ADVERTISEMENT

Module 4. Blood Glucose Monitoring
|
IMPORTANT DEFINITIONS
Glycosylated hemoglobin (HbA1c) - The HbA1c test is a measure of glycation of red blood cells. Glycation is a term used to describe the attachment of sugar to the red blood cells. Because the amount of sugar that "sticks" to a red blood cell is related to the average amount of glucose in the blood, this test is a measure or average of blood glucose for approximately three months, which is the life span of the red blood cell until new ones are generated. HbA1c levels are reported in "%" values. The target HbA1c for patients with diabetes varies, depending on the patient. For most patients the HbA1c target is between 6.5-7.0%. Glycosylated hemoglobin may also be written or referred to A1C.
Hypoglycemia-Low blood sugar, which generally equates to a blood glucose level of 70 mg/dl or below. Symptoms of low blood sugar can include shakiness, sweating, confusion, and in more serious cases, a patient can be rendered unconscious.
Hyperglycemia-High blood sugar, which generally equates to glucose levels above 250 mg/dl. When blood sugars get too high, fat starts to break down for energy, and ketones can form. When ketones start to spill in the urine, it can pose a dangerous situation causing body chemistry to be off balance. Some symptoms of hyperglycemia include lack of energy, excess hunger, and excess thirst. Hyperglycemia can also lead to dehydration.
SMBG- Refers to Self-Monitoring of Blood Glucose. This is a standard of care for patients with diabetes. Diabetes is a "take home" disease that requires patient education.
|
INTRODUCTION
Diabetes mellitus (DM) is a global epidemic that impacts millions of Americans every year. Based on data from 2012, the overall incidence of diabetes in the United States (US) is approaching 10% and continues to increase, with no apparent slowing down.1 DM is a complex chronic disease that requires a multifaceted approach to successful treatment that includes appropriate self-monitoring of disease through blood glucose (BG) monitoring, also known as self-monitoring of blood glucose (SMBG).2 A basic understanding of the function of BG meters is important for health care practitioners (HCPs) to effectively educate patients on their use in performing appropriate SMBG. Because pharmacy technicians will frequently encounter people purchasing and using blood glucose monitors, it is important to have a basic understanding of these devices and their use.
BG METERS
BG meters (also known as glucometers) are used to perform SMBG, with studies showing that appropriate SMBG is important in effectively controlling diabetes.3,4 Proper functioning and, thus, accurate results are dependent on the patient’s and caregiver’s appropriate use of the BG meter.5 There are an assortment of meters available for patients on the market, although most of the meters currently available have a very similar basic functionality. The essential supplies involved in the testing process are the glucose meter, a lancing device with disposable lancets (or single-use disposable lancets) used to pierce the skin to obtain blood for testing, test strips with a control solution (not always required), alcohol wipes (See Figure 1), and a sharps container or sharps-resistant vessel (See Figure 2).
The general steps involved in SMBG include6,7:
- Choose a finger in which to test the BG. Wash the site of testing with warm water and soap, or use an alcohol swab to clean the site. If using an alcohol swab, ensure that the site dries before testing, as the alcohol may impact the testing result. Please note that alternate-site testing is allowed with some meters, which may include using blood from the forearm or thigh, etc.
- Insert the test strip in the meter and wait for the confirmatory sign to test, which is usually a flashing illustration of a blood drop on the screen.
- Use the lancing device or single-use lancet to prick the finger according to the diagram in the package.
- Touch the drop of blood to the appropriate area of the test strip and wait for the result. A cotton ball or gauze pad may be used to wipe remaining blood from the finger.
- Once the result is received, remove the test strip from the meter and place in the trash along with the cotton ball. Put the lancet in a sharps-resistant container.
- Document the result in a BG diary.
