ADVERTISEMENT

Pharmacotherapeutic Agents for Smoking Cessation
INTRODUCTION
Nearly 18% of adults in the United States (U.S.) smoke cigarettes, and approximately 70% of these individuals want to quit.1,2However, smoking cessation is a challenging feat that often takes multiple attempts because of nicotine's addictive nature and the habits that smokers develop around smoking.Chemically, drug addiction occurs when substance use results in release of dopamine in the brain, which overstimulates the reward pathway and induces positive symptoms such as euphoria. This pathophysiological process reinforces behaviors that lead to nicotine addiction.3
Nicotine increases dopamine release by binding to nicotinic acetylcholine receptors, which leads to downstream synaptic changes that are similar to those induced by cocaine.4 Withdrawal occurs when the drug activating these pathways is unavailable. Signs and symptoms of nicotine withdrawal include craving, increased appetite, depressed mood, anxiety, irritability, and difficulty focusing.5,6 In general, these symptoms begin quickly after cessation (1 to 2 days), peak in the first week, and last on average between 2 to 4 weeks and are difficult to manage.6
Despite nicotine's propensity to be strongly addictive and the difficulty managing symptoms of withdrawal, today the U.S. has more former than current smokers.2 This statistic is due in part to increased public awareness that smoking cigarettes is harmful and can lead to a variety of cancers, lung disease, adverse pregnancy outcomes, and premature mortality. Figure 1 and Table 1 summarize numerous health consequences linked to smoking.5,7,8
Figure 1. Health consequences causally linked to smoking
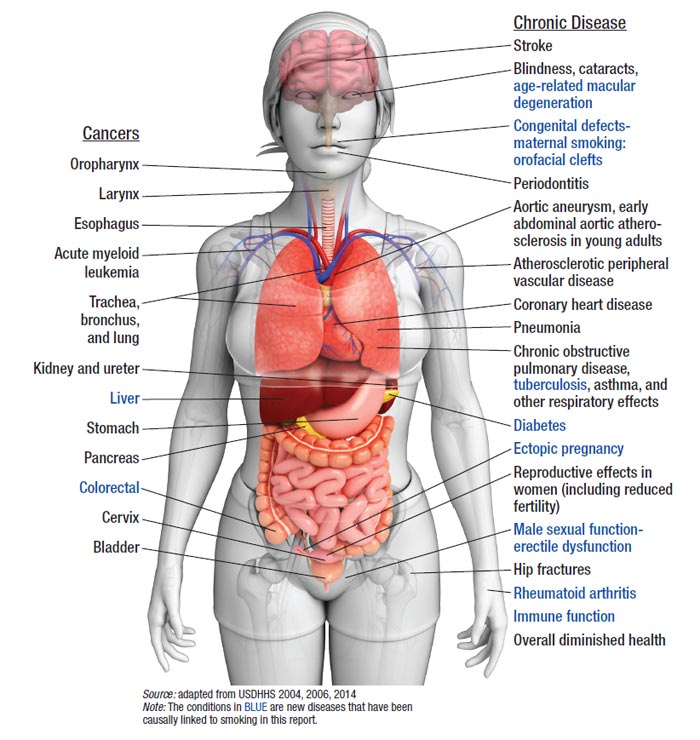
| Table 1. Summary of the Health Consequences of Smoking |
| Increased Risk Category |
Description |
| Cancer |
Increased risk of lung, larynx, oral cavity, pharynx, esophagus, pancreas, bladder, kidney, colorectal, cervix, and stomach cancer, as well as acute myeloid leukemia. |
| Cardiovascular disease |
Smoking causes about 140,000 premature deaths annually due to CVD and has a negative effect on many CVD risk factors (low high-density lipoprotein cholesterol, glucose intolerance). It has also been shown to increase risk of stroke and peripheral arterial disease. |
| Respiratory system |
In addition to increased risk of cancers in the respiratory system, cigarette smoking has been shown to cause chronic bronchitis, chronic obstructive pulmonary disease (the leading cause of this condition in the U.S.), emphysema, and asthma. |
| Reproductive system |
Smoking negatively affects fertility in both sexes, pregnancy outcomes (including low birth weight, congenital anomalies, and infant death), reproductive development in the child, irregular menstrual cycles, and younger age at menopause. |
Source: Reference 5
Abbreviation used: CVD, cardiovascular disease. |
Possibly less well known than the health consequences of smoking are that damage caused by cigarettes is potentially reversible. For example, cessation earlier in life results in greater health improvement, but quitting benefits smokers regardless of age. Risk of heart attack drops dramatically within 1 year of successful cessation, after 5 years, the risk of stroke is similar to that of nonsmokers, and after 10 years the risk of lung cancer is reduced by one-half.5 A review of national health survey data by Jha et al. showed that when all deaths due to cigarette use are considered, smoking cessation before age of 40 decreases the excess risk of death by 90%.9
In addition to improved health, patients may save a substantial amount of money after successful cessation. According to the American Lung Association, the average price per pack of cigarettes in the U.S. is $5.51 (slightly more than $2,000 per year for a pack-per-day smoker).10 States also benefit from decreased loss of productivity and health care expenditures that would have otherwise been caused by cigarette use–even when they are used to fund cessation treatment. The American Lung Association estimates that for every dollar states spend on smoking cessation treatments, they will ultimately save $1.26 (or $712 million annually).10
Pharmacists, with their background in pharmacokinetics, pharmacodynamics, and patient counseling and education, are well-suited to provide assistance with smoking cessation and product selection in all practice environments. In some states, such as California, pharmacists are allowed to provide nicotine replacement therapy (NRT) without a prescription. Numerous studies support the utility of pharmacist-led cessation services. For example, a recent meta-analysis of smoking cessation counseling by pharmacists in community settings found significantly improved quit rates, citing a relative risk of 2.21 (95% CI 1.49–3.29) when comparing abstinence rates of groups with pharmacist intervention with controls.11 This analysis included studies with or without NRT, interventions that ranged from 1 to 4 months in length, and smoking status assessed at dates 1 to 12 months after the initial intervention.11 A cost analysis of pharmacist-led cessation services in the Veterans Administration demonstrated that the 16% quit rate obtained by participating patients saved more money than the total cost of the program.12
Clearly, pharmacists are valuable providers of smoking cessation interventions. Increasing the number of pharmacists comfortable with providing cessation services is essential to decrease the morbidity and mortality associated with cigarette use. It would also contribute to meeting Healthy People 2020's goal of reducing the number of smokers in the United States to 12%.13
This continuing pharmacy education activity works toward increasing pharmacist comfort with providing cessation services by reviewing U.S. Food and Drug Administration (FDA)–approved NRTs for smoking cessation. It also addresses related topics:
- General concerns before starting an NRT
- Recent FDA-approved changes in NRT product labeling
- Combining smoking cessation products
- Use of electronic cigarettes as a smoking cessation tool
Since drug therapies used in smoking cessation are intended to be part of a comprehensive treatment plan that includes patient education and counseling, this activity begins with an overview of behavioral therapy.
