ADVERTISEMENT

Anemia: An Overlooked Aspect of Heart Failure Management
OVERVIEW OF HEART FAILURE IN AMERICA
Approximately 5.1 million people in the United States (U.S.) have heart failure (HF) and the prevalence continues to rise, with more than 650,000 new cases of HF diagnosed each year.1 Prognosis is poor, with approximately 50% of patients dying within 5 years of HF diagnosis. HF causes approximately 1 million hospitalizations annually (mean cost $23,077/admission), and, after being hospitalized, approximately 25% of patients are readmitted within 1 month. More than half of the $30 billion dollars spent on HF care in the U.S. every year is spent on hospitalizations.1
Approximately half of HF patients have HF with reduced ejection fraction (HFrEF).1 In these patients, the triple regimen of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) with or without sacubitril, a beta blocker, and either an aldosterone receptor antagonist (in patients of non-African descent) or hydralazine plus isosorbide (in patients of African descent) provides significant reductions in mortality and hospitalizations.1,2 These therapies make up the backbone of pharmacologic management of HFrEF. The pharmacologic management of HF with preserved ejection fraction (HFpEF) in not as progressive: blood pressure control to prevent disease progression and the use of diuretics for symptomatic relief are universally recommended. No therapy has been shown to decrease mortality in HFpEF, and the only therapy purported to reduce hospitalizations is treatment with an ARB, but the magnitude of benefit is small and the strength of evidence is weak.1,2
ANEMIA AND IRON DEFICIENCY IN HF
Anemia is a common finding in patients with chronic HF and the prevalence of anemia among patients with HF increases with HF severity.1 Between 25% and 40% of patients with HFrEF and HFpEF have anemia, which is defined as a hemoglobin (Hb) concentration less than 13 g/dL in men and less than 12 g/dL in women.1,3
Iron deficiency can be defined as having either a serum ferritin of less than 100 μg/L or a serum ferritin of 100 to 300 μg/L along with a percentage of transferrin saturation (serum iron divided by transferrin level multiplied by 100) of less than 20%.3,4 According to this definition, approximately 60% of HF patients with anemia and 40% of HF patients without anemia have iron deficiency.3,4
Causes and consequences of anemia and iron deficiency in HF
The analytic framework linking HF with the occurrence of anemia and/or iron deficiency is illustrated in Figure 1.3,5-7 Iron deficiency can precipitate anemia of chronic disease through a mechanism involving interleukin (IL) activation. IL-6 releases hepcidin from the liver, which subsequently impedes the functioning of ferroportin.3,5-7 Ferroportin inhibition suppresses iron absorption from the gastrointestinal tract and prevents iron release from storage sites in macrophages and hepatocytes.7 Since hepcidin undergoes glomerular filtration, concomitant renal failure can lead to further increases in its concentration compared to increases in HF alone. Approximately half of patients with HF also have renal insufficiency, as noted by a creatinine clearance of less than 60 mL/min/m2.7
Figure 1. Analytic Framework Linking Heart Failure to Anemia and Iron Deficiency3,5-7
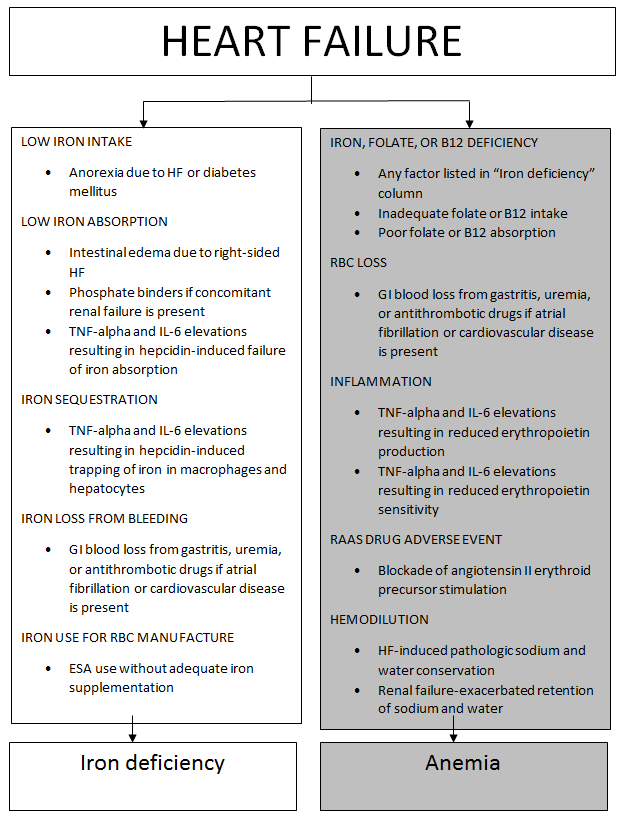
Abbreviations: ESA = erythropoiesis-stimulating agent; GI = gastrointestinal;
HF = heart failure; IL = interleukin; RAAS = renin-angiotensin-aldosterone system;
RBC = red blood cell; TNF = tumor necrosis factor.
