ADVERTISEMENT

The Changing Landscape in the Treatment of Rheumatoid Arthritis: An Update for Pharmacists
INTRODUCTION
Few areas of medicine have been more positively impacted by the development of biologic medications than rheumatology. Specifically, the treatment of rheumatoid arthritis (RA) has been revolutionized. In only 25 years, available treatments for RA have evolved from drugs that had no effect on the progression of joint destruction (e.g., aspirin and other non-steroidal anti-inflammatory drugs) and/or were poorly tolerated (e.g., gold salts and high-dose corticosteroids) to targeted therapies that are largely safe and capable of stopping disease progression.1 These specific therapies target key mediators of the inflammatory cascade that are responsible for the symptoms of RA, particularly the progressive destruction of joints that can cripple patients with advanced disease. However, the superior efficacy of new RA treatments comes with monetary costs and safety concerns, which mandate pharmacist involvement for optimal outcomes. The purpose of this module is to update pharmacists on the latest treatments in RA and provide a review of the recently released American College of Rheumatology (ACR) 2015 Guidelines.
EPIDEMIOLOGY AND PATHOGENESIS OF RA
RA is a chronic inflammatory form of arthritis that affects approximately 1% of the United States (U.S.) population.2 The onset of RA usually begins in individuals between 25 and 55 years of age. RA usually presents as peripheral polyarthritis and can result in physical disability and joint damage (primarily of the feet, fingers, and wrists). Arthropathy is the primary effect of RA, but it is important to realize that RA is a systemic inflammatory disease and other, extra-articular symptoms may also be present; fatigue, pulmonary disease, peripheral neuropathy, central nervous system effects, vasculitis, and pericarditis can all occur with RA. Patients with RA also have an increased risk of cardiovascular disease and increased mortality from cardiovascular causes.3
RA is a costly disease for the health care system. In 2003, the total cost of rheumatoid arthritis and other rheumatic conditions in the U.S. was approximately $128 billion, which was equivalent to 1.2% of the U.S. gross domestic product the same year. As with other autoimmune-type diseases, RA is more common in women, affecting them approximately 3 times more frequently than men. Studies indicate that there is a genetic component to RA and approximately 10% to 25% of cases have a genetic link. Additionally, environmental factors can increase the risk for developing RA. For example, women who smoke cigarettes have a 2.5 times higher risk of developing RA than women who do not smoke.
Still, despite decades of research, the exact cause of RA remains unknown. The primary mode of disease has long been thought to be a dysregulated immune system that leads to the body attacking its own tissues, but exactly how this dysregulation occurs is unclear. Laboratory markers that suggest general inflammation (e.g., C-reactive protein [CRP]) and specific autoimmune antibodies (e.g., rheumatoid factor [RF]) are positive in many, but not all, RA patients. Genome-wide association studies have found genetic markers that predispose for RA and may even play a role in the severity of the disease.4 Prediction models of RA have attempted to account for these genetic markers and the influence of environmental factors such as smoking, which have been found to correlate with age of onset of RA and disease severity.5 As genetic testing becomes more common, prediction models may be used in the future to more closely monitor patients and individuals at high risk for developing RA. Currently, genetic testing for these purposes is still considered experimental.
RA TREATMENT GUIDELINES AND TARGETS
In November 2015, the ACR updated guidelines for the treatment of RA. The guidelines were made available electronically ahead of print and then later published in January 2016.6 The ACR discussed the roles of therapies not approved when the previous guidelines were published, and it endorsed several key concepts in the treatment of RA, including the objective of “treat-to-target” in RA patients and using biomarkers to guide therapy. These principles will be discussed here, followed by a review of the pharmacotherapy of RA.
Treat-to-target strategy
In 2010, experts in the field of RA convened to consider advances in the pharmacotherapy of RA and establish the overarching goal of clinicians treating the disease. The convention produced the treat-to-target principles.7 These principles comprised 10 recommendations for the treatment of RA that informed rheumatologists, other health care professionals, and patients about strategies to achieve optimal outcomes (Table 1).8 The central theme of treat-to-target recommendations is aggressive therapy that is frequently monitored with a goal of low or no disease activity in RA patients. In other words, total remission of disease activity is the goal for most patients, since remission is important for preventing irreversible joint damage. Adhering to treat-to-target guidelines requires cooperation among rheumatologists, members of the health care team, and the patient. Formalized systems for monitoring RA such as the ACR percentage-based improvement scales have given way to easy-to-use assessments such as the Disease Activity Score 28 and the Simplified Disease Activity Index (Table 2).9 These scores should be assessed frequently to monitor disease activity and progression and to evaluate whether adjustments in pharmacotherapy are needed. Use of disease-modifying antirheumatic drugs (DMARDs), especially biologic DMARDs, should be considered early in therapy to achieve remission. Some rheumatologists have noted that a treat-to-target strategy has yet to be rigorously evaluated in a randomized controlled trial that compares it to the usual care of RA patients and that safety is still a consideration for many RA patients. Still, a treat-to-target approach to RA pharmacotherapy is a cornerstone of the 2015 ACR guidelines.