Error readings can be frequent occurrences when using BG meters. If there is an issue with the glucose result when using the meter, an error code will usually show on the screen, which will vary based on the type of meter used. One of the most common error codes is caused by an insufficient amount of blood applied to the test strip.8 Strategies to increase blood flow prior to lancing and, thus, improving the chances of avoiding this error include washing the hands with warm water, lowering the hand below heart level, and shaking the hand to improve blood flow. Avoid squeezing the finger too hard after lancing as this can affect the test results.9 Other possible error codes may be a result of expired or altered test strips, a low battery, or faulty meters. It is important to carefully read the manual that is provided with the meter to understand error messages. If a manual is misplaced or lost, manuals can frequently be accessed on the manufacturers’ website.
Patients may note pain at the site of lancing, especially when using the end of the finger to test BG. Some patients report that pricking the side of the finger by the nail bed reduces the pain, as the end of the finger is usually more sensitive compared with the side due to fewer nerve endings. In addition, many lancing devices may be adjusted to alter the depth of the lancet prick, thus potentially lessening the pain upon lancing.7 Patients should keep in mind, however, that reducing the depth of lancing may affect the ability to obtain an adequate-sized blood drop for testing and could result in errors. Some BG-meter kits might also include devices to test at alternate sites such as the thigh or forearm, although the testing method is slightly different and the results may not be as accurate as testing performed using the finger.9
Helping patients choose from among the various BG meters available in the marketplace includes providing the contrasting variables and factors involved that are specific to the patient’s situation and concerns. Such considerations include the cost of the meter, the accuracy of the results, the dimensions of the meter, and the size of the blood drop required for testing. Keep in mind that accuracy is difficult to gauge other than comparing a meter test at home with one done in the HCP’s office to ensure that the BG readings are not that different from each other. Many meters now come with special features that can link with smartphone applications to help track and store BG results. In most situations, it is important to instruct patients to call their insurance company or to check their insurance firm’s drug formulary to help them choose a meter (and testing strips) that is covered for them.10
BG-meter accuracy is a trending concern. Some literature, including a study published in Diabetes Technology and Therapeutics, raises concerns about the accuracy of BG meters, particularly in the “Low-glucose range.”11,12 Previous standards required that “95% of all meter test results to be within 20% of the actual BG level for results greater than 75 mg/dL and within 15 mg/dL for values below 75 mg/dL.”10 In response to accuracy concerns, the U.S. Food and Drug Administration (FDA) updated and improved the standards to ensure more precise results in those BG meters available. This is why it is important to try to stick with one meter, if possible.
SUGGESTIONS FOR SMBG TESTING FREQUENCY
The appropriate recommendation for frequency of SMBG testing in patients with DM is a complex issue that is often misunderstood in practice. How often to test is dependent on a number of factors, but the most important issue to consider is the patient’s safety, namely preventing severe episodes of hypoglycemia and hyperglycemia.
Testing more often is particularly important for those on multiple daily insulin (MDI) injections or other pharmacologic agents that may acutely lower BG, such as the sulfonylurea class.2 The ADA Standards of Medical Care in Diabetes recommend that patients on intensive insulin therapy, defined as patients on MDI or insulin pump therapy, should consider testing BG “prior to meals and snacks, after meals, at bedtime, before exercising, when low BG is suspected, after treating low BG until patients are back to an acceptable blood glucose, and prior to such critical tasks as driving.” This most common suggestion applies to patients with type 1 diabetes mellitus (T1DM), who are naturally more prone to significant fluctuations in BG because they are entirely dependent on insulin therapy.
Available literature does support benefits of more frequent SMBG testing in children and adolescents with T1DM,13 as this age group is particularly subject to glucose fluctuations. Evidence for patients who do not fit within the category of intensive insulin therapy, however, is far less clear. Studies have examined the benefits and cost-effectiveness of more frequent SMBG testing in people who do not use insulin therapy, with mixed results.14-16 How often to test blood sugars is based on many factors, including the willingness of the patient to do so. Without testing, however, it becomes impossible to understand how well a patient’s diabetes is controlled. Real time testing provides a lot of insight for a patient.