Behavioral Therapy
Behavioral therapy and patient education on smoking cessation tools are essential components of smoking cessation regimens. Studies have shown that counseling sessions (even when brief) in combination with medication improve quit rates over either individual intervention alone.14 The "5 As" is a well-known, efficient smoking cessation intervention. It includes the following:
- Asking patients about their smoking use
- Advising smokers to quit
- Assessing readiness (and providing brief motivational interviewing to individuals unwilling to quit)
- Assisting with a cessation plan
- Arranging for follow up to assess progress and provide encouragement
When possible, providers should use open-ended questions when covering the "5 As."
If very short on time, pharmacists can instead perform 3-step Ask-Advise-Refer interventions. In this model, Refer corresponds to helping the patient identify resources, such as local quit centers, to ensure they have the tools for finding support, designing a cessation plan, and identifying a follow-up provider.15 For additional reading, the American Congress of Obstetricians and Gynecologists (ACOG) provides a review of the "'5 As" with sample patient questions and further descriptive information.16 https://www.acog.org/-/media/Departments/Tobacco-Alcohol-and-Substance-Abuse/SCDP.pdf?dmc=1&ts=20161209T1034488715
Considerations Before Starting NRT
The health benefits of smoking cessation almost always outweigh the potential risks associated with NRT use.5 However, patients should be advised to speak with a health professional before use because they should consider the potential adverse effects related to excess nicotine carefully. High-risk patient populations include: (1) patients who have experienced a recent (within 2 weeks) myocardial infarction and those with serious or worsening angina and (2) patients with stomach ulcers, as nicotine decreases lower esophageal reflux pressure.5,17 Smokers younger than 18 years of age should also discuss smoking cessation strategies with health professionals because there is insufficient data on risk/benefit in this population.14
Additionally, pregnant and nursing women should attempt smoking cessation initially without NRTs because of limited safety data in these populations. Health professionals should recommend behavioral therapy providers initially, and if behavioral therapy is inadequate, further discussion of NRT use should include their primary care physicians or obstetricians. To date, at least 3 clinical trials have evaluated the efficacy of NRT options in pregnancy; the efficacy data were not overwhelming in any of the trials. Two studies that included pregnant women who used transdermal nicotine patches found no improvement in quit rates. Another study allowed pregnant women to select their preferred NRT and found improvement over placebo when combined with behavioral therapy.18-20 Although a single study was halted prematurely based on a higher rate of preterm labor in the NRT group that exceeded a priori criteria for study termination, the Data Safety Monitoring Board ultimately concluded preterm labor was unrelated to NRT use.20 Overall safety data from these studies suggest that NRT use during pregnancy does not worsen outcomes. For further reading, a recent Cochrane Review by Coleman et al. provides detailed discussion on studies assessing the safety and efficacy of all pharmacologic treatment options used for smoking cessation.21 Finally, all NRTs should be kept out of reach of children and pets to avoid toxicity secondary to nicotine overdose.
Another consideration is how existing drug levels may change once patients stop smoking. Pharmacokinetic drug interactions unique to cigarettes are largely related to induction of cytochrome P-450 (CYP450) drug metabolizing enzymes by the polycyclic aromatic hydrocarbons found in cigarette smoke.22 Patients who quit smoking may require dose reductions of medications metabolized by this enzyme. Medications most likely to be affected by a change in smoking status are those metabolized by CYP1A2, such as clozapine, olanzapine, theophylline, and caffeine. Close monitoring of response and tolerance will be especially important for drugs with heavily reliant on CYP1A2, such as clozapine. Additionally, if patients consume caffeine, they should be advised to decrease intake with smoking cessation, as caffeine concentrations will increase significantly as CYP1A2 activity falls.
An important pharmacodynamic drug interaction occurs between cigarette smoking and combination hormonal contraceptives. Concurrent use significantly increases risk of thromboembolism, ischemic stroke, and myocardial infarction, and the interaction is dose-dependent and age-related.23 High-dose oral contraceptive users and those who smoke 25 cigarettes or more daily are at highest risk.23 Combination hormonal contraceptives are contraindicated in women aged 35 and older who smoke 15 cigarettes or more daily.23 Pharmacists can identify and assist this high-risk population of women with smoking cessation.
Nicotine Replacement Therapies
NRTs improve cessation rates by delivering nicotine at doses lower than those delivered by cigarettes to ease withdrawal symptoms. Currently, 5 NRT dosage forms are available. Anyone aged 18 years or older can purchase nicotine gum, transdermal patches, and lozenges over the counter (OTC), but the nicotine inhaler and nasal spray require prescriptions. Pharmacists can help patients interested in prescription-only products through education and communication with other health professionals to request prescriptions when needed.
Few studies have compared NRT products directly, so little evidence suggests that any product is superior to another with respect to improving cessation rates.24 Product selection often comes down to individual patient preference, which may be based on taste, having something to occupy the mouth, a product form that more closely mimics a cigarette, onset of nicotine action and duration, and ease of use.
With cigarettes, nicotine delivery to the brain via pulmonary absorption is extremely rapid, occurring in as few as 10 to 20 seconds after inhalation and leading to peak plasma levels around 15 ng/mL after 1 cigarette.25,26 Nicotine's quick absorption leads to an equally rapid release of dopamine in the brain, which increases cigarettes' addictive potential. All NRT formulations have nicotine absorption rates that are slower than cigarettes, which is why they have less potential for dependence but still ease withdrawal symptoms. Time to peak nicotine plasma concentration can be an important variable for some patients when they select smoking cessation aids because rapid absorption will be related to rapid withdrawal-symptom relief.