| Table 1. Treat-to-Target Recommendations for Rheumatoid Arthritis (RA)8 |
| 1 |
The primary target for the treatment of RA should be a state of clinical remission |
| 2 |
Clinical remission is defined as the absence of signs and symptoms of significant inflammatory disease activity |
| 3 |
While remission should be a clear target, based on available evidence, low disease activity may be an acceptable alternative therapeutic goal, particularly in established, long-standing disease |
| 4 |
Until the desired treatment target is reached, drug therapy should be adjusted at least every 3 to 6 months |
| 5 |
Measures of disease activity must be obtained and documented regularly, as frequently as monthly for patients with high/moderate disease activity or less frequently (e.g., every 3 to 6 months) for patients in sustained low disease activity or remission |
| 6 |
The use of validated composite measures of disease activity, which include joint assessments, is needed in routine clinical practice to guide treatment decisions |
| 7 |
Structural changes and functional impairment should be considered when making clinical decisions, in addition to assessing composite measures of disease activity |
| 8 |
The desired treatment target should be maintained throughout the course of the disease |
| 9 |
The choice of the (composite) measure of disease activity and the level of the target value may be influenced by considering comorbidities, patient factors, and drug-related risks |
| 10 |
The patient must be appropriately informed about the treatment target and the planned strategy to reach this target under the supervision of the rheumatologist |
| Table 2. Treatment Assessment Scores in Rheumatoid Arthritis9 |
| Scale |
Description |
| ACR 20/50/70 |
American College of Rheumatology (ACR) criteria are defined as a percent improvement in tender and swollen joint count plus a percent improvement in 3 of 5 of the following criteria: patient pain assessment, patient global assessment, physician global assessment, patient self-assessed disability, and acute phase reactants (erythrocyte sedimentation rate [ESR] or C-reactive protein [CRP]). The number (20, 50, 70) represents the percentage improvement required for each measure (i.e., ACR20 represents a 20% improvement). |
| DAS 28 (ESR or CRP) |
The disease activity score (DAS) is a calculated measure of disease activity in patients with RA. The combined index includes the number of swollen and tender joints, ESR or CRP, and a subjective measure of the general health of the patient. |
| SDAI |
The Simple Disease Activity Index (SDAI) is a calculated measure of disease activity using swollen and tender joint counts, patient and provider global assessment of disease activity, and CRP levels. |
Use of biomarkers to direct pharmacotherapy
While no laboratory test is completely sensitive or specific for RA, some biomarkers are valuable for assessing general levels of inflammation and disease activity, including CRP and erythrocyte sedimentation rate. Considering the advent of the treat-to-target strategy and a goal of low or no disease activity for most RA patients, several studies have examined if biomarkers can play a role in identifying patients at risk for aggressive RA who would benefit from early use of biologic DMARDs. The BeST trial, which was published in 2010, reported that tailored but aggressive therapy in early RA maintained joint function and improved symptoms and quality of life.10 However, the question remains: if patients do not currently have aggressive disease, can laboratory biomarkers identify patients with early RA that is likely to develop into aggressive disease? Both RF and autoantibodies against cyclic citrullinated peptides (CCP; anti-CCP antibodies) have been examined as possible biomarkers for aggressive RA. Studies have suggested that RA patients who are positive for one or both of these biomarkers are at higher risk of developing joint erosion and destruction than RA patients who are not positive for the markers.11 Whether treatment guided by early examination of these biomarkers actually leads to improved outcomes is the subject of ongoing research. However, anti-CCP antibodies are now routinely used in the assessment of RA. As more sensitive biomarkers are discovered, a more personalized approach to the treatment of RA can be expected.12
Review of 2015 ACR guidelines
The 2015 ACR guidelines were developed by a group of expert rheumatologists and other health care providers who completed a thorough review and assessment of the current literature surrounding RA treatment. Recommendations were decided by consensus of the group members and were reviewed using the Grading of Recommendations Assessment, Development, and Evaluation methodology.13 Each recommendation was given a strong or conditional endorsement from the working groups involved in guideline development.
To focus the recommendations, the guidelines divide RA into “early disease” and “established disease.” Early disease describes RA with a duration of disease/symptoms of less than 6 months. Established disease describes RA with a duration of disease/symptoms of more than 6 months or meeting 1987 ACR RA classification criteria. (“Duration” denotes the length of time the patient has had symptoms or disease, not the length of time since RA diagnosis.)6 An algorithmic approach to defining early and established RA is provided in the guidelines. The guidelines also provide vaccination recommendations for RA patients and offer recommendations for treating RA in patients with comorbid diseases.
The treatment algorithms outlined in the 2015 ACR guidelines are detailed in Figures 1 and 2, and key points are summarized in the following paragraphs. For early symptomatic RA, the guidelines stress the treat-to-target approach. Monotherapy with a synthetic DMARD is usually first-line therapy for these patients, and methotrexate is the preferred agent. If synthetic DMARD monotherapy is not tolerated or fails to achieve low disease activity or remission, the guidelines state that combination synthetic DMARDs, a tumor necrosis factor (TNF) inhibitor (TNFi), or a non-TNF biologic agent may be considered instead of continuing DMARD monotherapy. Methotrexate can be continued or discontinued when the therapy is changed. Corticosteroids also play a role in early RA. If disease activity is high despite first-line monotherapy or combination or second-line therapy, the addition of a low-dose corticosteroid is conditionally recommended by the guidelines. Corticosteroids can be used at any time for acute flares of the disease.
| Figure 1. American College of Rheumatology Algorithm for Treating Early Rheumatoid Arthritis |
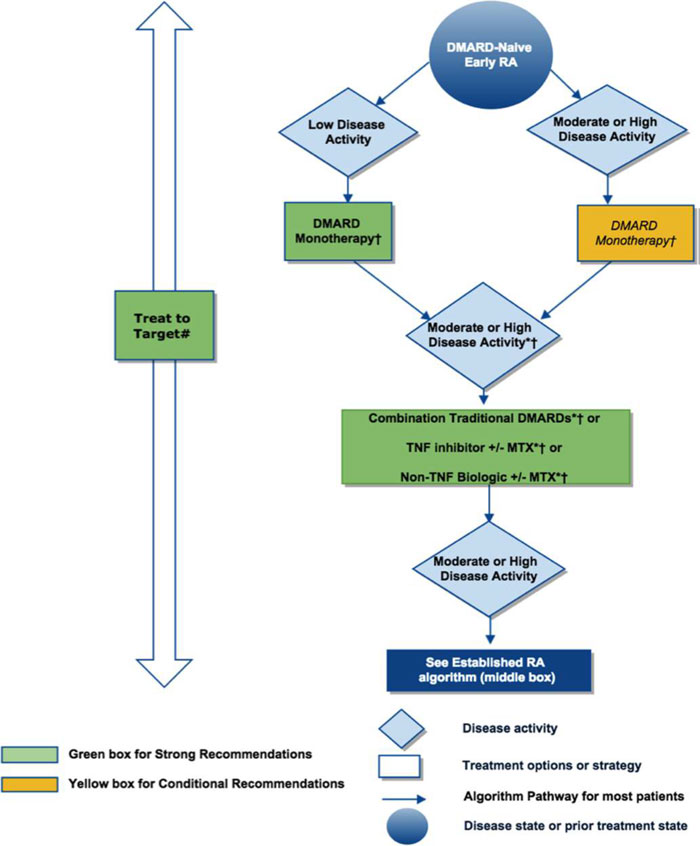 |
Figure 1. 2015 American College of Rheumatology recommendations for the treatment of Early rheumatoid arthritis (RA), defined as disease duration <6 months. * = consider adding low-dose glucocorticoids (≤10 mg/day of prednisone or equivalent) in patients with moderate or high RA disease activity when starting disease-modifying antirheumatic drugs (DMARDs) and in patients with DMARD failure or biologic failure.