INTERPRETATION OF SMBG RESULTS
A basic understanding of the general diagnostic criteria and blood glucose target ranges for patients are important before interpretation of results is possible. See Table 1 for a summary of diagnostic criteria. The ADA guidelines distinguish between FBG (fasting blood glucose) and postprandial or post-meal blood glucose (PPG). In most cases, HCPs will target the HbA1c (discussed below) and FBG goals initially and then progress to targeting PPG if the FBG readings are at goal concurrently with an elevated HbA1C.2 See Table 1 for a summary of glycemic targets in DM. HCPs may consider individualization of glycemic targets based on various factors, as discussed more in the section on HbA1c (below).
| Table 1. Glycemic Diagnostic Criteria and Targets (non-pregnant adults) as per the ADA Standards of Medical Care in Diabetes |
| Laboratory Test |
Diagnostic Criteria |
Target Range |
Factors Impacting Individualization of Target Range |
| FBG lab draw/SMBG |
≥126 mg/dL |
80 – 130 mg/dL |
- Duration of disease
- Patient life expectancy
- Patient motivation
- DM complications
- CV comorbidities
- Hypoglycemic history
|
| 2-hour PPG lab (using OGTT) |
≥200 mg/dL |
<180 mg/dL |
| Random BG |
≥200 mg/dL with concurrent symptoms of hyperglycemia |
Not applicable |
| A1C |
≥6.5% |
<7% |
A1C = glycosylated hemoglobin; BG = blood glucose; CV = cardiovascular; FBG = fasting blood glucose; OGTT = oral glucose tolerance test; PPG = postprandial glucose; SMBG = self-monitoring of blood glucose
Adapted from Tables 2.1 and 6.2 in the Standards of Medical Care in Diabetes—20162 |
Proper interpretation of SMBG results requires that all parties involved have a complete understanding of all the factors that may cause changes in BG. Assuming that BG testing is properly done, many circumstances may affect the end result, including physical activity, changes in carbohydrate content in meals, compliance with medications that lower BG, and concurrent stress or illness.2
Physical activity can affect BG results in 2 major ways. In most individuals, physical activity will lower BG during and after exercise.17 When physical activity is combined with insulin or insulin secretagogues, drugs that increase insulin secretion from the pancreas, (e.g., sulfonylureas), or a significant reduction in carbohydrate consumption pre-exercise, the combination of factors can cause hypoglycemia. These patients should be counseled to pretreat with a serving of carbohydrates before exercise, especially if the pre-exercise BG is less than 100 mg/dL. This will help prevent exercise-induced hypoglycemia. This situation occurs less frequently in those not taking insulin or secretagogues, such as glipizide, and pretreatment is not usually needed.2
The second, and much less common, manner in which physical activity can have a significant impact on BG readings is when blood sugars are high and ketones are present in the urine. This was discussed in the exercise module. In this instance, physical activity can actually increase BG levels,18 causing hospitalization in worst-case scenarios.
Abrupt changes in food (carbohydrate) consumption may also significantly alter SMBG results, especially for patients receiving insulin therapy. Consistent carbohydrate intake will help avoid fluctuations in SMBG and may help improve overall glycemic control.19 Significant reductions in the quantity of carbohydrates, particularly in patients on mealtime insulin, may result in hypoglycemia, while increases in carbohydrate content may lead to hyperglycemia. Foods with high sugar content, such as non-diet soft drinks or sweets, are more likely to cause acute hyperglycemia when ingested, given their rapid absorption, especially when patients forget or neglect to take extra insulin. HCPs should remember to inquire about carbohydrate intake and adherence with medications when interpreting SMBG results.
Concurrent stress and illness, such as infection, trauma, or surgery, can worsen hyperglycemia in patients with diabetes.2 Incidences of severe hyperglycemia, including progression to diabetic ketoacidosis, are not uncommon in those with T1DM, and people with T2DM on insulin therapy. Patients should be counseled to frequently monitor SMBG daily during concurrent illness to avoid hospitalization as well as to maintain adequate hydration and other treatment modalities.