A recent Cochrane meta-analysis assessed efficacy of NRT therapies by examining randomized clinical trials that met the following criteria: NRT was compared with placebo or a no-NRT control group, follow up was longer than 6 months, and studies focused on smoking cessation as the primary outcome (rather than improvement in nicotine withdrawal).27 Anyone lost to follow up was considered to have continued smoking.27 In order, the relative risk (RR) meta-analysis cessation results for the gum, lozenge, patch, inhaler, and nasal spray dosage forms as compared with no therapy or placebo were as follows: 1.49 (95% CI 1.40– 1.60), 1.95 (95% CI 1.61– 2.36), 1.64 (95% CI 1.52–.78), 1.90 (95% CI 1.36–2.67), and 2.02 (95% CI 1.49– 2.73).27 While at first glance it may appear that some therapies are more effective than others (for example, the gum has a lower RR than the lozenge), it would be incorrect to say that any therapy is more effective than another because these studies did not compare dosage forms directly. Furthermore, the number of studies meeting inclusion criteria varied by NRT formulation. For example, 56 studies were included in the gum trial meta-analysis (enrolling more than 22,000 participants) versus 7 studies in the lozenge trial meta-analysis (enrolling more than 3,000 participants).27
Again, few studies compare NRT dosage forms directly, and patient preference based on product characteristics influences NRT selection heavily. The following sections review key aspects of NRT product labeling and pharmacokinetics in an effort to help pharmacists work with their patients to select the most suitable NRT. Table 2 summarizes this information and serves as a quick reference for clinicians.
| Table 2. Key Characteristics of Pharmacotherapeutic Agents Approved by FDA for Smoking Cessation |
| Nicotine Replacement Therapies |
|
Formulation
Brand Name
Generic Availability
|
Administration Directions
|
Recommended Maximum and Minimum Dose
Duration of Therapya
|
Nicotine PK Notes
|
Common AEs
|
Special Considerations and Notesb
|
|
Gum
Nicorette
Generic available
|
Use 4-mg gum if patient generally smokes within 30 minutes of awakening; others should use the 2-mg gum.
Chew slowly until a tingling sensation develops, then park gum between the cheek and the gums for nicotine absorption until the tingling sensation fades. Repeat the process until chewing the gum does not release any more nicotine (as noted by minimal tingling after chewing).
|
Minimum 9 pieces of gum/day for the first 6 weeks
Maximum 24 pieces/day
Duration: 12 weeks
|
Cmax occurs in 30 minutes
Each piece should last ~30 minutes
|
Jaw muscle ache
Sore throat or mouth and increased saliva production
|
Avoid if extensive dental work, TMJ
|
|
Lozenge
Nicorette, COMMIT
Generic available
|
Use 4-mg lozenge if patient generally smokes within 30 minutes of awakening; others should use the 2-mg lozenges.
Place a lozenge in the mouth and allowing it to dissolve slowly, without swallowing or chewing, and occasionally moving it from 1 side of the mouth to the other.
|
Minimum 9 lozenges/day for the first 6 weeks
Maximum 20 lozenges/day, 5 lozenges/6 hours
Duration: 12 weeks
|
Cmax occurs in 30 minutes
Each lozenge should last ~20 to 30 minutes
|
Sore throat or mouth and increased saliva production
|
Do not chew or swallow, will increase potential for AEs
Warmth or tingling in the mouth may occur
|
|
Transdermal patch
Nicoderm CQ
Generic available
|
Patch is supplied in 21 mg/day, 14 mg/day, and 7 mg/day nicotine release systems (also referred to as step 1, 2, and 3 patch strengths)
If patient smokes ≥ 10 cigarettes daily, start with step 1 patches; those smoking fewer than 10 cigarettes daily should start with step 2 patches.
Remain on first patch strength for 6 weeks, and then use each consecutive patch for 2 weeks.
Apply to nonhairy, clean, dry skin on the upper body or upper outer arm, immediately after removing backing. Hold on skin for 10 seconds and leave on for 16–24 hours. Wash hands after application or removal. When placing a new patch, the application site should be rotated.
|
Duration: 8–10 weeks
|
Cmax occurs in 2–8 hours
|
Limited to local skin irritation, erythema, and pruritus
|
Take off before bed if causing sleep disturbances, may want to avoid with sensitive skin
Patch is waterproof
Never cut patch (nicotine will evaporate)
Original pouch can be saved for patch disposal
|
|
Inhaler
Nicotrol
|
Insert new cartridge and puff frequently on the inhaler.
Find daily dose that optimizes withdrawal management while avoiding symptoms of nicotine overdose, and maintain dose for 12 weeks.
After 12 weeks, a gradual reduction in daily dose should be attempted over a period of up to an additional 12 weeks. Manufacturer recommends that health professionals keep track of daily use and set a quit date to best plan a gradual taper.
|
Minimum 6 cartridges per day for the first 3–6 weeks
Maximum 16 cartridges per day
Duration: 24 weeks
|
About 4 mg of the total dose is delivered, another 2 mg is absorbed through oral mucosa
Cartridges last about 20 minutes
Cmax occurs in 20 minutes
|
Local irritation in the mouth and throat, coughing, and rhinitis that generally decline in frequency with use
|
Contraindicated if allergic to menthol
Mouthpiece should be cleaned regularly with soap and water
Some patients may not require taper after 12 weeks of use
If unable to stop smoking by week 4 of inhaler use, discontinuation of treatment should be considered
Prescription only
|
|
Nasal spray
Nicotrol NS
|
Spray the drug into the nasal cavity with the head tilted back slightly and no sniffing, swallowing, or inhaling during administration.
One dose of the nasal spray is equivalent to 1spray per nostril.
Continue at the initial dose for 8 weeks (optimizing withdrawal management while avoiding symptoms of nicotine overdose), and then slowly titrate the daily dose down until product has been discontinued in another 4–6 weeks. Tapering advice provided by the manufacturer include using 1 spray per dose, skipping every other dose, and setting a quit date with a personalized, preplanned taper.
|
Minimum 8 doses/day for at least the first week
Maximum is 5 doses/hour or 40 doses/day
Duration: recommended ~3.5 months; has been studied up to 6 months
|
Cmax occurs in 4-15 minutes
|
Irritation in the nasal cavity described as moderate to severe in 94% of users in clinical trials that often improves, but persists
Runny nose, watering eyes, sneezing, throat irritation, and coughing
|
Less common adverse reactions unique to spray include nasal congestion, altered taste and scent perception, epistaxis, eye irritation, and facial flushing
Consider avoiding in patient populations that would be most impacted by nasal irritation (e.g., polyps, sinusitis)
Prescription only
|
| Non-NRT Options for Smoking Cessation |
|
Generic
Brand Name
Generic Availability
|
Administration Directions
|
Duration of Therapya
|
Mechanism of Action
|
Common AEs
|
Special Considerations
|
|
Varenicline
Chantix
|
Set a smoking quit date for 1week after starting varenicline.