†=also consider using short-term glucocorticoids (defined as <3 months treatment) for RA disease flares. Glucocorticoids should be used at the lowest possible dose and for the shortest possible duration to provide the best benefit-risk ratio for the patient.
#= treatment target should ideally be low disease activity or remission. For the level of evidence supporting each recommendation, see the related section in the Results. This figure is derived from recommendations based on PICO (population, intervention, comparator, and outcomes) questions A.1 to A.12. For definitions of disease activity (categorized as low, moderate, or high) and descriptions, see Tables 1 and 2 in Reference6. MTX=methotrexate.
Adapted from Reference6 |
| Figure 2. American College of Rheumatology Algorithm for Treating Established Rheumatoid Arthritis |
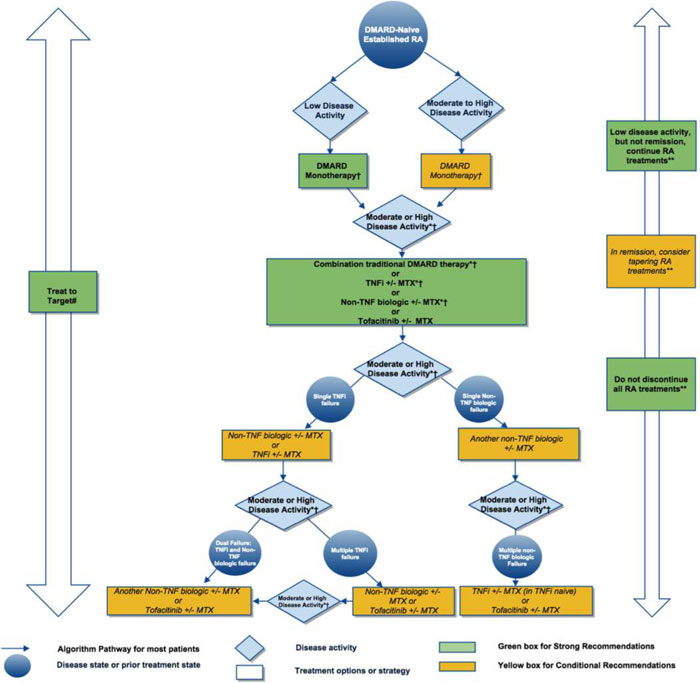 |
Figure 2. 2015 American College of Rheumatology (ACR) recommendations for the treatment of Established rheumatoid arthritis (RA), defined as disease duration ≥6 months, or meeting the 1987 ACR classification criteria. Due to complexity of management of established RA, not all clinical situations and choices could be depicted in this flow chart, and therefore we show the key recommendations. For a complete list of recommendations, please refer to the Results.
*= consider adding low-dose glucocorticoids (≤10 mg/day of prednisone or equivalent) in patients with moderate or high RA disease activity when starting traditional disease-modifying antirheumatic drugs (DMARDs) and in patients with DMARD failure or biologic failure.
†= also consider using short-term glucocorticoids (defined as <3 months treatment) for RA disease flares. Glucocorticoids should be used at the lowest possible dose and for the shortest possible duration to provide the best benefit-risk ratio for the patient.
#= treatment target should ideally be low disease activity or remission. **= tapering denotes scaling back therapy (reducing dose or dosing frequency), not discontinuing it and if done, must be conducted slowly and carefully. For the level of evidence supporting each recommendation, see the related section in the Results. This figure is derived from recommendations based on PICO (population, intervention, comparator, and outcomes) questions B.1 to B.38. For definitions of disease activity (categorized as low, moderate, or high) and descriptions, see Tables 1 and 2 in Reference 6. MTX=methotrexate; TNFi=tumornecrosis factor inhibitor.
Adapted from Reference6 |
Patients with established RA have likely been on previous therapies, perhaps even biologic drugs. Therefore, the choices for therapy to achieve low or no disease activity in these patients may be limited. Still, the recommendations for treatment of established RA are similar to those for early RA. For patients with low to high disease activity who have not taken synthetic DMARDs in the past, methotrexate monotherapy is preferred. Patients with established RA who cannot tolerate methotrexate or continue to have moderate to high disease activity while taking methotrexate should consider combination synthetic DMARDs, a TNFi, a non-TNF biologic, or tofacitinib. Methotrexate therapy may or may not be continued when these second-line therapies are initiated. If disease activity remains moderate or high despite TNFi therapy, a non-TNF biologic (with or without methotrexate) is preferred over other therapies. If a patient fails TNFi therapy and a non-TNF biologic, tofacitinib (with or without methotrexate) is recommended. The recommendations for the use of corticosteroids in established RA are similar to those in early RA. The guidelines emphasize that, with the exception of methotrexate as monotherapy, no single drug is considered more effective in RA than another. The guidelines also state that patient preference and goals must be key considerations when determining therapy. The pharmacist can play a role in educating patients with RA on the various types of therapy, including formulations, risk and benefits, and monetary costs.
PHARMACOTHERAPY OPTIONS IN RA
Synthetic DMARDs
Methotrexate. For more than 30 years, methotrexate has been the cornerstone of RA therapy. Due to its relatively low cost and favorable efficacy and safety profiles, methotrexate is commonly used both as first-line monotherapy in treatment-naïve patients and as an anchor drug in methotrexate-insufficient responders in combination with other synthetic or biologic DMARDs. Multiple studies have found that low-dose methotrexate (usually 15 to 25 mg weekly) is effective and well tolerated. A recent meta-analysis of 158 trials found that methotrexate had a probability of achieving a 50% improvement in signs and symptoms of RA (ACR50) of 41% when used as monotherapy and as high as 67% when combined with other drugs.14
Methotrexate is generally well tolerated, with minor adverse effects such as nausea, mouth ulcers, and fatigue being the most commonly reported.15 More significant adverse effects include neutropenia and liver toxicity, both of which mandate periodic laboratory monitoring (usually every 6 to 12 weeks). Adverse effects such as nausea and hepatotoxicity can be ameliorated by the addition of daily folic acid.18 Methotrexate can be given parenterally (usually subcutaneously or intramuscularly) and orally. Oral absorption of methotrexate is somewhat variable, and recent studies have investigated differences in outcomes between oral and subcutaneous administration. These studies have largely found that the subcutaneous route is associated with improved outcomes with no difference in toxicity.17 Therefore, the subcutaneous route, which can be self-administered by patients, is the preferred mode of administration. Methotrexate is teratogenic, so patients should be instructed to use appropriate contraceptive measures.
