ADVERTISEMENT

Immunoglobulin G: A Primary Strategy in Secondary Immunodeficiency
Introduction
The challenges of treating people with immunodeficiencies reached most people’s awareness in 1971
through the experiences of David Vetter, “the boy in the bubble” who was born with severe combined
immune deficiency (SCID). Because of his immediate risk of death from infection caused by even low
levels of microbes, David lived in a sterile cocoon environment during the several years he stayed at
Texas Childrens Hospital in Houston. Eventually able to live at home but still inside a bubble, David died after a bone marrow transplant at age 12 resulted in his acquiring Epstein–Barr virus and developing
fatal lymphoma.
Today, a variety of immunoglobulin G (IgG) products are available and beneficial in patients with both
primary immunodeficiencies like the one David had and the more common secondary immune deficiencies that place people at risk of infections. This article will highlight the use of IgG products in secondary immunodeficiencies (SIDs), detail how these products can best be used based on patient comorbidities, and introduce contemporary concepts about intravenous (IV) and subcutaneous (SC) routes of administration.
Types of Immunodeficiencies
The human immune system is complex. Some components provide innate immunity in which the body reacts nonspecifically and immediately to pathogens through processes such as phagocytosis (chemotaxis, migration, ingestion, and microbial killing). The stronger and more specific components of the immune system provide adaptive immunity. On a second or subsequent exposure of an antigen through antigen-presenting cells, the body mounts an antibody-mediated immune response involving memory B cells and many other immune cytokines, other factors, and cells.1
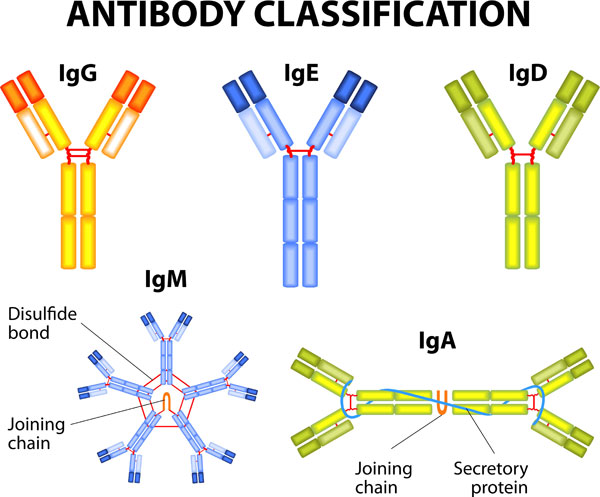
Antibodies (Figure 1) are immunoglobulins (Ig), and the light chain types are categorized as IgG, IgM, IgA, IgD, and IgE (Figure 2).2 Of these types, IgG makes up about 80% of total body immunoglobulins and thus is considered the most important contributor to the specific immune response to previously encountered antigens. IgG antibodies are also involved in immunomodulation, increasing the immune response to foreign antigens and suppressing the body’s reactions to its own proteins.3
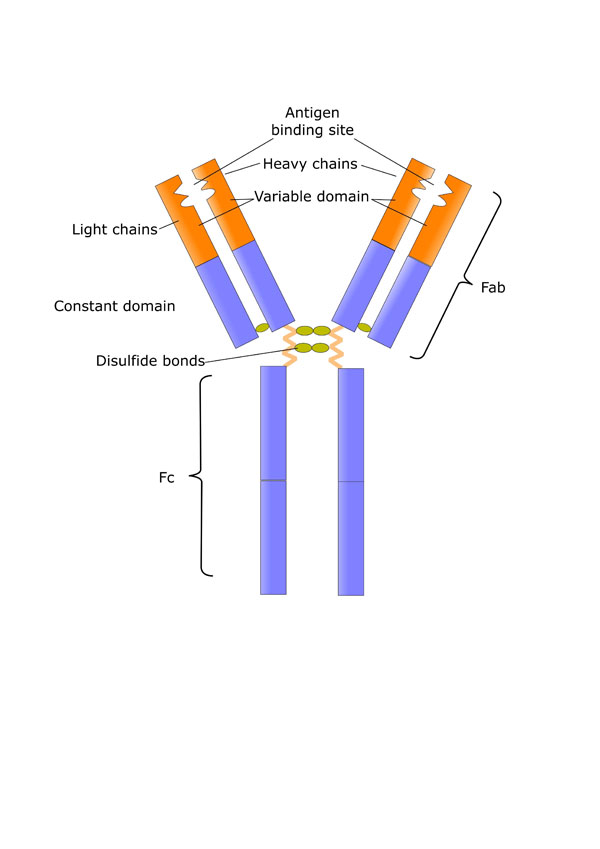 |
Functions of the two Fab arms can include a wide variety of immunomodulatory effects, such as modulation of apoptosis and cell cycle; antiproliferative effects; effects on cell adhesion and cytokine levels; and importantly, antibodies to pathogens, superantigens, and immunoregulatory molecules (e.g., cytokines, T-cell receptors, CD4, and CD5).