Take with a full glass of water after eating a meal per the following dosing regimen:
Days 1–3: 0.5 mg daily
Days 4–7: 0.5 mg twice daily
Days 8 until 12 weeks: 1 mg twice daily
If successful at week 12, an additional 12 weeks at the maintenance dose should be done to encourage long-term abstinence.
Reduce dose if recommended dose is not tolerated, and do not exceed 0.5 mg twice daily if CrCl <30 mL/min.
|
Duration: 24 weeks
|
Binds to nicotinic acetylcholine receptors, effectively blocking nicotine and acting as a less potent agonist than nicotine
|
Nausea is the most common AE
Additional common AEs include abnormal dreams, constipation, and vomiting
|
Low potential for drug interactions
Black box warning for serious neuropsychiatric AEs; be extremely cautious of use in patients with uncontrolled psychiatric disorders
|
|
Bupropion
Zyban, Wellbutrin
Generic available
|
Set a smoking quit date for 1–2 weeks after beginning bupropion.
Start with 150 mg once daily for 3 days, then 150 mg twice daily for at 7–12 weeks.
For older patients and those with renal dysfunction, follow a slower taper and start at lower doses. Reach 150 mg twice daily only if tolerated.
Liver dysfunction: mild hepatic impairment (Child–Pugh score 5 or 6) consider a lower dose, moderate to severe impairment (Child–Pugh 7–15) maximum dose is 150 mg every other day.
|
Duration: 12 weeks
|
Increases the availability of NE and DA in neuronal synapse by blocking reuptake
|
Tachycardia, agitation, dizziness, anxiety, headache, insomnia, diaphoresis, weight loss, constipation, nausea, xerostomia, and tremor
|
Potential for drug interactions: metabolized primarily by CYP2B6 and inhibits CYP2D6
Black box warning for serious neuropsychiatric events
Avoid if seizure disorder, anorexia, or bulimia
Chance of increasing blood pressure increases with concomitant NRT
|
|
Source: References 24, 25, 28–30, 33–35, 39
Abbreviations used: AEs, adverse events; Cmax, maximum plasma concentration; CrCl, creatinine clearance; DA: dopamine; NE, norepinephrine; NRT, nicotine replacement therapy; PK, pharmacokinetic; TMJ, temporomandibular joint dysfunction.
aConsult with health professional about extending duration of therapy past recommended date.
bSigns of nicotine overdose/toxicity are possible with NRT products, but far more likely with overuse or misuse and include: dizziness, headache, diarrhea, and tachycardia.
|
Nicotine Gum and Lozenge (Nicorette, Commit, generic)
Nicotine gum and lozenge share many features. Both have been approved for OTC availability and come in a variety of flavors and 2 strengths, 2 mg and 4 mg nicotine per piece of gum or per individual lozenge. Patients may find these products appealing for any number of reasons, including their OTC accessibility and dosage forms that occupy the mouth (and therefore may help avoid weight gain related to smoking cessation). Compared with the lozenge, the gum is likely not the best option for those with dental conditions that would be aggravated by chewing (e.g., temporomandibular joint disease) or those with extensive dental work or dentures. The gum is unusually sticky due to product excipients, and this can damage crowns and fillings. The lozenge may be inappropriate for individuals with sensitive mucosal tissue such as oral lesions or infection. Both rely on nicotine absorption through the oral mucosa.
Gum and lozenge dosage is based on the time period patients generally wait before smoking their first cigarette in the morning. Those who smoke their first cigarette within 30 minutes of waking should use the 4 mg product, and those who wait longer than 30 minutes should use the 2 mg lozenge.
A key difference between the gum and the lozenge is their administration directions, mostly because of the nature of the gum matrix. Unlike nonmedicated gum, NRT gum is not intended to be chewed continuously, and patients are advised to "park" the gum between the cheek and the oral cavity's gums intermittently. Specifically, patients should chew the gum slowly until a tingling sensation develops, which indicates that nicotine is being released. For maximum nicotine absorption, they should then park the gum between the cheek and the gums until the tingling sensation fades. This lack of action indicates that nicotine is no longer being released. The patient should repeat the process until chewing the gum does not release any more nicotine, which manifests as limited tingling after chewing. Each piece lasts an average of 30 minutes.28
The lozenge directions are more straightforward and recommend simply placing a lozenge in the mouth and allowing it to dissolve slowly, without swallowing or chewing. Patients should occasionally move it around the mouth to avoid irritation due to nicotine, which may cause warmth or tingling in the mouth. Average lozenge dissolution time is approximately 20 to 30 minutes. When used as directed, both the lozenges and gum result in similar peak nicotine levels (for example approximately 10 ng/mL with the 4 mg gum or lozenge) that occur 60 minutes after starting use.29
The rest of lozenge and gum directions are similar. Because scheduled administration of nicotine replacement products have been observed to improve quit rates, patients should schedule at least 9 pieces of gum or lozenges daily for the first 6 weeks. Patients should avoid eating or drinking anything 15 minutes before or during use because acidic conditions in the mouth will lower nicotine absorption. The recommended dosing regimen for both products proposes slow downward titration over 12 weeks: During weeks 1 through 6, use1 piece every 1 to 2 hours; weeks 7 through 9, use1 piece every 2 to 4 hours; and weeks 10 through 12, use 1 piece every 4 to 8 hours. After 12 weeks, patients should consult with health professionals regarding continued use. For strong cravings, patients can use a second piece within the hour as long as they do not exceed 24 pieces of gum or 20 lozenges in a 24-hour period.28,29 The lozenge product labeling also states that patients should not exceed 5 lozenges in 6 hours.29
The most common adverse reaction associated with nicotine gum is jaw muscle ache. Other adverse reactions for both the lozenge and gum include sore throat or mouth and increased saliva production. Cardinal indicators of NRT overuse include stomach upset and dizziness, which will lessen with decreased frequency of product use. Less frequent adverse reactions that are more likely with product overuse (or misuse) include hiccups and heartburn; signs of nicotine overdose include dizziness, headache, diarrhea, tachycardia, and vomiting.