Sulfasalazine, hydroxychloroquine, and leflunomide. Other synthetic DMARDs used in clinical practice and referenced by the ACR guidelines include sulfasalazine, hydroxychloroquine, and leflunomide. In the past, these medications were used as monotherapy if methotrexate was not effective or not tolerated. Today, however, the recommended approach is to use multiple synthetic DMARDs in combination (so-called “triple therapy” with sulfasalazine, hydroxychloroquine, and methotrexate) as an aggressive approach to reach treat-to-target goals. Clinical trials have found that triple therapy (with a prednisone taper at the beginning of therapy) has sustained radiographic and symptom benefits compared to monotherapy with fairly comparable discontinuation rates due to adverse effects.18 Despite comparable rates of discontinuation, sulfasalazine has numerous adverse effects, especially at high doses, including nausea, rash, renal toxicity, hepatic toxicity, and bone marrow suppression.19
Hydroxychloroquine was previously used in mild or early RA. Its ability to modify disease progression as monotherapy is limited, but its adverse effect profile is favorable. Ocular toxicity has been reported at high doses but is rare at clinically used doses.20 Leflunomide is a pyrimidine synthesis inhibitor that acts as an immunomodulator similar to methotrexate. It also shares several adverse effects with methotrexate, including hepatotoxicity, pancytopenia, and agranulocytosis. It is more costly than other synthetic DMARDs and is also teratogenic.19 Finally, pulmonary toxicity has been reported with leflunomide, and patients should be counseled to report any shortness of breath or long-standing cough to their rheumatologist.21 Leflunomide can be used as monotherapy for RA in patients who have failed or cannot tolerate methotrexate. In general, synthetic DMARDs are less costly than biologic DMARD regimens, and synthetic DMARDs can be considered for patients who do not have access to biologic DMARDs. Adverse effects, such as infection, can occur with both biologic and synthetic DMARDs.19
Biologic DMARDs
Tumor necrosis factor inhibitors. TNFis were the first class of biologic DMARDs approved for RA in the U.S. The efficacy of these drugs is predicated on the fact that TNF is a potent pro-inflammatory cytokine that plays a key role in joint inflammation in RA.There are 5 drugs in this class (Table 3): adalimumab, certolizumab pegol, etanercept, infliximab, and golimumab. Clinical experience in RA is most extensive with etanercept and infliximab, and these are often the TNFi agents chosen first by rheumatologists.22 All TNFis have been studied as monotherapy and in combination with methotrexate. Therapy is selected on the basis of symptom severity, markers for severe disease, and adverse effects. A confounding problem in evaluating the role of biologic DMARDs in RA is the lack of head-to-head clinical trials evaluating the efficacy and safety of all the agents. Although some agents have been examined in randomized controlled trials, most comparative data related to biologic DMARDs comes from numerous meta-analyses and retrospective indirect pair-wise comparisons. A large network meta-analysis of 28 trials published in 2015 examined the comparative efficacies of all biologic DMARDs, including the TNFis, either alone or in combination with methotrexate.23 The authors found that all TNFis had roughly equivalent likelihoods (30%) of producing an ACR50 response when used as monotherapy; the likelihoods of achieving ACR50 were 55% when a TNFi was used with methotrexate and only 19% with methotrexate alone (odds ratio [OR] of ACR50 response of combination therapy compared to methotrexate alone, 5.16 [95% CI 3.74 - 7.53]). Therefore, regardless of the TNFi used with methotrexate, roughly half of patients can expect a 50% improvement in signs of symptoms of RA with the use of any TNFi. Since these drugs are roughly equal in efficacy, differences in administration (e.g., intravenous infusion versus subcutaneous injection), costs, and safety will determine the selection of an agent.
| Table 3. Tumor Necrosis Factor Inhibitor (TNFi) Agents in Rheumatoid Arthritis2,6 |
| Drugs |
Role in therapy |
Suggested monitoring |
Adverse effects |
Comments |
| Infliximab, Etanercept, Adalimumab, Golimumab, Certolizumab |
Usually the first biologic DMARDs used; recommended by both American and European Guidelines, especially in rapidly progressing disease or in patients with poor prognostic markers |
Initial: CBC, LFTs, SCr, PPD, and/or QFT-GIT; screen for hepatitis B/C; consider administering all needed vaccines before initiating treatment
Continued: CBC and LFTs every 1-3 months; yearly PPD; routine cancer screening including full dermatologic exam yearly |
Increased risk of bacterial and fungal infections; slightly increased risk of skin cancers; infusion reactions (infliximab) or injection site reactions; lupus-like reactions reported
Contraindicated in patients with heart failure or CNS demyelination disease |
Anti-drug antibodies can form over time and may increase risks of both treatment failure and adverse effects
Therapeutic drug monitoring is an emerging strategy to optimize dosing
Most TNFis can be self-administered by SC injection |
| Abbreviations: CBC, complete blood count; CNS, central nervous system; DMARD, disease-modifying antirheumatic drug; LFT, liver function test; PPD, tuberculin skin test; QFT-GIT, QuantiFERON-TB Gold In-Tube; SC, subcutaneous; SCr, serum creatinine. |
Safety of the TNFis is a crucial issue when determining therapy. TNFis increase the risk for bacterial and other infections, and rare fungal infections, tuberculosis, and hepatitis reactivation have been reported for all TNFis. Additionally, severe sepsis and worsening of common bacterial infections have been reported; all 5 TNFis carry warnings concerning the risk of infection. Infusion reactions can also occur with TNFis administered intravenously. Patients with certain diseases or conditions such as heart failure or neuromuscular disease (e.g., multiple sclerosis) may experience worsening symptoms with TNFis and, as such, should avoid this class of drugs. A meta-analysis published in 2008 found that all TNFis have been associated with side effects requiring discontinuation, including infusion reactions and infections; etanercept has the lowest discontinuation rate from these effects.24
A final safety concern of the TNFis is the potential risk for malignancy. Given the role of TNF in screening and eliminating neoplastic cells, the concern that the use of agents that inhibit this compound may increase the risk for malignancy is understandable; numerous retrospective registry and case control studies have been conducted to assess this aspect of TNFis. The most recent review of these studies, published in 2013, did not find an increased risk of solid tumor malignancies or lymphoma in RA patients.25 However, other retrospective trials have suggested an increased risk of skin cancer related to TNFi therapy. In fact, a recent study from Sweden that included more than 12,000 RA patients who were receiving TNFi therapy found a small but significant increase in squamous cell cancer rates (hazard ratio 1.88, 95% CI 1.74 - 2.03) compared to biologics-naïve RA patients. This translated to an annual number needed to harm of approximately 1 in 1600.26 Yearly skin examinations performed by an experienced health professional should be a standard recommendation for RA patients receiving TNFi therapy. Another study from the same group in Sweden did not find an increase in overall mortality in RA patients using TNFi therapy compared to RA patients who did not use TNFi therapy.27 In the absence of longer-term studies, TNFis appear to be safe if appropriate monitoring and preparation for drug use are considered, including receiving recommended vaccines and screening for indolent infections such as tuberculosis.