Anemia compromises the delivery of oxygen to tissues.3 In observational studies, patients with HF and anemia have been shown to have worse quality of life (QOL) and functional capacity and more frequent hospitalizations than patients with HF but without anemia.8 In a large observational study of 150,000 patients, the mortality risk was approximately doubled in HF patients with anemia compared to those without anemia, and this risk persisted after controlling for other confounders, including renal dysfunction and HF severity.1
Anemia is not the only consequence of iron deficiency: many enzymes depend on iron for proper functioning, including oxidative metabolism in mitochondria and cytochrome P450 oxidative metabolism.3,5-7 Iron in myoglobin is involved in oxygen storage in striated muscle, and iron is involved in the synthesis and degradation of proteins, lipids, and (deoxy)ribonucleic acids. Without adequate iron, all cells will experience disruptions in normal functioning, ranging from inefficient respiration and cellular construction to improperly functioning enzymes.3,5-7 In animal models, iron deficiency results in the release of oxygen free radicals and the disruption of sarcomeres, as well as left ventricular hypertrophy and mitochondrial swelling.3 Approximately two-thirds of patients with HF have concomitant coronary artery disease, so the thrombocytosis and increased platelet aggregation associated with iron deficiency in patients with chronic kidney disease and pulmonary hypertension might also enhance the risk of acute coronary syndromes and strokes in patients with HF.3 In a pooled observational analysis of 1,506 patients with HFrEF or HFpEF, iron deficiency was significantly related to mortality (hazard ratio [HR] 1.42, 95% confidence interval [CI] 1.30 - 2.33) and impaired health-related QOL (p = 0.016).7
PREVENTION OF ANEMIA AND IRON DEFICIENCY IN PATIENTS WITH HF: ERYTHROPOIESIS- STIMULATING AGENTS AND IRON SUPPLEMENTATION
Small clinical trials suggest that erythropoiesis-stimulating agents (ESAs) might improve functional capacity in patients with HF and anemia.8 The best understanding of the role of ESAs in HF comes from the Reduction of Events by Darbepoetin-alfa in Heart Failure (RED-HF) trial: RED-HF was a randomized, double-blind, placebo-controlled trial of 2278 patients with New York Heart Association (NYHA) class II to IV HFrEF and mild to moderate anemia (Hb 9 - 12 g/dL).8 Patients (41% women; 65% NYHA class III or IV) had a median age of 72 years, an average ejection fraction of 31%, and an average glomerular filtration rate of 46 mL/min/m2. Patients had an average transferrin saturation of 24% and an average Hb concentration of 11 g/dL at baseline. Darbepoetin-alfa was dosed to achieve a target Hb of 13 g/dL. The median Hb achieved over 60 months was 13 g/dL in the darbepoetin-alfa group (median dose of 167 μg given once per month) and 11.5 g/dL in the placebo group. Serum transferrin was assessed every 3 months and iron supplementation was indicated if the concentration fell below 20%. Anemia treatments, other than darbepoetin-alfa, were used in some patients in both the treatment group and the placebo group: intravenous (IV) iron (4.9% and 5.6%, respectively; p = 0.42), oral iron (72.3% and 73.5%, respectively; p = 0.52), and blood transfusions (10.9% and 16.5%, respectively; p < 0.001). There were no differences between the darbepoetin-alfa group and the placebo group in the composite primary endpoint of death from any cause or hospitalization for worsening HF (50.7% vs. 49.5%, respectively; p = 0.87) or for the 2 endpoints evaluated separately (HR 1.04, 95% CI 0.92 - 1.19; and HR 0.99, 95% CI 0.85 - 1.16, respectively). The risk of embolic and thrombotic events was significantly higher in the darbepoetin-alfa group than in the placebo group (13.5% vs. 10.0%, p = 0.009), including a significant increase in ischemic strokes (4.5% vs. 2.8%, p = 0.03) and 3 concerning trends in the risks of unstable angina (HR 1.49, 95% CI 0.72 - 3.009), any stroke (HR 1.33, 95% CI 0.83 - 2.12), and pulmonary embolism (HR 3.20, 95% CI 0.64 - 15.90). Darbepoetin-alfa did improve QOL as evidenced by changes in the Kansas City Cardiomyopathy Questionnaire Score compared to placebo (mean difference 2.2, 95% CI 0.65 - 3.75).8
According to transferrin saturation percentage, the patients in the trial were in the normal therapeutic range (15% - 50% for men and 12% - 45% for women) at baseline and received iron supplementation if the transferrin saturation decreased to below 20%.8 However, the supplementation was with oral iron sulfate 325 mg 3 times daily and iron indices were not evaluated for 3 months after supplementation began. If the decreased transferrin saturation percentage remained unresolved or worsened, ascorbic acid was administered to enhance iron absorption and other causes of iron deficiency were explored. Only then could IV iron be initiated. As such, the increases in embolic and thrombotic vascular events associated with darbepoetin-alfa might be due to enhanced platelet aggregation in the face of absolute or relative iron deficiency.8