Nicotine overdose, or toxicity, is relatively rare with all NRTs, and as such will not be discussed in further sections. Patients should be advised to stop use and contact their physician about signs of serious adverse reactions, such as palpitations, severe headache, or apparent allergic reaction to any product excipient.
Transdermal Nicotine Patch (NicoDerm CQ, generic)
The transdermal nicotine patch is the only other FDA-approved OTC nicotine replacement product for use in adults aged 18 years and older. Unlike the lozenge and gum, the patch delivers nicotine transdermally at a continuous rate and reaches a peak plasma level more slowly than cigarettes, lozenge, or gum.30-32 Plasma concentrations vary by manufacturer because nicotine release is inherently dependent on the patch matrix.32 Patients may find transdermal nicotine appealing because of the once-daily administration and steady release of nicotine. The patch has also been studied in conjunction with other forms of NRT, and the results benefit patients who are struggling to quit with single product therapy. More consideration will be given to combination therapies later in this learning activity.
The patch is supplied by a number of manufacturers in 21 mg/day, 14 mg/day, and 7 mg/day nicotine release systems—also referred to as step 1, 2, and 3 patch strengths. The initial transdermal system dosage should be chosen based on the number of cigarettes that the patient smokes per day. Patients who smoke 10 or more cigarettes daily should start with step 1 patches, and those who smoke fewer than 10 cigarettes per day should start with step 2 patches. Directions for use indicate that patients should remain on the initial patch strength for the first 6 weeks, and then 2 weeks on each successive patch.30
Patients should place transdermal patches on nonhairy, clean, dry skin on the upper body or upper outer arm immediately after removing the backing. The patch should then be pressed onto the skin for 10 seconds to ensure proper adherence; each patch should be left on for 16 to 24 hours. The patch is waterproof and should not fall off when bathing or swimming. After applying or removing the patch, patients should wash hands their hands. When placing a new patch, patients should rotate application sites, using the original pouch for patch disposal. The patch should never be cut as this will encourage nicotine evaporation and make the patch less effective. Additional guidelines suggest patients should use the patch for 24 hours if they normally wake with cigarette cravings. However, if the nicotine results in sleep disturbances or vivid dreams, patients should remove the patch before bed and place a new patch upon waking.30
If the patch causes skin irritation, the patient may want to try a different manufacturer's product before switching NRT formulation. Manufacturers use different product excipients. Other adverse reactions unique to the nicotine patch include erythema, pruritus, or burning at the application site.
Nicotine Inhaler (Nicotrol)
The nicotine inhaler is 1 of 2 prescription-only NRTs. The inhaler device is pen-shaped, and its use provides patients with a hand-to-mouth ritual they may find helpful in smoking cessation. The nicotine inhaler also contains menthol, and therefore should not be used in patients with menthol allergies.
Unlike asthma inhalers or cigarettes, where drug absorption occurs within the lung, the nicotine inhaler delivers drug to the oral mucosa. Inhalers are available in a single strength: 10 mg of nicotine per cartridge. Of the 10 mg contained in the cartridge, 4 mg is delivered and 2 mg is absorbed in the mouth.33 With proper technique, peak nicotine plasma concentrations reach 6 to 8 ng/mL (approximately one-third peak levels of a cigarette) after 15 to 20 minutes.33
The recommended method of use is to puff frequently on the inhaler, which will deliver nicotine for about 20 minutes. As previously discussed with other NRT products, patients should use the inhaler regularly at the beginning of treatment to mitigate withdrawal symptoms. The product insert recommends using at least 6 cartridges per day for the first 3 to 6 weeks of treatment (usually 1 cartridge every 1 to 2 hours while awake), without exceeding 16 cartridges daily for the first 12 weeks. The daily dose can be adjusted to optimize withdrawal symptom management while avoiding symptoms of nicotine overdose. After 12 weeks at an optimized dose, a gradual reduction in daily dose should be attempted over a period of up to another 12 weeks. The manufacturer does not provide a titration scheme, instead recommending that health professionals encourage patients to track daily use and set a quit date to best plan a gradual taper.33
Unlike other nicotine replacement therapies, the inhaler's product labeling includes a disclaimer that states if a patient is unable to stop smoking by week 4 of inhaler use, he or she should consider discontinuing treatment. Common adverse reactions unique to this deliver system include local irritation in the mouth and throat, coughing, and rhinitis, which generally decline with frequency with use.33 Patients should be advised to contact their medical providers if symptoms become intolerable or severe.
Nicotine Nasal Spray (Nicotrol NS)
The prescription-only nicotine nasal spray delivers more rapid and elevated nicotine plasma concentrations than other available NRTs. In clinical studies, average peak plasma concentrations ranged from 2 ng/mL to 12 ng/mL over 4 to 15 minutes.34 Nicotine nasal spray's enhanced bioavailability may be particularly helpful to patients who experienced strong cravings and/or were not successful with previous NRTs. Although the only contraindication noted by the manufacturer is allergy to product excipients, patients who may want to avoid this product include those with nasal conditions, such as sinusitis, allergic rhinitis, and nasal polyps.
Patients should be advised to spray the drug into the nasal cavity with their head tilted back slightly, and to breathe normally. One dose of the nasal spray is equivalent to 1spray per nostril, for a total delivery of 1 mg of nicotine. Only about 50% of a dose is absorbed. Patients should use at least 8 doses per day the first week. Otherwise, the recommended number of doses per hour is 1 to 2, with a maximum of 5 doses per hour and 40 doses per day (which equates to 80 sprays or roughly one-half bottle).34 Patients should be advised to continue at their initial dose for 8 weeks, and then slowly titrate the daily dose down over 4 to 6 weeks until they have discontinued use. No safety data supports using the nasal spray for periods longer than 6 months. Tapering tips provided by the manufacturer include using 1 spray (instead of 2) per dose, skipping every other dose, and setting a quit date with a personalized, pre-planned taper.34
Adverse reactions unique to nicotine nasal spray include local irritation to the nasal cavity. This reaction was described as moderate to severe in 94% of users in clinical trials and 81% still noted nasal irritation after 3 weeks.34 Additional common adverse reactions include runny nose, watering eyes, sneezing, throat irritation, and coughing. Less common adverse reactions include nasal congestion, altered taste and scent perception, epistaxis, eye irritation, and facial flushing.34 The adverse reaction profile likely contributes to the rare use of the nasal spray for smoking cessation.