Therapeutic drug monitoring is an emerging strategy to appropriately dose TNFis, particularly infliximab and adalimumab. Patients receiving infliximab may develop anti-infliximab antibodies (sometimes called human anti-chimeric antibodies, [HACA]). These antibodies develop in a significant minority of patients (some studies have suggested 30% to 50% of patients receiving the drug) and are associated with both a loss of efficacy over time and an increased risk for adverse effects.28 Monitoring serum trough levels of TNFis and HACA levels are associated with prolonged efficacy of infliximab in RA patients. As testing for TNFi levels and antibodies becomes more widely available, pharmacists may assist in the interpretation of these values and corresponding dose adjustments.
Anakinra. Anakinra is a recombinant, non-glycosylated form of the human interleukin (IL)-1 receptor antagonist. (IL-1 is a potent pro-inflammatory mediator in RA.) Anakinra was the first non-TNF biologic agent approved for RA in the U.S. and it is approved for monotherapy or in combination with methotrexate in RA. The overall safety of anakinra is comparable to other biologic DMARDs, with injection site reactions being the most common adverse effect in clinical trials. Precautions with this drug are similar to the TNFis. In clinical practice, the use of anakinra for RA has decreased substantially as other non-TNF biologics have been developed. Several investigations, as well as clinical experience, suggest that anakinra is less effective than both TNFis and other non-TNF biologics. A network meta-analysis published in 2009 found that ACR50 was less likely to be achieved with anakinra compared to other biologics, particularly adalimumab (OR 0.45, 95% CI 0.21 - 0.99) and etanercept (OR 0.34, 95% CI 0.14 - 0.81).29
Rituximab. Rituximab is a chimeric monoclonal antibody against the protein CD20, which inhibits B cell production and, thus, auto-antibody development. It is approved for combination therapy with methotrexate in patients with moderately to severely active RA who have had an inadequate response to one or more TNFis. A recent Cochrane review that evaluated rituximab and methotrexate versus methotrexate alone found that 29% of patients receiving combination therapy achieved ACR50 and 9% of patients receiving methotrexate only achieved the same outcome.30 Studies have also suggested that certain patient factors, most notably seropositivity to RF or anti-CCP antibodies, are associated with successful treatment with rituximab.31 Rituximab is administered less frequently than other biologic DMARDs: patients usually only receive 2 doses per year of rituximab. Among the most feared adverse effects of rituximab are infusion reactions, which can be fatal. The manufacturer recommends that corticosteroids be administered 30 minutes before each infusion to decrease the severity of these reactions. A boxed warning in rituximab’s product labeling warns of fatal infusion reactions, severe mucocutaneous reactions, hepatitis B virus reactivation, and reports of progressive multifocal leukoencephalopathy resulting in death.32 Due to these safety concerns, rituximab will likely continue to be reserved for patients who have failed other biologic DMARDs.
Abatacept . Abatacept exhibits potent anti-inflammatory effects by acting as a selective inhibitor of the co-stimulation of T cells. It can be administered as an intravenous or a subcutaneous injection. In general, data suggest that the efficacy of abatacept in RA is similar to other biologic DMARDs. A meta-analysis published in 2011 compared data from 4 studies and found that patients taking abatacept achieved ACR50 rates of 31.7% (95% CI 15.9% - 50.6%), which was higher than the rate for patients taking placebo (11.9%, 95% CI 9.7% - 14.0%) and comparable to the rates for patients taking other biologic agents (26.0% to 57.3%).33 Long-term data suggest that 20% to 30% of patients who failed a TNFi biologic will achieve low disease activity or remission for up to 5 years with abatacept with no significant increase in adverse effects such as serious infections, malignant neoplasms, or autoimmune events. Common adverse effects reported with abatacept include headache, nausea, and nasopharyngitis. In clinical studies, patients with chronic obstructive pulmonary disease (COPD) were more likely to have COPD-related adverse effects; abatacept should be used with caution in patients with this disease.34 Other adverse effects reported with abatacept are similar to other biologic DMARDs and include an increased risk of infections. As with most biologic DMARDs, abatacept can be taken with or without methotrexate. Abatacept can be considered a first-line biologic DMARD in RA patients, but most patients who receive the drug have previously failed TNFi therapy.
Tocilizumab. Tocilizumab is a humanized monoclonal antibody against the IL-6 receptor. (IL-6 is a potent pro-inflammatory mediator in RA.) Tocilizumab is specifically approved for patients with moderately to severely active RA who have had an inadequate response to one or more DMARDs. Like abatacept, it can be given as either an intravenous infusion or subcutaneous injection and as monotherapy or combined with methotrexate. The ADACTA trial compared tocilizumab and adalimumab in patients with RA in whom methotrexate was not a viable option.35 In this study, over 24 weeks, the ACR50 rates were 47.2% in the tocilizumab group and 27.8% in the adalimumab group (p = 0.0002). ADACTA is one of the few examples of a prospective head-to-head trial showing that a non-TNF biologic DMARD has superior efficacy to a TNFi. A meta-analysis published in 2014 reported similar ACR50 response rates when evaluating 8 other tocilizumab studies.36 Both of these investigations highlight several unique adverse effects associated with tocilizumab, including increased liver enzymes, neutropenia, and increased low-density lipoprotein levels. Patients receiving this drug will require more laboratory tests than those receiving TNFi therapy in order to monitor for these unique adverse effects. A risk of infection is also associated with tocilizumab, as it is with all DMARDs. Tocilizumab is at least as, or possibly more, effective than other DMARDs, but this benefit may be somewhat offset by safety concerns.