The occurrence of these negative vascular effects is consistent with similar events in 2 major clinical trials in patients without HF.9 In the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) trial, patients with chronic kidney disease received erythropoietin targeted to achieve an Hb concentration of 13.5 g/dL or 11.3 g/dL. This trial was stopped early because the group with the higher Hb level also had a higher composite endpoint of death, myocardial infarction, stroke, and HF hospitalization. In the Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT), patients randomized to receive darbepoetin-alfa to achieve an Hb of 13 g/dL had double the risk of stroke compared with patients receiving placebo.9 Considering these findings together, the U.S. Food and Drug Administration mandated the addition of a boxed warning on ESA labelling information. ESAs should be reserved until the Hb is less than 10 g/dL and the lowest dose required to prevent blood transfusions should be used.9
Table 1 summarizes the controlled trials assessing the use of iron without ESAs in patients with HF who reported HF endpoints.10-16 The impact of iron supplementation on the treatment of HF in patients with iron deficiency was explored in a meta-analysis from 2016. The analysis included all of the trials listed in Table 1 except for the Effect of Ferric Carboxymaltose on Exercise Capacity in Patients with Iron Deficiency and Chronic Heart Failure (EFFECT-HF) trial and the results from Toblli 2015, which were not yet available; the analysis also included an additional underpowered trial (not included in Table 1) that only reported survival.10-16 The meta-analysis included 851 patients with HFrEF and most patients had NYHA class II or III disease. All patients had iron deficiency, diagnosed by ferritin and transferrin saturation indices, and most patients had anemia, diagnosed by Hb less than 12.5 g/dL. Only IV iron preparations, including iron sucrose and ferric carboxymaltose, were used, with total iron doses between 1000 and 2000 mg. Follow-up was conducted for 18 or 24 weeks for most of the studies. There were fewer HF hospitalizations (odds ratio [OR] 0.28, 95% CI 0.16 - 0.50) and greater improvements in the 6-minute walking distance test (median improvement 30.8 m, 95% CI 18.2 - 43.4 m) and NYHA classification (median change -0.54, 95% CI -0.87 - -0.21) in the IV iron-treated patients than in patients in the control group.17
| Table 1. Outcomes Studies Assessing the Use of Iron Supplementation in Heart Failure (HF) 10-16 |
| Study author (name), year, n |
Design and baseline iron indices |
HF type and severity |
Active treatment |
Follow-up |
Significantly improved outcomes or indices |
| Iron sucrose |
Toblli, 2007 and 2015, n = 60
(40 of 60 patients already represented in Toblli 2007) |
R, DB, PC;
Hb < 12.5 g/dL (men),
Hb < 11.5 g/dlL (women); ferritin < 100 ng/mL and/or transferrin saturation < 20%;
CrCl < 90 mL/min |
HFrEF,
NYHA II - IV |
Iron sucrose 200 mg/week for 5 weeks |
6 months |
Quality of life
NYHA class
Exercise capacity |
| Okonko (FERRIC-HF), 2008, n = 35 |
R, OL, PC;
Hb < 12.5 g/dL (men); ferritin < 100 ng/mL and/or transferrin saturation < 20% |
HFrEF,
NYHA II - III |
Iron sucrose |
18 weeks |
Quality of life
NYHA class |
| Ferric carboxymaltose |
| Anker (FAIR-HF), 2009, n = 459 |
R, DB, PC;
ferritin < 100 μg/L or 100 - 299 μg/mL if transferrin saturation < 20%; patients with and without anemia |
HFrEF,
NYHA II - III |
Ferric carboxymaltose 200 mg/week |
24 weeks |
Patient global assessment
NYHA class
Exercise capacity |
| Ponikowski (CONFIRM-HF), 2014, n = 304 |
R, DB, PC;
Hb < 15 g/dL; ferritin < 100 μg/L or 100 - 299 μg/mL if transferrin saturation < 20% |
HFrEF,
NYHA II - III |
Ferric carboxymaltose 500 - 2000 mg (weeks 0 and 6) in therapy phase and 500 mg iron (weeks 12, 24, and 36) in maintenance phase |
52 weeks |
Quality of life
NYHA class
Exercise capacity |
Van Veldhuisen
(EFFECT-HF), 2016,
n = 174 |
R, OL, C;
Hb < 15 g/dL; ferritin < 100 μg/L or 100 - 300 μg/mL if transferrin saturation < 20% |
HFrEF,
NYHA II - III |
Ferric carboxymaltose, total dose administered 1,204 mg (weeks 0, 6, and 12) |
24 weeks |
Patient global assessment
NYHA class
VO2 max |
Abbreviations: AHA = American Heart Association; C = controlled; CrCl = creatinine clearance; DB = double blind; Hb = hemoglobin concentration; HFrEF = heart failure with reduced ejection fraction; NYHA = New York Heart Association; OL = open label; PC = placebo controlled; R = randomized; VO2 max = maximum amount of oxygen that an individual can utilize during intense exercise.