Varenicline (Chantix)
Varenicline is a prescription-only, non-NRT medication that is FDA-approved to assist with smoking cessation. Like the NRT formulations it is intended to be used with counseling and education. Varenicline binds to the same receptors in the brain as nicotine (nicotinic acetylcholine receptors), but is a less potent agonist and therefore does not elicit the same response.35 Once varenicline is bound to these receptors, it cannot be displaced by nicotine. Varenicline also has moderate affinity for a subtype of serotonin receptor (5-HT3) that is of unknown significance.35
Patients are explicitly permitted to smoke for the first week while using varenicline, and some may prefer this option over NRTs because of convenient once-daily dosing. Varenicline smoking cessation rates appear to be dose dependent.36 A study by Nakamura et al. noted cessation improvements trend positively with dose when assessed during weeks 9 through 12 of treatment and up to 24 weeks following treatment .36 When compared with placebo, this translates to a roughly 3-fold improvement in quit rate for the 2 mg per day dose, and a 2-fold improvement for the 1 mg/day dose.14
Varenicline may not be the ideal first-line agent for smoking cessation for patients with mood disorders that are not well managed. This medication has a boxed warning for serious neuropsychiatric events, including depression and suicidal ideation, and its risk evaluation and mitigation strategy requires pharmacists to provide a medication guide to patients each time they dispense the drug.37 Worsening of pre-existing mood disorders or new psychiatric symptoms should be monitored carefully. Precautions must be taken when considering varenicline use in pregnant or lactating women as safety data in these populations are limited. Animal studies have associated varenicline with decreased fetal weight when administered in concentrations higher than human exposure.35 Varenicline is excreted in breast milk with unknown effect.35
Varenicline therapy should be initiated 1 week before the patient's predetermined stop smoking date. The recommended dose titration of varenicline follows35:
- Days 1 through 3, 0.5 mg daily
- Days 4 through 7, 0.5 mg twice daily
- Days 8 through 12 weeks, 1 mg twice daily
If a patient has successfully stopped smoking at week 12, an additional 12 weeks at the patient's maintenance dose is recommended to encourage long-term abstinence.35 Dose reductions may be necessary if the patient cannot tolerate the medication at the recommended dose. In patients whose estimated creatinine clearance is less than 30 mL/min, the maximum recommended dose is 0.5 mg twice daily.35 The dose does not need to be adjusted for liver dysfunction.
The most common adverse reaction associated with varenicline in clinical studies was nausea (30% of the treatment group and 10% in the placebo group).35 To decrease nausea and stomach upset, patients can take varenicline with food and a full glass of water. Additional adverse reactions that occurred in more than 5% of study participants include abnormal dreams, insomnia, constipation, and vomiting.35 Examples of rare adverse reactions (described as occurring in less than 1% of users) include rash, diarrhea, hiccup, abnormal vision, and peripheral edema.35
Sustained-Release Bupropion (Zyban)
Bupropion is an antidepressant that increases dopamine and norepinephrine concentrations in the neuronal synapse. It is marketed under 2 brand names: Zyban (for smoking cessation) and Wellbutrin (antidepressant). They are the same generic drug entity and come in a variety of release forms. Generic bupropion for smoking cessation is a sustained-release version intended to be dosed twice daily; it was the formulation used in the original clinical trials that ultimately lead to the drug's approval for smoking cessation. Patients do not need to be experiencing depressive symptoms for this medication to be effective.
A recent Cochrane review examined the efficacy of antidepressants used in smoking cessation trials that compared the drug with a placebo or a control that did not include pharmacotherapy, and follow up at a minimum of 6 months after antidepressant initiation (the majority of studies included cessation rates at 12 months).38 When assessing bupropion's efficacy as reported in 44 trials, the RR was 1.62 (95% CI 1.49–1.76).38 Studies that assessed efficacy at different doses (150 and 300 mg daily) were pooled and cessation rates were not significantly different between these 2 doses at 12 months.38
Patients may find this medication appealing if they are interested in treating comorbid depressive symptoms, prefer a tablet dosage form, or are particularly worried about smoking cessation resulting in weight gain or loss of concentration. Weight loss is a common adverse reaction with bupropion,39 and this medication is used off-label for attention-deficit/hyperactivity disorder.
Like varenicline, bupropion has a boxed warning describing potentially increased risk of serious neuropsychiatric adverse reactions (similar to all antidepressants).39 Special consideration before use should be given to patients who are experiencing significant anxiety, as bupropion can cause or worsen anxiety (especially early in treatment), and those with uncontrolled hypertension, as this medication may increase blood pressure.39 The potential for worsening anxiety may be lessened by a slower titration to the target dose than generally recommended (as described below). Bupropion should not be used in patients with a history of seizures, anorexia, or bulimia.39
Patients who are prescribed bupropion for smoking cessation should be advised to set a smoking quit date that is approximately 1 to 2 weeks after the initiation of therapy.39 Unless there is a reason for a lower daily dose or slower titration, the patient is advised to start at 150 mg once daily for 3 days, then 150 mg twice daily for 7 to 12 weeks.39 Because bupropion may cause insomnia, the second dose should be spaced at least 8 hours after the first dose, but not at bedtime (dosing with breakfast and dinner may help adherence and encourage proper medication timing). Continuing therapy past 12 weeks may be beneficial for some patients after discussion with their health professionals. When patients are ready to stop bupropion therapy, tapering is unnecessary but may be beneficial in patients with comorbid anxiety. Dosing adjustments may be required for older patients and those with renal or hepatic dysfunction (Table 2), and dose titration should be slowed in these patients.
Compared with varenicline, bupropion has an increased potential for drug interactions related to its extensive metabolism by CYP450 enzymes. Bupropion is also a strong inhibitor of CYP2D6, which is responsible for metabolizing many medications. As a result, pharmacists should screen each patient's medication profile to reduce the possibility of drug–drug interactions.