Janus kinase inhibitors
Tofacitinib. Protein kinases are small, intracellular molecular enzymes that modify the function of other proteins by attaching phosphate groups to them. Janus kinases (JAKs) play a key role in activating a host of immune responses because of their ability to interact with a variety of molecules, including ILs, interferon, and white cells. JAK inhibitors act to block this activation and are currently being studied in a number of inflammatory conditions.37 Tofacitinib is the first JAK inhibitor approved for the treatment of moderately to severely active RA in patients who have had an inadequate response or intolerance to methotrexate. Unlike the biologic DMARDs discussed previously, JAK inhibitors can be given orally.
A 2015 meta-analysis examined the efficacy and safety of tofacitinib with or without methotrexate by analyzing 10 published studies.38 The efficacy outcome of this study was a 20% improvement in signs and symptoms of RA (ACR20) and tofacitinib was significantly more likely to achieve this goal with and without methotrexate compared to placebo (OR 7.56, 95% CI 3.07 - 21.16 and OR 3.67, 95% CI 2.60 - 5.71, respectively). A Phase III trial that examined tofacitnib compared to adalimumab or placebo in patients with moderate to severe RA found that ACR20 rates were higher in the tofacitnib arms compared to the other arms of the study, with roughly 55% of patients responding to tofacitnib.39 This was statistically superior to placebo but not adalimumab.
JAK inhibitors are unique entries into the armamentarium of RA treatment, and new and emerging safety issues are of critical concern. In trials conducted to date, tofacitinib has been well tolerated; nausea, diarrhea, and headache are the most common side effects. More serious adverse effects, such as infection (including tuberculosis), lymphopenia, anemia, alterations in serum cholesterol levels, and increased liver function tests, have also been reported.40 Additionally, the risk of herpes zoster reactivation is concerning, with one trial finding twice the risk of this viral infection occurring with tofacitinib compared to other biologic drugs.41 Finally, the product information for tofacitinib lists intestinal perforation as a possible adverse effect that was reported in the Phase III studies of the drug.40 The mechanism of this effect remains unknown, but patients experiencing sudden abdominal pain should contact their rheumatologist immediately. Patients taking methotrexate or corticosteroids concomitantly may be an increased risk for this adverse effect. Considering the safety issues listed above, the ACR guidelines recommend that other therapies, including synthetic DMARDs and biologic DMARDs, are preferred over tofacitinib, despite the positive efficacy results seen in tofacitinib trials.6 Tofacitinib blocks JAK-1 and JAK-3 preferentially. Other JAK inhibitors being studied for RA include decernotinib and baricitinib, which are more selective for a single JAK isoform than tofacitinib.42 Whether this selectivity results in improved safety remains to be seen. To date, all JAK inhibitors have been studied for use with or without methotrexate, similar to tofacitinib. Table 4 lists the safety and monitoring parameters for each non-TNF biologic DMARD and tofacitinib.
| Table 4. Non-Tumor Necrosis Factor (TNF) Biological Disease-modifying Antirheumatic Drugs (DMARDs) and Janus Kinase (JAK) Inhibitors for Rheumatoid Arthritis (RA)2,6
|
| Drugs |
Role in therapy |
Suggested monitoring |
Adverse effects |
Comments |
| Anakinra |
Infrequently used due to decreased effectiveness compared to other biologic DMARDs |
Initial: CBC, LFTs, SCr, PPD, and/or QFT-GIT; screen for hepatitis B/C; consider giving all needed vaccines before initiating treatment
Continued: CBC and LFTs every 1-3 months |
Injection site reactions, cytopenias |
Requires daily SC injections |
Abatacept
|
Effective in patients who are nonresponsive to methotrexate and in patients who have failed to respond to TNF agents; some data suggest it may be a preferred agent after TNF agent failure |
Initial: CBC, LFTs, SCr, PPD, and/or QFT-GIT; screen for hepatitis B/C; consider giving all needed vaccines before initiating treatment
Continued: CBC and LFTs every 1-3 months |
Mild-to-moderate infusion or injection site reactions; increased risk for bacterial infection (especially in patients with underlying lung disease) |
Use with caution in patients with chronic obstructive pulmonary disease |
| Rituximab |
Effective in long-standing, active RA with inadequate response to TNF inhibitor therapy when used in combination with methotrexate; efficacy may persist many months after intravenous administration; patients with RA seropositivity may be more likely to respond |
Initial: CBC, LFTs, SCr, PPD and/or QFT-GIT; screen for hepatitis B/C; consider giving all needed vaccines before initiating treatment
Continued: CBC every 1-3 months |
Mild-to-moderate infusion reactions; increased risk for bacterial infection; FDA warning of severe mucocutaneous reactions, hepatitis B reactivation, and progressive multifocal leukoencephalopathy |
Methylprednisolone or equivalent recommended 30 minutes before infusion to prevent serious infusion reactions; medications and supportive care measures should be available during infusion |
| Tocilizumab |
Effective in patients who are nonresponsive to methotrexate, and in patients who have failed to respond to TNF agents; some data suggest it may be a preferred agent after TNF agent failure |
Initial: CBC, LFTs, SCr, PPD and/or QFT-GIT, serum lipid panel; screen for hepatitis B/C; consider giving all needed vaccines before initiating treatment
Continued: CBC and LFTs every 1-3 months; periodic lipid panel |
Mild-to-moderate infusion or injection site reactions; increased risk for bacterial infection and TB; cytopenias; increasing cholesterol levels; liver dysfunction
Strict monitoring and dose adjustment based on laboratory results recommended |
May be more effective in methotrexate non-responders than other biologic DMARDs
Long-term safety is unknown, making its use as a first-line biologic DMARD uncertain |
| Tofacitinib |
New JAK inhibitor approved for RA patients who have failed to respond to other DMARDs; can be used with methotrexate |
Initial: CBC, LFTs, SCr, PPD and/or QFT-GIT, serum lipid panel; screen for hepatitis B/C; consider giving all needed vaccines before initiating treatment, particularly herpes zoster vaccine in at-risk patients
Continued: CBC and LFTs every 3 months; periodic lipid panel |
Similar risk of infection, including TB, as other biologic DMARDs; increased LFTs; cytopenias; dyslipidemias; bowel perforations have been reported in studies, but the role of JAK inhibitors is unknown; risk of malignancy is unknown |
ACR guidelines currently suggest using tofacitinib only in patients who have failed other therapy due to lack of safety information in this new class of drug; herpes zoster reactivation is a particular concern and at-risk patients should be vaccinated before beginning treatment |
| Abbreviations: ACR: American College of Rheumatology; CBC, complete blood count; FDA: U.S. Food and Drug Administration; LFT, liver function test; PPD, tuberculin skin test; QFT-GIT, QuantiFERON-TB Gold In-Tube; SC, subcutaneous; SCr, serum creatinine; TB, tuberculosis. |
THE ROLE OF THE PHARMACIST IN RA TREATMENT
Pharmacists in a variety of settings interact with RA patients. As such, pharmacists are well-poised to aid patients and rheumatologists in several aspects of RA therapy, including treatment monitoring, vaccination recommendations, and medication adherence.