Note: Serum ferritin levels can be measured in micrograms per liter (µg/L) or nanograms per milliliter (ng/mL). The units presented in the table are the units originally used in the study. |
These results are similar to an earlier meta-analysis from 2012; the analysis did not include results of the Cardiac Compass with Optival to Negate Future Inpatient Re-admissions Through Monitoring in HF Patients (CONFIRM-HF) trial but it did analyze other endpoints of importance.18 Iron supplementation improved health-related QOL (median change -2.66, 95% CI -5.40 - 0.07); fewer cardiac adverse events (relative risk [RR] 0.45, 95% CI 0.32 - 0.63) and no increase in neurological adverse events (RR 0.78, 95% CI 0.47 - 1.31) were observed. In this earlier meta-analysis, 290 of the 594 patients had anemia—defined as an Hb concentration less than 12.5 g/dL. In patients without anemia, the Hb concentrations were unchanged (median change 0.13, 95% CI -0.14 - 0.39) with IV iron supplementation, but there was a trend towards improved Hb concentrations in patients with anemia (median change 1.35, 95% CI -0.09 - 2.78). All patients had significant improvements in iron indices.18
In the aforementioned meta-analyses, 459 of the patients came from the Ferrinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure (FAIR-HF) trial.13 Among the patients receiving ferric carboxymaltose, significant improvements in global assessments of disease severity were achieved (OR 2.51, 95% CI 1.75 - 3.61), as well as improvements of at least 1 NYHA class (OR 2.40, 95% CI 1.55 - 3.71) and improvements in 6-minute walking distance (313 ± 7 m vs. 277 ± 10 m, p < 0.001). Importantly, no differences in death (3.4 vs. 5.5 events/100 patient-years, p = 0.47) or hospitalizations (17.7 vs. 24.8 events/100 patient-years, p = 0.30) occurred between the treatment and placebo groups. There were significant differences in the risks of cardiac events (27.6 vs. 50.2 events/100 patient-years, p = 0.01) and gastrointestinal events (16.9 vs. 6.9 events/100 patient years, p = 0.06). Results were similar in patients with and without anemia.13
In a randomized, double-blind, placebo-controlled trial by Toblli et al in 2015 (an extension of Toblli’s 2007 trial that was included in the meta-analysis), patients received IV iron sucrose 200 mg/mL weekly for 5 weeks.10 At 6 months after treatment initiation, IV iron was associated with reduced severity of the symptoms of chronic HF and improved renal function (both p < 0.001 vs. control). Left ventricular systolic and diastolic diameters increased, and improved left ventricular function correlated with iron status in patients receiving IV iron but not in patients in the control group.10 In an important contrast, erythropoietin-alfa was given to 22 HF patients in another trial and did not improve ejection fraction compared to placebo (p = 0.91) or any other index of left ventricular structure or function, even though Hb concentrations improved (p < 0.0001).19 At the time this module was published, the EFFECT-HF trial was only available in abstract form, and no information from the study, other than what is presented in Table 1, is available.15,16