HBA1C TESTING
The HbA1C test is routinely done in practice to evaluate long-term glycemic control, as it represents an estimation of the average BG over approximately 3 months. Table 2 shows the estimation of average BG correlated with the corresponding HbA1C value, as derived from clinical studies.20 As noted above and in Table 1, the general target for most adults is an HbA1C of less than 7.0%, although individualization of this goal is essential for effective management of diabetes. Given the nature of the test, in general, it is appropriate to recommend quarterly testing in patients who are not at their individualized goal or those who have had therapy adjustments, while people at goal may be tested less frequently.2
| Table 2. Correlation of HbA1c with Estimated Average BG |
| A1C (%) |
Estimated Average BG |
| mg/dL |
mmol/L |
| 6 |
126 |
7.0 |
| 7 |
154 |
8.6 |
| 8 |
183 |
10.2 |
| 9 |
212 |
11.8 |
| 10 |
240 |
13.4 |
| 11 |
269 |
14.9 |
| 12 |
298 |
16.5 |
A1C = glycosylated hemoglobin; BG = blood glucose
Adapted from Table 6.1 in the Standards of Medical Care in Diabetes—20162 |
The HbA1c test is considered a valuable glycemic control marker as it is strongly associated with diabetes complications; thus, a reduction in HbA1c can decrease the risk of morbidity and mortality related to diabetes.21,22
Interpreting HbA1c Results
The interpretation of HbA1C results requires the ability to identify diagnostic criteria (Table 1) and evaluate appropriate individualization of HbA1C goals. While ADA guidelines recommend a general goal of below 7%, certain studies suggest less stringent goals based on a number of modifiable and non-modifiable patient-and disease-specific factors.2 As represented in Figure 3, factors that would cause HCPs to target less aggressive HbA1C goals (such as below 8%), include long duration of the disease, extensive cardiovascular complications or known cardiovascular disease, along with short life expectancy. These patients are representative of those encompassed by a large clinical trial that showed more aggressive targets (e.g., HbA1c below 6%) could actually cause more harm than benefit.23 It is also reasonable, however, for HCPs to assign more aggressive HbA1C goals (such as below 6.5%) for patients who are young, newly diagnosed, and those who are relatively healthy with few comorbidities as long as doing so does not create an inappropriate risk of hypoglycemia.2
| Figure 3. Factors to Consider for Individualization of A1C Targets |
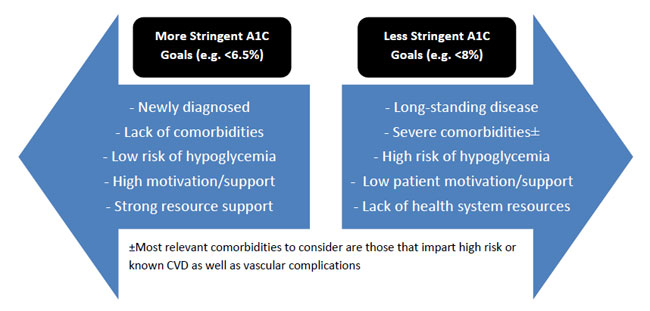 |
A1C= glycosylated hemoglobin; CVD = cardiovascular disease
Adapted from Figure 6.1 in the Standards of Medical Care in Diabetes—2016 |
HbA1c evaluation at the patient’s initial diagnosis, particularly in T2DM, may suggest important characteristics about the individual’s state of disease. For instance, those with mild elevations in the HbA1c above diagnostic criteria (e.g., 7%) at baseline may indicate short-standing disease. Given that diabetes is often asymptomatic in patients with mild elevations in BG along with symptoms of hyperglycemia potentially being insidious, it is not unusual for patients to be diagnosed with an HbA1C that is significantly elevated at baseline, such as 9-10%. This may indicate longer-standing disease or more acute progression of underlying insulin deficiency or resistance. Certainly, patients presenting with more significant hikes in the HbA1c at baseline who have multiple comorbid complications associated with diabetes (such as retinopathy or neuropathy, for example) suggest more long-standing disease. In patients with T2DM, significantly elevated HbA1C at baseline may necessitate initiation of insulin therapy with oral medication when appropriate.