Monitoring of synthetic and biologic DMARDs is crucial to the safe use of these potent medications, and routine laboratory monitoring is needed for all these medications. Tables 3 and 4 provide recommended laboratory monitoring parameters for available treatments. Pharmacists can query patients about symptoms that may indicate an adverse effect (e.g., night sweats and low-grade fevers may be symptoms of an indolent infection in a patient taking a biologic DMARD) and remind patients of the importance of keeping laboratory appointments. Pharmacists can also discuss with patients expected results from DMARD therapy. No agent can achieve ACR50 improvement in all patients, so discussing realistic expectations of therapy while emphasizing the need for medications to reach treat-to-target goals is important for patient education.
Adherence to RA medications has traditionally been low when studied in clinical trials. Again, pharmacists can play a key role in improving this important treatment-related factor. One study examined a U.S. pharmacy benefits management company that implemented an RA medication therapy management (MTM) program for patients receiving specialty pharmacy services and parenteral medications.43 Participants in this MTM program received a patient-centric model of care that provided education, a brochure containing information on medication use and disposal, access to a pharmacist for questions 24 hours a day, regular refill reminders, and communications concerning related conditions that require significant self-care efforts. A study of the MTM program found that RA patients enrolled in the service had improved adherence rates compared to control community pharmacy patients, as well as improved scores on several self-reported measures of disease activity. MTM programs delivered by pharmacists and designed to target adherence can be successful, if sufficient resources and education are made available. Additionally, pharmacists can help patients who have trouble accessing or paying for medications learn about and enroll in patient assistance programs.
Both synthetic and biologic DMARDs carry a risk of infection, so immunizations are essential for avoiding a potentially dangerous exposure to disease. Since vaccine recommendations are critically important, the ACR guidelines devote a special section to discussing them.6 In general, RA patients should receive all age-appropriate vaccines before starting DMARD therapy, including yearly (parenteral) influenza, pneumococcal, and hepatitis B vaccines. Live vaccines should be avoided during DMARD therapy due to the theoretical risk of causing the disease itself in patients using these immunosuppressive therapies. The herpes zoster vaccine should be considered in patients over 50 years of age who are scheduled to receive any DMARD, particularly tofacitinib. In many states, pharmacists provide immunizations and can work with rheumatologists to ensure that patients receive appropriate vaccinations before and during DMARD therapy. Pharmacists should also act as a resource about additional vaccines needed in special situations (e.g., an RA patient using a DMARD and travelling to a part of the world that requires further vaccinations). Finally, as part of a comprehensive approach to RA treatment, pharmacists can aid in educating patients, selecting therapy, and monitoring for comorbidities related to RA such as potential cardiovascular disease risks (e.g., dyslipidemia and hypertension) and risk of osteoporotic fracture.
CONCLUSION
RA is just one of several immunologic diseases for which treatment has undergone vast changes in recent decades. More potent biologic DMARDs and aggressive use of synthetic DMARDs have allowed rheumatologists to achieve outcomes in many patients that would have been unattainable only a generation ago, including complete disease remission. Although effective, biologic DMARDs have a host of concerns and unresolved issues, including potential adverse effects, development of laboratory markers to guide therapy, cost, and adherence barriers, that add significant complexity to their use. New drug entities, such as JAK inhibitors, are continuing to enter the market for RA, each with its own safety and monitoring concerns. Pharmacists can aid patients with RA by acting as a source of information concerning adverse effects, monitoring, and adherence of RA therapy. Pharmacists should also offer vaccination recommendations and patient counseling for RA patients to maximize the safety and efficacy of the potent agents now available to treat their disease.
References
- Lahaye C, Tatar Z, Dubost JJ, et al. Overview of biologic treatments in the elderly. Joint Bone Spine . 2015;82(3):154-60.
- Wahl K, Schuna AA. Rheumatoid Arthritis. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill; 2014.
- Mikuls TR, Saag KG. Comorbidity in rheumatoid arthritis. Rheum Dis Clin North Am . 2001;27(2):283-303.
- Barton A, Worthington J. Genetic susceptibility to rheumatoid arthritis: an emerging picture. Arthritis Rheum. 2009;61(10):1441-1446.
- Scott IC, Seegobin SD, Steer S, et al. Predicting the risk of rheumatoid arthritis and its age of onset through modelling genetic risk variants with smoking. PLoS Genet. 2013;9(9):e1003808.
- Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol . 2016;68(1):1-26.
- Smolen JS, Aletaha D, Bijlsma JWJ, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis . 2010;69(3):631-637.
- Treat to Target Recommendations. AbbVie, Inc. http://www.t2t-ra.com/recommendations/target-to-treat-statements. Accessed June 1, 2016.
- Anderson J, Caplan L, Yazdany J, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken). 2012;64(5):640-647.
- Allaart CF, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. Aiming at low disease activity in rheumatoid arthritis with initial combination therapy or initial monotherapy strategies: the BeSt study. Clin Exp Rheumatol . 2006;24(6 Suppl 43):S-77-82.