One clinical trial has been conducted to assess outcomes with oral iron supplementation.15 The Oral Iron Repletion Effects on Oxygen Uptake in Heart Failure (IRONOUT-HF) trial also assessed VO2 max, but no difference was found (0.3 mL/kg/min improvement, p = 0.30) when oral iron polysaccharide (150 mg twice daily) was compared to placebo. This finding is qualitatively much lower than the results seen in EFFECT-HF when ferric carboxymaltose was compared to control treatment (1.04 mL/kg/min).15 This is likely because of poor correction of iron deficiency, since the ferritin concentration only rose by 11 ng/mL and the transferrin saturation only increased by 3%; more specific information is not available. This seems reasonable, since oral iron products only have approximately 10% bioavailability in normal individuals and HF patients experience a reduction of as much as 50% in carrier-mediated intestinal absorption capacity.7
There is also a body of literature assessing concomitant iron and ESA therapy. An observational study of 30 HF patients with both anemia (Hb < 12 g/dL for men and < 11.5 g/dL for women) and iron deficiency assessed the impact of IV iron sucrose 300 mg weekly or the same iron sucrose dose plus darbepoetin- alfa 50 μg weekly for 6 weeks on Hb concentration.20 No baseline difference in serum ferritin was noted (121.1 ± 114.2 ng/mL vs. 139.8 ± 163.9 ng/mL, p = 0.523). Significant increases in Hb concentrations occurred over the 6 weeks in the IV iron group (10.6 ± 0.9 g/dL to 12.8 ± 1.4 g/dL, p < 0.05) and in the combination therapy group (10.9 ± 1.1 g/dL to 12.6 ± 0.8 g/dL, p < 0.01) with no discernible differences between them.20 In another observational study, the impact of IV iron alone in improving anemia in patients with HF and renal dysfunction (cardiorenal syndrome) was explored.21 Of the 81 patients in the study, 34 received IV iron (iron sucrose 200 mg/week) and 47 received both IV iron (same drug and dose) and an ESA (epoetin-beta 10,000 units/week) for 6 weeks. The Hb levels increased in the iron group (10.6 ± 1.1 g/dL to 11.9 ± 1.1 g/dL, p < 0.002) and in the combination therapy group (10.2 ± 0.9 g/dL to 12.4 ± 1.3 g/dL, p = 0.001), but the net ferritin increase from baseline was significantly greater in patients receiving IV iron alone (308 ± 219 ng/mL vs. 161 ± 133 ng/mL, p = 0.002). This suggests that iron is cleared faster with concomitant ESA use without dramatically different results in Hb improvements. Interestingly, the use of iron supplementation alone significantly reduced the platelet count (225 ± 69 x 109 cells/L to 199 ± 51 x 109 cells/L, p = 0.002), while combination therapy did not impact platelet count (219 ± 63 x 109 cells/L to 216 ± 54 x 109 cells/L, p = 0.540). Whether this portends less vascular risk with IV iron alone compared to combination therapy in HF patients is unclear.21 However, in people without HF but with iron deficiency due to inflammatory bowel disease, thrombocytosis can occur and increase the thromboembolic risk.22 In a randomized, single-blind, placebo-controlled trial in patients with inflammatory bowel disease and abnormally high platelet counts (> 500,000 cells/μL), the use of IV ferric carboxymaltose (up to 1,500 mg total repletion) reduced platelet counts; no change in platelet count was noted in the placebo group (p < 0.01).22
Implementing an iron supplementation strategy
For best results, iron supplementation needs to be administered intravenously, which creates logistical challenges for health systems. Only 2 IV products with evidence-based regimens have been studied in HF patients.10-18 In order to begin using IV iron in HF, the appropriate drug(s) need to be available on a formulary and a specific protocol needs to be created so that:
- Proper patients can be identified
- Administration regimens have an appropriate balance of benefit and harm
- Acute monitoring is provided to ensure patient safety is maximized and risks are mitigated