The most common adverse reactions associated with bupropion include tachycardia, agitation, dizziness, anxiety, headache, insomnia, diaphoresis, weight loss, constipation, nausea, xerostomia, and tremor.39 Among the above adverse reactions, insomnia and xerostomia are particularly common among those using bupropion for smoking cessation.
CHANGES IN NRT PRODUCT LABELING AND COMBINATION THERAPY
In 2013–after considering several studies that supported the safe and effective use of NRT therapies outside of original labeling–FDA recommended several seemingly subtle but highly important changes to NRT drug labeling.40 These changes focused on 3 central areas:
- Extending the length of NRT product use
- Using NRT products while still smoking
- Combining NRT dosage forms
Several studies examined the efficacy and safety of extending NRT use. These demonstrated that extending the length of therapy is safe and may improve cessation maintenance.14, 41 This research is reflected in product labeling changes by changing the hard stop date (variably by product) to a recommendation that encourages completion of treatment course followed by speaking with a health professional before continuing NRT use.40
Regarding continued cigarette use in conjunction with NRT therapy, FDA recommended removing language that states patients should not use NRT therapy while smoking.40 This practice was discouraged originally because of a concern about nicotine toxicity, but this has not been supported in clinical trials investigating this combination. Recent research shows that smoking while using NRTs is actually well tolerated.41
In a review of the evidence supporting FDA recommendations, Fucito et al.41 acknowledged that the body of research studying cigarette use concomitantly with NRT products has been somewhat contradictory with respect to quit rates, but that overall the effect of combination therapy is positive (particularly when considering precessation patch use) and may be more effective than abruptly stopping cigarette use prior to NRT initiation. One of the largest studies supporting the use of prequit NRT use found that starting nicotine patches 2 weeks before a selected cessation date resulted in significantly better quit rates 6 months after treatment initiation than abrupt smoking cessation followed by patch use.42 If this strategy is preferred, patients should be encouraged to decrease the number of cigarettes they smoke when beginning an NRT therapy, and then taper off so they are only using the NRT as a source of nicotine by their selected cessation date.
The last change approved by FDA concerned NRT combination therapy. To date, NRT combination therapies have largely focused on using a short-acting and long-acting formulation concurrently, such as the transdermal patch in combination with gum, nasal spray, lozenge, or inhaler, and have found them to be safe and effective.14 Similar to strategies used for pain control, the patch provides baseline nicotine while the short-acting product is used for sudden cravings. One large, randomized controlled trial investigated the use of the NRT patch and nasal spray together, and found that the combination was more effective than either therapy alone in achieving cessation at 6 weeks.43 All combination studies assessed as part of the FDA label update included patients who smoked 10 or more cigarettes daily.41 The product labeling was changed to reflect this evidence by deleting the statement stating patients should not use NRT products in combination with other NRT agents or tobacco-containing products.40
Based on the average cigarette use of 10 or more cigarettes daily in these combination studies, it may be most appropriate to attempt these regimens in patients who are heavier smokers, or those who have previously failed single NRT therapy cessation attempts. Furthermore, other medication combinations have been studied, but as they do not involve multiple NRTs they are not discussed in detail here. Examples of combinations that have shown potential in clinical trials include bupropion with varenicline44 and varenicline in combination with NRT products.45 Additionally, bupropion used with the nicotine patch is an FDA-approved combination for treatment of smoking cessation.14
Pharmacists who are aware of NRT label changes and the research supporting the variable smoking cessation medication combinations can help patients find their best regimens. For example, patients who do not feel ready to stop smoking abruptly may benefit from tapering off cigarettes while on a nicotine patch, and those who smoke more than 10 cigarettes per day may find it easier to quit using both the patch and the lozenge.
ELECTRONIC NICOTINE DELIVERY SYSTEMS
E-cigarettes are cigarette-shaped, nicotine-inhalation devices that are generally designed and marketed to look and feel like traditional tobacco-based cigarettes. E-cigarettes use disposable cartridges or refillable fluid cases that contain flavoring agents and nicotine (although some options are nicotine-free). Some manufacturers market them as safer alternatives to tobacco-based cigarettes. However, they are not regulated by FDA as an OTC agent for smoking cessation.
Although e-cigarettes contain fewer toxins than cigarettes, studies have identified several chemicals in several brands' vaporizing liquid, including formaldehyde and cyclohexane, that should not be inhaled.46 Early research has demonstrated that e-cigarettes may have utility as a smoking cessation agent.47 However, because of the lack of FDA regulation and the inability to guarantee safety of the vaporizing liquid content, it would be more prudent to direct patients toward FDA-approved smoking cessation products before recommending cessation attempts using e-cigarettes.
CONCLUSION
Smoking continues to be a leading cause of preventable death worldwide. Pharmacists are highly accessible health professionals with extensive knowledge. As such, they are ideally suited to provide smoking cessation counseling and education and to assist with NRT selection. Multiple studies that weighed health care expenditure against intervention cost have demonstrated pharmacists deliver smoking cessation services effectively. Their interventions save health systems money.12
Considering FDA's 2015 changes to NRT product labeling, pharmacists have more options than ever to help their patients select optimal NRT therapy. Pharmacists are also located in the community where many NRT options are available without a prescription. Ultimately, all pharmacists involved in patient care should be comfortable providing smoking cessation services to decrease the preventable morbidity and mortality associated with cigarette use.
REFERENCES
- Centers for Disease Control and Prevention. Current cigarette smoking among adults—United States, 2005–2013. MMWR Morb Mortal Wkly Rep. 2014;63(47):1108–1112.
- Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2001–2015. MMWR Morb Mortal Wkly Rep. 2011;60(44):1513–1519.
- National Institute of Drug Abuse. Drugs, brains, and behavior: the science of addiction. http://www.drugabuse.gov/publications/drugs-brains-behavior-science-addiction/drugs-brain. Accessed November 29, 2016.
- Mao D, Gallagher K, McGehee DS. Nicotine potentiation of excitatory inputs to ventral tegmental area dopamine neurons. J Neurosci. 2011;31(18):6710-6720.
- U.S. Department of Health and Human Services. The health consequences of smoking—50 years of progress: a report of the surgeon general. 2014. http://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/index.htm. Accessed November 29, 2016.
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res [Internet]. 2007;9(3):315–327.
- U.S. Department of Health and Human Services. The health consequences of smoking: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2004.
- U.S. Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General–executive summary. Rockville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2006.
- Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341-350.