- Syversen SW, Gaarder PI, Goll GL, et al. High anti-cyclic citrullinated peptide levels and an algorithm of four variables predict radiographic progression in patients with rheumatoid arthritis: results from a 10-year longitudinal study. Ann Rheum Dis . 2008;67(2):212-217.
- Huizinga TW. Personalized medicine in rheumatoid arthritis: is the glass half full or half empty? J Intern Med. 2015;277(2):178-187.
- GRADE Methodology. www.gradeworkinggroup.org. Accessed May 19, 2016.
- Hazlewood GS, Barnabe C, Tomlinson G et al. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: abridged Cochrane systematic review and network meta-analysis. BMJ . 2016;353:i1777.
- Katchamart W, Trudeau J, Phumethum V, et al. Efficacy and toxicity of methotrexate (MTX) monotherapy versus MTX combination therapy with non-biological disease-modifying antirheumatic drugs in rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis . 2009;68(7):1105-1112.
- Morgan SL, Baggott JE. Folate supplementation during methotrexate therapy for rheumatoid arthritis. Clin Exp Rheumatol. 2010;28(5 Suppl 61):S102-109.
- Hazlewood GS, Thorne JC, Pope JE, et al. The comparative effectiveness of oral versus subcutaneous methotrexate for the treatment of early rheumatoid arthritis. Ann Rheum Dis . 2016;75(6):1003-1008.
- de Jong PH, Hazes JM, Han HK, et al. Randomised comparison of initial triple DMARD therapy with methotrexate monotherapy in combination with low-dose glucocorticoid bridging therapy; 1-year data of the tREACH trial. Ann Rheum Dis . 2014;73(7):1331-1339.
- Ramiro S, Gaujoux-Viala C, Nam JL, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis . 2014;73(3):529-535.
- Ding HJ, Denniston AK, Rao VK et al. Hydroxychloroquine-related retinal toxicity. Rheumatology. 2016;55(6):957-967.
- Raj R, Nugent K. Leflunomide-induced interstitial lung disease (a systematic review). Sarcoidosis Vasc Diffuse Lung Dis. 2013;30(3):167-176.
- Gibofsky A, Palmer WR, Keystone EC, et al. Rheumatoid arthritis disease-modifying antirheumatic drug intervention and utilization study: safety and etanercept utilization analyses from the RADIUS 1 and RADIUS 2 registries. J Rheumatol . 2011;38(1):21-28.
- Buckley F, Finckh A, Huizinga TW, et al. Comparative efficacy of novel DMARDs as monotherapy and in combination with methotrexate in rheumatoid arthritis patients with inadequate response to conventional DMARDs: a network meta-analysis. J Manag Care Spec Pharm . 2015;21(5):409-423.
- Alonso-Ruiz A, Pijoan JI, Ansuategui E, et al. Tumor necrosis factor alpha drugs in rheumatoid arthritis: systematic review and metaanalysis of efficacy and safety. BMC Musculoskelet Disord . 2008;9:52.
- Chen Y, Sun J, Yang Y, et al. Malignancy risk of anti-tumor necrosis factor alpha blockers: an overview of systematic reviews and meta-analyses. Clin Rheumatol . 2016;35(1):1-18.
- Raaschou P, Simard JF, Asker Hagelberg C, et al. Rheumatoid arthritis, anti-tumour necrosis factor treatment, and risk of squamous cell and basal cell skin cancer: cohort study based on nationwide prospectively recorded data from Sweden. BMJ . 2016;352:i262.
- Simard JF, Neovius M, Askling J, ARTIS Study Group. Mortality rates in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors: drug-specific comparisons in the Swedish Biologics Register. Arthritis Rheum . 2012;64(11):3502-3510.
- Pascual-Salcedo D, Plasencia C, Ramiro S, et al. Influence of immunogenicity on the efficacy of long-term treatment with infliximab in rheumatoid arthritis. Rheumatology. 2011;50(8):1445-52.
- Singh JA, Christensen R, Wells GA, et al. A network meta-analysis of randomized controlled trials of biologics for rheumatoid arthritis: a Cochrane overview. CMAJ . 2009;181(11):787-796.
- Lopez-Olivo MA, Amezaga Urruela M, McGahan L, et al. Rituximab for rheumatoid arthritis. Cochrane Database Syst Rev . 2015;1:CD007356.
- Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum . 2006;54(9):2793-2806.
- Rituxan [prescribing information]. South San Francisco, CA: Genentech, Inc.; 2016.
- Guyot P, Taylor P, Christensen R, et al. Abatacept with methotrexate versus other biologic agents in treatment of patients with active rheumatoid arthritis despite methotrexate: a network meta-analysis. Arthritis Res Ther. 2011;13(6):R204.
- Orencia [prescribing Information]. Princeton, NJ: Bristol-Myers Squibb Company; 2016.
- Gabay C, Emery P, van Vollenhoven R, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381(9877):1541-1550.
- Navarro G, Taroumian S, Barroso N, et al. Tocilizumab in rheumatoid arthritis: a meta-analysis of efficacy and selected clinical conundrums. Semin Arthritis Rheum . 2014;43(4):458-456.
- Hodge JA, Kawabata TT, Krishnaswami S, et al. The mechanism of action of tofacitinib - an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol . 2016;34(2):318-328.
- Lee YH, Bae SC, Song GG. Comparative efficacy and safety of tofacitinib, with or without methotrexate, in patients with active rheumatoid arthritis: a Bayesian network meta-analysis of randomized controlled trials. Rheumatol Int. 2015;35(12):1965-1974.
- van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med . 2012;367(6):508-19.
- Xeljanz [prescribing information]. New York, NY: Pfizer, Inc.; 2016.
- Curtis JR, Xie F, Yun H, Bernatsky S. Real-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis. Ann Rheum Dis . 2016;pii: annrheumdis-2016-209131.
- Yamaoka K. Janus kinase inhibitors for rheumatoid arthritis. Curr Opin Chem Biol . 2016;32:29-33.
- Stockl KM, Shin JS, Lew HC, et al. Outcomes of a rheumatoid arthritis disease therapy management program focusing on medication adherence. J Manag Care Pharm . 2010;16(8):593-604.
Back to Top