Fc region functions can include inhibition of phagocytosis, deposition of activated complement, and antibody-dependent cellular cytotoxicity; effects on glucocorticoid receptor binding affinity and dendritic cell maturation; and blockade of access of immune complexes to Fc receptors.
|
|
Figure 1. Structure of immunoglobulins.
The various parts of the immunoglobulin serve different immunomodulatory functions. Commercially
available immunoglobulin products also contain other components, including cytokines, cytokine
receptors, CD4, MHC Class II and stabilizing agents, and sugars.
Source: Shutterstock and Reference 2.
|
 |
|
Figure 2. Classifications of human antibodies.
Source: Shutterstock.
|
Primary immunodeficiencies (PIDs) comprise disorders in which intrinsic defects in the human immune system prevent a person from mounting an immunologic response to microorganisms and other foreign matter. These genetic conditions result from an inability to produce components of the immune system, such as antibodies or B-cells (quantity defects), or deficiencies in function of antibodies, cells, phagocytic cells, complement, or other immunologic components (quality defects).
Classifications of Antibodies
Examples of PIDs illustrate the potential defects in the body’s systems. In common variable immune deficiency (CVID), B cells are present to produce antibodies, but both the amount and quality of IgG produced is low. In X-linked or autosomal recessive agammaglobulinemia, B-cell production by the bone marrow is low, and IgG quantity and quality are very low. In people with deficiencies of specific antibodies, IgG quantity and B-cell counts are normal, but IgG quality is low for specific antigens.4
Secondary immunodeficiencies (SIDs; serum IgG concentrations <5 g/L) can be caused by infectious, malignant, or iatrogenic insults to an innately functional immune system. These can result in general or specific antibody deficiencies, depending on the cause. There is a relationship between serum IgG trough levels and the risk for infection; one evaluation found a 5-fold increased rate of pneumonia in patients with levels below 5 g/L compared with those whose levels were 10 g/L or higher.5 British and American guidelines state that serum IgG levels below 5 g/L in combination with infection should warrant IgG replacement therapy.6,7
Epidemiology and Etiology
Despite the extent and nature of the effects that immunodeficiencies can have on people’s lives, a
surprising number of cases are not diagnosed. About 10% of people with immunodeficiencies have the
primary condition. While there are more than 300 different disorders described as PIDs, most people
have either common variable, severe combined, or specific antibody deficiencies.
A 2007 Immune Deficiency Foundation survey of 10,000 U.S. households showed a prevalence of PID of
83 per 100,000 people. An estimated 250,000 people have been diagnosed with PID in the United
States, but more than 500,000 people are not diagnosed.8 Some 37% of patients with PID reported impairment as a result of infections prior to diagnosis, 23% reported permanent loss of lung function prior to diagnosis, and 11% reported loss of hearing.9
Secondary immunodeficiencies can result from a host of other conditions or infections (Table 1).10–12 They are much more common than PIDs — on the order of 10-fold higher — but prevalence can change based on the prevalence of cancers that affect the immune system, the emergence of pathogens such as the human immunodeficiency virus (HIV) and associated conditions such as the acquired immunodeficiency syndrome (AIDS), and the use of immunosuppressant medications. Normally when
the production of antibodies is low, the end result is chronic respiratory infections such as otitis media, bronchitis, and pneumonia, or gastrointestinal infections resulting in chronic diarrhea. Other sequelae include autoimmune disorders, allergies, skin conditions, and even meningitis.
| Table 1. General and Specific Causes of Secondary Immunodeficiencies |
| Oncologic conditions |
Chronic lymphocytic leukemia
Mulitple myeloma
Hodgkin lymphoma
Non-Hodgkin B-cell lymphoma
Good’s syndrome (thymoma with hypogammaglobulinemia) |
| Hematologic conditions/causes |
Aplastic anemia
Graft-versus-host disease
Sickle cell disease
Splenectomy |
| Nutritional and metabolic conditions |
Malnutrition
Alcoholism
Diabetes mellitus |
| Infectious diseases |
Viruses (e.g., human immunodeficiency virus, Epstein–Barr virus, cytomegalovirus, measles virus, varicella zoster virus)
Bacteria (including rare bacterial infections with superantigens, including Staphylococcus aureus)
Mycobacteria |
| Gastrointestinal disorders |
Hepatic insufficiency
Hepatitis
Intestinal lymphangiectasia
Protein-losing enteropathy |
| Renal disorders |
Nephrotic syndrome
Renal insufficiency
Uremia |
| Other diseases/disorders |
Systemic lupus erythematosus
Burns
Chromosomal abnormalities
Congenital asplenia
Histiocytosis
Sarcoidosis |
| Medications |
|
| Anticonvulsants |
Carbamazepine, lamotrigine, phenytoin, valproate |
| Immunosuppressants |
Azathioprine, cyclosporine, gold salts, muromonab, mycophenolate mofetil, sirolimus, tacrolimus |
| Corticosteroids |
Methylprednisolone, prednisone |
| Chemotherapeutic agents |
Alemtuzumab, busulfan, cyclophosphamide, melphalan |
| Tyrosine kinase inhibitors |
Imitinib, dasatinib |
| Biologic disease-modifying antirheumatic agents |
Adalimumab, etanercept, infliximab, rituximab, tocilizumab |
| Others (rare) |
Ramipril, aspirin |
| Sources: References 10–12. |
Malignancy-Related Causes
Hematologic malignancies and certain agents used to treat various oncologic and autoimmune disorders
are common causes of SID (Table 1).10–12 Some insults to the immune system are specific and can be countered with specific treatments, as with a low neutrophil count that is improved by colony-stimulating factors. Cancers or cytotoxic drugs that kill B lymphocytes or produce
hypogammaglobulinemia are central to this discussion of IgG and immunoglobulin therapies; some of
these cytotoxic drugs are also used for immune-mediated disorders such as rheumatoid arthritis and
multiple sclerosis (e.g., rituximab, alemtuzumab).