- Chronic monitoring is provided to ensure that benefits are achieved and chronic adverse effects are detected and mitigated appropriately
There are numerous options available for IV iron supplementation in patients without HF, so why should a health system utilize a drug specifically studied in clinical trials? Using evidence-based products provides the best level of confidence that the cost of the product will yield the results seen in clinical trials for the health systems’ patients.7
An algorithm for determining when iron deficiency exists and choosing an evidence-based IV iron regimen is presented in Figure 2.7,10-18 The 3 IV iron regimens each have advantages and disadvantages: regimens 1 and 3 use weekly dosing, which can be inconvenient, and regimen 2, which is derived from the CONFIRM-HF trial, is more convenient, but it requires initial dosing above the maximum single recommended dose of 750 mg provided in the package insert.7,10-18 Table 2 and Figure 3 provide critical features and comparisons between ferric carboxymaltose and iron sucrose that health systems need to understand.23,24
Figure 2. Assessment for Iron Deficiency and Evidence-based Treatments in Patients with HFrEF and HFpEF7,10-18
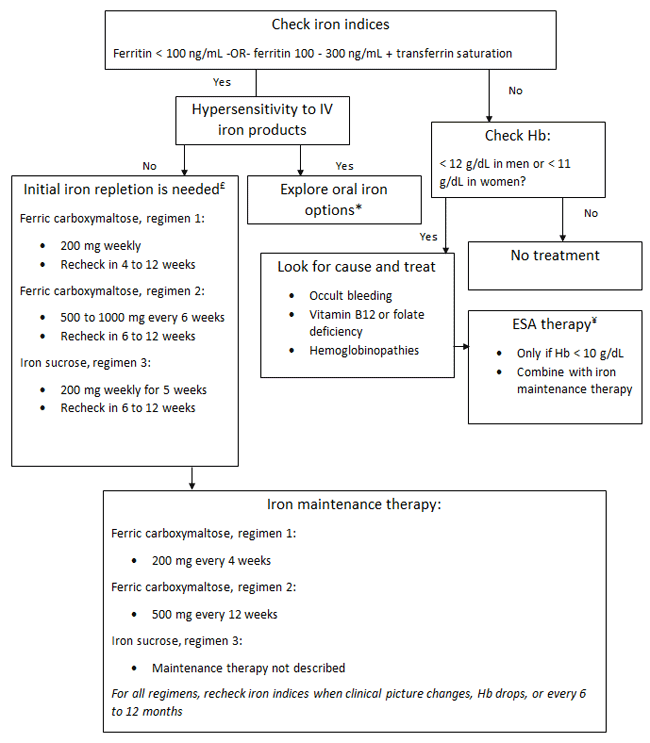
Abbreviations: ESA = erythropoietin-stimulating agent; Hb = hemoglobin concentration; IV = intravenous.
*Oral iron supplementation has not been well studied; prolonged therapy is likely needed,
and its efficacy might not match that of IV therapy.
£Other IV iron options may also replete iron, but dosing has not been studied in the heart failure population.
¥ESAs have not been shown to enhance the efficacy of iron supplementation in heart failure patients with iron deficiency,
but ESAs are indicated to prevent significant anemia in patients without iron deficiency, regardless of etiology.
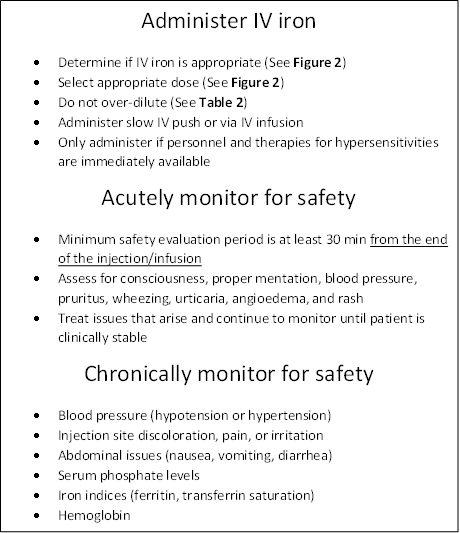
Figure 3. Administration and Monitoring Algorithm for Intravenous (IV) Iron23,24
| Table 2. Comparison of Intravenous (IV) Iron Repletion Products 7,10-18,23,24 |
| |
Ferric carboxymaltose |
Iron sucrose |
| IV push administration speed |
Undiluted at 100 mg/min |
Undiluted at 100 mg over 2 to 5 min |
| IV infusion administration speed |
Dilute in 0.9% sodium chloride to 2 - 4 mg/mL and infuse over at least 15 min |
Dilute in 0.9% sodium chloride to 1 mg/mL and infuse over at least 15 min |
| Dose alterations in geriatric patients |
None |
None |
| Post-administration monitoring |
Evaluate for at least 30 min for hypersensitivity and hypertension. Treat issues that arise and continue monitoring until clinically stable. |
Evaluate for at least 30 min for hypersensitivity and hypotension. Treat issues that arise and continue monitoring until clinically stable. |
| Contraindications |
Hypersensitivity |
Hypersensitivity |
| Pregnancy category |
C |
B |
| Most common adverse events |
Nausea
Vomiting
Dizziness
Hypertension
Injection site discoloration
Headache
Skin flushing
Pruritis
Blood phosphorous decrease |
Diarrhea
Vomiting
Dizziness
Hypotension
Injection site discoloration
Headache
Nasopharyngitis/sinusitis/URTI/cough
Pruritis
Chest pain |
| Hypersensitivity adverse events |
Hypotension
Shock
Unconsciousness
Collapse |
Hypotension
Shock
Unconsciousness
Collapse |
| Time to laboratory testing |
Do not monitor serum iron for 24 h after dosing (overestimates actual levels) |
Do not monitor serum iron for 48 h after dosing (overestimate actual levels) |
Abbreviations: URTI = upper respiratory tract infection.