In T2DM, an elevated HbA1c may indicate that the disease is progressing, and insulin production is continuing to decline.24,25 New treatment options may need to be considered, such as adding insulin to the treatment plan.
It is also important to consider individuals with HbA1c test results that may be too “low.” The main concern in this situation is that the diabetes treatment regimen may be too aggressive and could contribute to hypoglycemia. In patients who are reporting hypoglycemic symptoms such as shakiness, sweating, and tachycardia,26 in combination with an HbA1c significantly below goal (e.g., an HbA1c of less than 6 to 6.5%), it is possible that the drugs being used to treat the patient are leading to frequent and consistent episodes of hypoglycemia. This is particularly notable in patients on regimens with agents that have a higher risk of causing hypoglycemia, such as sulfonylureas, glinides, and, of course, insulin. In this case, HCPs must note the considerable dangers that come with severe hypoglycemia, such as coma, hospitalization, and even death.
ADJUSTING DIABETES TREATMENT REGIMENS BASED ON RESULTS
The adjustment of therapy regimens requires careful consideration by trained HCPs in diabetes care, considering a multitude of factors such as those described above, and incorporating a strong understanding of the pharmacotherapy involved in treating diabetes. This is particularly important for patients with T1DM, given their propensity for more acute fluctuations in BG after changes in treatment; thus, these patients should always be referred to their endocrinologist or other clinical specialist for recommendations on titrating insulin doses. In any event, considering treatment adjustments in diabetes must always include assessment of all factors described above that could impact glycemic control.
The ADA and the European Association for the Study of Diabetes have valuable summaries of the tactics to use when considering revisions or advancements in treatment regimens. The ADA Standards of Care contains information that describes the basic principles to consider when weighing treatment decisions in patients with T2DM, noting such key factors as cardiovascular effects, drug efficacy, adverse events, cost, risk of hypoglycemia, and effects on weight when deciding how to advance therapy. These guidelines also have recommendations on initial therapy in T2DM, which in most cases involves initiation of metformin before advancement to additional therapies.2,24
HCPs who are not authorized to make independent adjustments to treatment regimens can still help patients by asking about their blood glucose levels and helping to communicate with other healthcare providers when necessary. For example, pharmacy staff in a retail setting who does not have a diabetes treatment protocol in place may encounter patients with mildly uncontrolled type 2 diabetes on metformin (Fortamet, Glucophage, Glucophage, Glumetza). If the patient presents to the pharmacy with high blood glucose values in their log book, the pharmacy technician should refer the patient to the pharmacist. The pharmacist may want to have a conversation with the provider to see if therapy changes need to be made.
Also note that the literature has suggested each additional agent added on after metformin can lower the HbA1c by approximately 1%.27 This may cause the HCP to elect moving to more aggressive therapy such as insulin if it seems unlikely that the patient will achieve glycemic targets with multiple oral and/or noninsulin injectable agents. A full and complete discussion of approaches to glycemic treatment requires a more detailed discussion, which is outside the scope of this module. You can get more information in the module discussing non-insulin injectables in this series.
CONCLUSIONS
Diabetes continues to be a significant health problem that affects millions of people in this nation and worldwide. One important component of the comprehensive care required to properly manage patients with diabetes is an understanding of BG monitoring and how to instruct people with diabetes on the appropriate use and frequency of testing. In addition, an understanding of how to interpret BG results in the context of individual treatment goals, taking into account the numerous key factors that can impact results, is imperative for HCPs to make appropriate recommendations or effectively intervene in care.
|
As pharmacy technicians, asking appropriate questions of patients with diabetes can lead to a better understanding of how well they know how to interpret their blood glucose results. Some questions you may want to ask patients using a blood glucose meter are:
- How often do you test your blood sugars?
- Does your meter work well for you?
- Do you know what your blood sugar values should be?
These basic questions mean that you are taking an active interest in your patients’ health, and may lead to a consult with a pharmacist where appropriate.
|
References
- American Diabetes Association (ADA). Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics. Accessed December 7, 2015.