- American Lung Association. Smoking cessation: the economic benefits. http://www.lung.org/our-initiatives/tobacco/cessation-and-prevention/smoking-cessation-economic-benefits.html. Accessed November 29, 2016.
- Saba M, Diep J, Saini B, Dhippayom T. Meta-analysis of the effectiveness of smoking cessation interventions in community pharmacy. J Clin Pharm Ther. 2014;39(3):240-247.
- Manolakis PG, Skelton JB. Pharmacists' contributions to primary care in the United States collaborating to address unmet patient care needs: the emerging role for pharmacists to address the shortage of primary care providers. Am J Pharm Educ. 2010;74(10):S7.
- Healthy People 2020. Tobacco use. https://www.cdc.gov/nchs/data/hpdata2020/HP2020MCR-C41-TU.pdf Accessed December 28, 2018.
- Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. U.S. Department of Health and Human Services, Public Health Service. http://www.ncbi.nlm.nih.gov/books/NBK63952/. Accessed November 29, 2016.
- McBane SE, Corelli RL, Albano CB, et al. The role of academic pharmacy in tobacco cessation and control. Am J Pharm Educ. 2013;77(5):93.
- American Congress of Obstetricians and Gynecologists. The first method: the 5 A's—for those ready to quit. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Smoking-Cessation-During-Pregnancy. Accessed December 28, 2018.
- Kadakia SC, De La Baume HR, Shaffer RT. Effects of transdermal nicotine on lower esophageal sphincter and esophageal motility. Dig Dis Sci. 1996;41(11):2130-2134.
- Wisborg K, Henriksen TB, Jespersen LB, et al. Nicotine patches for pregnant smokers: a randomized controlled study. Obstet Gynecol. 2000;96(6):967–971.
- Kapur B, Hackman R, Selby P, et al. Randomized, double-blind, placebo-controlled trial of nicotine replacement therapy in pregnancy. Curr Ther Res Clin Exp. 2001;62:274–278.
- Pollak KI, Oncken CA, Lipkus IM, et al. Nicotine replacement and behavioral therapy for smoking cessation in pregnancy. Am J Prev Med. 2007;33(4):297-305.
- Coleman T, Chamberlain C, Davey M-A, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane database Syst Rev. 201512.
- Zevin S, Benowitz NL. Drug interactions with tobacco smoking. an update. Clin Pharmacokinet. 1999;36(6):425-438.
- Kroon LA. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64(18):1917–1921.
- American Cancer Society. Choosing and using nicotine replacement therapy. 2014. http://www.cancer.org/healthy/stayawayfromtobacco/guidetoquittingsmoking/guide-to-quitting-smoking-choosing-and-using-nrt. Accessed November 29, 2016.
- Le Houezec J. Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: a review. Int J Tuberc Lung Dis. 2003;7(9):811-819.
- Benowitz NL, Porchet H, Sheiner L, Jacob P. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther. 1988;44(1):23-28.
- Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane database Syst Rev. 2012;11.
- Nicotine Polacrilex (Nicotine resin complex). In: Drug Facts and Comparisons. St. Louis, MO: Wolters Kluwer Health. Updated Apr 2014. Accessed November 29, 2016.
- Choi JH, Dresler CM, Norton MR, Strahs KR. Pharmacokinetics of a nicotine polacrilex lozenge. Nicotine Tob Res. 2003;5(5):635-644.
- Nicotine transdermal system. In: Drug Facts and Comparisons. St. Louis, MO: Wolters Kluwer Health. Updated Apr 201. Accessed November 29, 2016.
- Fant RV, Henningfield JE, Shiffman S, Strahs KR, Reitberg DP. A pharmacokinetic crossover study to compare the absorption characteristics of 3 transdermal nicotine patches. Pharmacol Biochem Behav. 2000;67(3):479-482.
- Gupta SK, Okerholm RA, Eller M, Wei G, Rolf CN, Gorsline J. Comparison of the pharmacokinetics of two nicotine transdermal systems: Nicoderm and Habitrol. J Clin Pharmacol. 1995;35(5):493-498.
- Nicotrol [product labeling]. New York, NY: Pfizer; 2008.
- Nicotrol NS [product labeling]. New York, NY: Pfizer; 2010.
- Chantix [product labeling]. New York, NY: Pfizer; 2014.
- Nakamura M, Oshima A, Fujimoto Y, Maruyama N, Ishibashi T, Reeves KR. Efficacy and tolerability of varenicline, an alpha-4, beta-2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clin Ther. 2007;29(6):1040-1056.
- Chantix Risk Evaluation and Mitigation Strategy (REMS). https://www.fda.gov/Drugs/DrugSafety/REMS/ucm592677.htm Accessed December 28, 2018.
- Hughes JR, Stead LF, Hartmann-Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane database Syst Rev. 2014;1.
- Zyban [product labeling]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
- Modifications to labeling of nicotine replacement therapy products for over-the-counter human use. https://www.federalregister.gov/articles/2013/04/02/2013-07528/modifications-to-labeling-of-nicotine-replacement-therapy-products-for-over-the-counter-human-use. Accessed November 29, 2016.
- Fucito LM, Bars MP, Forray A, et al. Addressing the evidence for FDA nicotine replacement therapy label changes: a policy statement of the Association for the Treatment of Tobacco use and Dependence and the Society for Research on Nicotine and Tobacco. Nicotine Tob Res. 2014;16(7):909-914.
- Rose JE, Herskovic JE, Behm FM, Westman EC. Precessation treatment with nicotine patch significantly increases abstinence rates relative to conventional treatment. Nicotine Tob Res. 2009;11(9):1067-1075.
- Croghan GA, Sloan JA, Croghan IT, et al. Comparison of nicotine patch alone versus nicotine nasal spray alone versus a combination for treating smokers: a minimal intervention, randomized multicenter trial in a nonspecialized setting. Nicotine Tob Res. 2003;5(2):181-187.
- Rose JE, Behm FM. Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm. Am J Psychiatry. 2014;171(11):1199-1205.
- Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA. 2014;312(2):155-161.
- Varlet V, Farsalinos K, Augsburger M, Thomas A, Etter J-F. Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health. 2015;12(5):4796-4815.
- Adriaens K, Van Gucht D, Declerck P, Baeyens F. Effectiveness of the electronic cigarette: an eight-week flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220-11248.
Back to Top