Note: Bolded text indicates differences between the IV iron products. |
The information provided in Figures 2 and 3 and Table 2 present important considerations and information that should be included in a health system protocol for iron supplementation in HF patients.7,10-18,23,24 These iron products should not be overdiluted or administered too rapidly, and the correct personnel and treatments need to be acutely available should hypersensitivity reactions or alterations in blood pressure occur.23,24 Patients need to be monitored long enough to preclude the occurrence of acute adverse events; they can then leave the health system and follow-up can be conducted periodically to monitor for efficacy and adverse events and to administer subsequent doses. This is the best way to maximize efficacy and minimize adverse events that can arise from IV iron therapy.23,24
ROLE OF THE PHARMACIST IN IRON SUPPLEMENTATION
Pharmacists in all practice settings can use the information in this module to enhance patient care. Community pharmacists interact with HF patients and, through patient counseling and health discussions, can alert patients that iron deficiency can result from chronic disease and can worsen their quality of life and physical functioning while simultaneously increasing their risks of hospitalization. This knowledge can help patients engage in discussions with clinicians and health care providers about iron status and the role of IV iron in treating iron deficiencies and anemia. Health system pharmacists can assess IV iron products for formulary review and design protocols for their safe use. They can also conduct drug use evaluations to ensure that important treatment and safety principles are being followed. Clinical pharmacists in hospitals and HF clinics can screen patients to see if iron therapy is appropriate. Iron supplementation may be especially important in patients who are hospitalized for an HF exacerbation. In this setting, the 30-day readmission risk is high and those readmissions are subject to public reporting and financial disincentives. Pharmacists in all settings can counsel patients about the benefits of iron therapy and the need for proper compliance in order to derive those benefits, as well as alert patients about possible adverse events. Finally, pharmacists can help ensure that proper monitoring is taking place.
Case example
ED is a 68-year-old male with HFrEF for 6 years who is complaining of fatigue despite optimized triple therapy with an ACE inhibitor, beta-blocker, and aldosterone antagonist. The community pharmacist discusses the possibility that iron deficiency could be a cause of his symptoms and that he should ask his physician about his iron levels. Upon testing, he has a transferrin level of 75 ng/mL and an Hb concentration of 11 g/dL. He has no past hypersensitivity to iron products. The cardiologist refers to the pharmacist-driven protocol and determines that, since his Hb is above 10 g/dL, he does not need ESA therapy but that he should receive 200 mg of ferric carboxymaltose weekly.10-18 ED receives the first dose, undiluted, administered by IV push over 2 minutes in the HF clinic, which has a crash cart available.23,24 ED experiences no adverse effects, but he is kept in the clinic for observation by the nurse for 30 minutes. He is scheduled to return for 3 more weekly injections before he will have his iron and Hb levels rechecked. After 4 injections, his ferritin is 200 ng/mL, transferrin saturation is 23%, and Hb is 12 g/dL. He no longer needs repletion, but he is scheduled to return in 4 weeks to receive a 200-mg maintenance injection, to recheck iron indices, and to check for chronic adverse events.10-18,23,24 He reports that he feels better and that he has more energy now. He is referred to the clinical pharmacist in the clinic for counseling about the importance of taking prescribed HF medications and continuing to receive his IV iron as long as it is indicated. He admits that he was considering stopping his beta-blocker now that he is feeling better, but, after discussing the mortality benefits associated with that drug and how its benefits exceed those of other therapeutic options, he agrees to take all his medications as prescribed.
CONCLUSIONS
In patients with HFrEF, correcting iron deficiency with IV iron sucrose or ferric carboxymaltose can improve physical functioning and QOL while reducing the risk of hospitalization.10-19 These benefits are achieved without an increase in vascular events. Oral iron products have not been shown to provide these benefits and, unless the Hb concentration is below 10 g/dL, there is no role for adjunctive ESAs.8,9 ESAs used with iron supplementation can improve Hb concentrations in patients with Hb concentrations of approximately 10 to 12 g/dL, but it may also increase the risk of vascular events, making iron supplementation alone a superior option.8,9 Like those with HFrEF, patients with HFpEF may benefit from IV iron supplementation, but this hypothesis needs to be proven in clinical trials.17,18
IV iron supplementation can predispose some patients to acute adverse events, but this can be minimized by monitoring patients for at least 30 minutes after the drug is administered, checking the iron indices at the appropriate times to see if repletion has occurred, not over-diluting the product and administering it by slow IV push or infusion using normal saline, and by assessing and treating chronic adverse events that arise.23,24 Pharmacists have an important role in triaging patients who might benefit from iron therapy. Through protocol generation and implementation, drug use evaluation and monitoring, and clinical activities, pharmacists can stress the need for compliance and help ensure that the benefits of iron therapy are maximized while adverse events are minimized.