- American Diabetes Association Clinical Diabetes 2016 Jan; 34(1): 3-21. http://dx.doi.org/10.2337/diaclin.34.1.3. Accessed November 4th, 2016.
- Miller KM, Beck RW, Bergenstal RM, et al; T1D Exchange Clinic Network. Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care. 2013;36(7):2009-2014.
- Polonsky WH, Fisher L, Schikman CH, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34(2):262-267.
- Sacks DB, Arnold M, Bakris GL, et al; National Academy of Clinical Biochemistry. Position statement executive summary: guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):1419-1423.
- Becton Dickinson and Company (BD): How to Test Your Blood Glucose. https://www.bd.com/us/diabetes/blood-glucose-monitoring/how-to-test. Accessed December 9, 2015.
- ADA. Checking Your Blood Glucose. http://www.diabetes.org/living-with-diabetes/treatment-and-care/blood-glucose-control/checking-your-blood-glucose.html. Accessed December 9, 2015.
- U.S. Food and Drug Administration. Common Problems with the Use of Blood Glucose Meters at the Point of Care. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/TipsandArticlesonDeviceSafety/ucm109449.html. Accessed December 9, 2015.
- ADA/Diabetes Forecast. 10 Ways to Master Your Blood Glucose Meter. http://www.diabetesforecast.org/2012/apr/10-ways-to-master-your-blood-glucose-meter.html. Accessed December 9, 2015.
- ADA/Diabetes Forecast. Meters: Does Your Device Measure Up? http://www.diabetesforecast.org/2015/mar-apr/meters-does-your-device-measure-up.html. Accessed December 9, 2015.
- Klaff LJ, Brazg R, Hughes K, et al. Accuracy evaluation of contour next compared with five blood glucose monitoring systems across a wide range of blood glucose concentrations occurring in a clinical research setting. Diabetes Technol Ther. 2015;17(1):8-15.
- Heinemann L, Zijlstra E, Pleus S, Freckmann G. Performance of blood glucose meters in the low-glucose range: current evaluations indicate that it is not sufficient from a clinical point of view. Diabetes Care. 2015;38(9):e139-e140.
- Ziegler R, Heidtmann B, Hilgard D, et al. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes 2011;12(1): 11-17.
- Farmer A, Wade A, Goyder E, et al. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007;335:132.
- Simon J, Gray A, Clarke P, et al; Diabetes Glycaemic Education and Monitoring Trial Group. Cost effectiveness of self monitoring of blood glucose in patients with non-insulin treated type 2 diabetes: economic evaluation of data from DiGEM trial. BMJ. 2008;336(7654):1177-1180.
- Willett LR. ACP Journal Club. Meta-analysis: self-monitoring in non-insulin-treated type 2 diabetes improved HbA1c by 0.25%. Ann Intern Med. 2012;156(12):JC6-JC12.
- Derouich M, Boutayeb A. The effect of physical exercise on the dynamics of glucose and insulin. J Biomech. 2002;35(7):911-917.
- Colberg SR. Exercise and Diabetes: A Clinician's Guide to Prescribing Physical Activity. 1st ed. Alexandria, VA: American Diabetes Association; 2013.
- Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2014;37 Suppl 1:S120-S143.
- Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ; A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473-1478.
- Albers JW, Herman WH, Pop-Busui R, et al; Diabetes Control and Complications Trial/ Epidemiology of Diabetes Interventions and Complications Research Group. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) study. Diabetes Care. 2010;33(5):1090-1096.
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405-412.
- Gerstein HC, Miller ME, Byington RP, et al; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140-149.
- Kahn SE, Haffner SM, Heise MA, et al; ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427-2443.
- ADA. Hypoglycemia (Low Blood Glucose) http://www.diabetes.org/living-with-diabetes/treatment-and-care/blood-glucose-control/hypoglycemia-low-blood.html. Accessed December 19, 2015.
- Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154(9):602-613.
Back to Top