.12013
48. Reyes ST, Deacon RMJ, Guo SG, et al. Effects of the sigma-1 receptor agonist blarcamesine in a murine model of fragile X syndrome: neurobehavioral phenotypes and receptor occupancy. Sci Rep. 2021;11(1):17150. doi:10.1038/s41598-021-94079-7
49. ANAVEX2-73 study in pediatric patients with Rett syndrome (EXCELLENCE). ClinicalTrials.gov identifier: NCT04304482. Updated February 8, 2023. Accessed January 25, 2023. https://clinicaltrials.gov/ct2/show/NCT04304482
50. ANAVEX2-73 study in patients with Rett syndrome (AVATAR). ClinicalTrials.gov identifier: NCT03941444. Updated January 27, 2022. Accessed January 25, 2023. https://clinicaltrials.gov/ct2/show/NCT03941444
51. BioSpace. ANAVEX®2-73 (blarcamesine) AVATAR phase 3 trial met primary and secondary efficacy endpoints for the treatment of adult patients with Rett syndrome. February 1, 2022. Accessed January 8, 2023. https://www.biospace.com/article/anavex-2-73-blarcamesine-avatar-phase-3-trial-met-primary-and-secondary-efficacy-endpoints-for-the-treatment-of-adult-patients-with-rett-syndrome/
52. Davidson BL, Gao G, Berry-Kravis E, et al. Gene-based therapeutics for rare genetic neurodevelopmental psychiatric disorders. Mol Ther. 2022;30(7):2416-2428. doi:10.1016/j.ymthe.2022.05.014
53. Coorey B, Haase F, Ellaway C, et al. Gene editing and Rett syndrome: does it make the cut? CRISPR J. 2022;5(4):490-499. doi:10.1089/crispr.2022.0020
54. Taysha Gene Therapies. Taysha Gene Therapies announces initiation of clinical development of TSHA-102 in Rett syndrome. March 29, 2022. Accessed January 25, 2023. https://ir.tayshagtx.com/news-releases/news-release-details/taysha-gene-therapies-announces-initiation-clinical-0/
55. Safety and efficacy of TSHA-102 in adult females with Rett syndrome (REVEAL adult study). ClinicalTrials.gov identifier: NCT05606614. Updated November 9, 2022. Accessed January 24, 2023. https://clinicaltrials.gov/ct2/show/NCT05606614
56. Neurogene Inc. Neurogene announces FDA clearance of IND for NGN-401 gene therapy for children with Rett syndrome. January 23, 2023. Accessed February 27, 2023. https://www.neurogene.com/press-releases/neurogene-announces-fda-clearance-of-ind-for-ngn-401-gene-therapy-for-children-with-rett-syndrome/
57. Chambers JD, Panzer AD, Kim DD, et al. Variation in US private health plans’ coverage of orphan drugs. Am J Manag Care. 2019;25(10):508-512.
58. AMCP Partnership Forum: principles for sound Pharmacy and Therapeutics (P&T) committee practices: what’s next? J Manag Care Spec Pharm. 2020;26(1):48-53. doi:10.18553/jmcp.2020.26.1.48
59. National Association of Insurance Commissioners. US insurance industry analysis report: 2020 annual results. 2021. Accessed January 31, 2023. https://content.naic.org/sites/default/files/inline-files/2020-Annual-Health-Insurance-Industry-Analysis-Report.pdf
60. Yehia F, Segal JB, Anderson GF. Predictors of orphan drug coverage restrictions in Medicare Part D. Am J Manag Care. 2020;26(9):e289-e294. doi:10.37765/ajmc.2020.88494
61. Cohen JP, Felix A. Are payers treating orphan drugs differently? J Mark Access Health Policy. 2014;2(1):23513. doi:10.3402/jmahp.v2.23513
62. Pinson N, Thielke A, King V, et al. Medicaid and specialty drugs: current policy options. Center for Evidence-based Policy, Oregon Health & Science University. September 9, 2016. Accessed March 16, 2023. https://centerforevidencebasedpolicy.org/wp-content/uploads/2021/12/MED_Medicaid_and_Specialty_Drugs_Current_Policy_Options_Final_Sept-9-2016.pdf
63. Dolan R, Tian M. Management and delivery of the Medicaid pharmacy benefit. Kaiser Family Foundation. December 6, 2019. Accessed March 13, 2023. https://www.kff.org/medicaid/issue-brief/management-and-delivery-of-the-medicaid-pharmacy-benefit/
64. Dolan R. Understanding the Medicaid Prescription Drug Rebate Program. Kaiser Family Foundation. November 12, 2019. Accessed March 16, 2023. https://www.kff.org/medicaid/issue-brief/understanding-the-medicaid-prescription-drug-rebate-program/
65. Barrington CJ, Burruano M, Carney C, et al. Addressing the role of edaravone in the management of amyotrophic lateral sclerosis and gaps in care and access: expert panel recommendations. Am J Manag Care. 2021;27(12 suppl):S231-S237. doi:10.37765/ajmc.2021.88732
66. Pasquini TLS, Goff SL, Whitehill JM. Navigating the U.S. health insurance landscape for children with rare diseases: a qualitative study of parents’ experiences. Orphanet J Rare Dis. 2021;16(1):313. doi:10.1186/s13023-021-01943-w