REFERENCES
- Yancy CW, Jessup M, Bozkurt B, et al; and American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128(16):e240-327.
- Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure. Circulation. 2016;134(13):e282-293.
- Silverberg DS, Wexler D, Schwartz D. Is correction of iron deficiency a new addition to the treatment of the heart failure? Int J Mol Sci. 2015;16(6):14056-14074.
- Belmar Vega L, de Francisco A, Albines Fiestas Z, et al. Investigation of iron deficiency in patients with congestive heart failure: a medical practice that requires greater attention. Nefrologia. 2016;36(3):249-254.
- Nakagawa Y, Yasuno S, Kuwahara K. Differential relationships between anemia and outcomes in subgroups of patients with chronic heart failure. Circ J. 2015;79(9):1893-1894.
- Ishani A, Weinhandl E, Zhao Z, et al. Angiotensin-converting enzyme inhibitor as a risk factor for the development of anemia, and the impact of incident anemia on mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2005;45(3):391-399.
- McDonagh T, Macdougall IC. Iron therapy for the treatment of iron deficiency in chronic heart failure: intravenous or oral? Eur J Heart Fail. 2015;17(3):248-262.
- Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013;368(13):1210-1219.
- Volpe M, Santolamazza C, Mastromarino V. Erythropoiesis-stimulating agents in heart failure: leave it or retake it? Eur J Heart Fail. 2015;17(11):1089-1090.
- Toblli JE, Di Gennaro F, Rivas C. Changes in echocardiographic parameters in iron deficiency patients with heart failure and chronic kidney disease treated with intravenous iron. Heart Lung Circ. 2015;24(70):686-695.
- Toblli JE, Lombraña A, Duarte P, Di Gennaro F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50(17):1657-1665.
- Okonko DO, Grzeslo A, Witkowski T, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51(2):103-112.
- Anker SD, Comin Colet J, Filippatos G, et al; and FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361(25):2436-2448.
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al; and CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36(11):657-668.
- Wendling P. Iron supplementation in HF: trials support IV but not oral. http://www.medscape.com/viewarticle/872088. Published November 18, 2016. Accessed December 22, 2016.
- Van Veldhuisen DJ. Effect of ferric carboxymaltose on exercise capacity in patients with iron deficiency and chronic heart failure (EFFECT-HF) [abstract]. Presented at the American Heart Association Annual Conference; November 2016; New Orleans, LA.
- Jankowska EA, Tkaczyszyn M, Suchocki T, et al. Effects of intravenous iron therapy in iron-deficient patients with systolic heart failure: a meta-analysis of randomized controlled trials. Eur J Heart Fail. 2016;18(7):786-795.
- Avni T, Leibovici L, Gafter-Gvili A. Iron supplementation for the treatment of chronic heart failure and iron deficiency: systematic review and meta-analysis. Eur J Heart Fail. 2012;14(4):423-429.
- Green P, Babu BA, Teruya S, et al. Impact of epoetin alfa on left ventricular structure, function, and pressure volume relations as assessed by cardiac magnetic resonance – the heart failure preserved ejection fraction (HFPEF) anemia trial. Congest Heart Fail. 2013;19(4):172-179.
- Terrovitis JV, Kaldara E, Ntalianis A, et al. Intravenous iron alone is equally effective with the combination of iron and erythropoietin for the treatment of iron-deficiency anemia in advanced heart failure. J Am Coll Cardiol. 2012;60(21):2255-2256.
- Ben-Assa E, Shacham Y, Shashar M, et al. Target hemoglobin may be achieved with intravenous iron alone in anemic patients with cardiorenal syndrome: an observational study. Cardiorenal Med. 2015;5(4):246-253.
- Toblli JE, Angerosa M. Optimizing iron delivery in the management of anemia: patient considerations and the role of ferric carboxymaltose. Drug Des Devel Ther. 2014;8:2475-2491.
- Infectafer [prescribing information]. Shirley, NY: American Regent, Inc.; 2013.
- Venofer [prescribing information]. Shirley, NY: American Regent, Inc.; 2014.
Back to Top