A condition very relevant to the use of immunoglobulin replacement therapy is chronic lymphocytic
leukemia (CLL). The most common cause of death in patients with CLL is infection and infective
complications. The disease itself produces immune defects, with hypogammaglobulinemia in up to 70%
of patients with CLL, and chemotherapy exacerbates the problem. The damage to the immune system is
irreversible in most patients, including many of those who achieve complete disease remission. Low
levels of IgG are directly proportional to the frequency and severity of infections.12
Another oncologic condition in which infections are the primary cause of death is multiple myeloma. As
with CLL, effects of the disease and chemotherapy are involved in the increased susceptibility to
infections. Susceptibility is greatest in the early stages of the disease and during the first months of
induction chemotherapy; early infections involve the respiratory tract, including bronchitis and
pneumonia. Involved pathogens are most frequently Haemophilus influenzae and Streptococcus
pneumoniae, suggesting hypogammaglobulinemia as a contributing etiologic factor.12
Nonmalignancy-Related Causes
The immunosuppression needed in patients undergoing bone-marrow or solid-organ transplantation
presents unique challenges in infection prevention and control.
Often used for hematologic malignancies, allogeneic bone-marrow transplantation can produce
persistent immune defects. Hypogammaglobulinemia is a common feature of this clinical scenario,
creating a need for long-term replacement immunoglobulins regardless of the success of treatment for
the underlying malignancy.12
The HIV makes its hosts susceptible to a variety of opportunistic infections, but its actions are on T
rather than B cells. Infection causes hypergammaglobulinemia but also a defect involving a failure to
mount specific antibody responses to T-cell-dependent antigens. Effects differ between children and
adults, resulting in different pathogens creating infections in the two groups. Recurrent bacterial
infections are more common in children, while opportunistic infections and cancers tend to occur in
infected adults.12
Other secondary causes of immunodeficiency with hypogammaglobulinemia are shown in Table 1.10–12These include diseases with Ig loss such as burns, renal, and intestinal diseases, and Good’s syndrome (thymoma, carcinoma cells on the outside of the thymus—the gland that produces lymphocytes).12
Drug-Induced Secondary Immunodeficiency
Medications are a common cause of iatrogenic immunodeficiency. As listed in Table 1, these include agents used for treatment of oncologic disorders but also therapies for rheumatoid arthritis, other conditions with autoimmune features, and anticonvulsants.10–12 For some patients, discontinuation of the offending drug may be sufficient to correct the SID; for others, damage to the immune system could be permanent and require lifelong support for prevention or treatment of infections.
Rituximab in particular has been identified as routine agent used in both combination chemotherapy
and nonmalignant therapies that may lead to profound immune depression. The agent binds to the CD-20 antigen of normal and malignant B cells and pre-B cells, initiating a cascade that reduces the number
and function of B cells, which can lead to hypogammaglobulinemia. In addition, neutropenia occurs
more frequently, adding to the risk of infection. These effects are especially problematic when rituximab
is used for maintenance therapy of oncologic or autoimmune diseases, and in patients who receive
several courses of therapy; the risk of symptomatic hypogammaglobulinemia has been noteworthy with
rituximab, occurring in 6.6% of patients with lymphoma in one report who were treated with the
monoclonal antibody.13,14
Immune Globulins
Originally developed in the 1970s and marketed in the 1980s to meet the needs of patients such as
David, the “bubble boy,” intravenous immunoglobulins (IVIG) have been refined pharmaceutically and
studied extensively in the intervening years. The products approved by FDA in the 1980s for patients
with PID would today not be considered acceptable. Considerable cleavage of the Fc moiety occurred
during the manufacturing process, and contaminating viruses – including hepatitis C – were recognized
and removed in the 1990s. In 2002, thrombosis was recognized as an adverse effect of IVIG use.15
Mechanisms of Action
Pharmaceutical products containing IgG have complex effects when administered as replacement
therapy to patients with PIDs and SIDs or as immunotherapy in other conditions. Derived from sera of thousands of patients, the products contain antibodies against many diseases and have immense
immunologic power of potential benefit to recipients with hypogammaglobulinemia, regardless of
cause. These effects and secondary indications of the products derive from the structure of the IgG
molecule as depicted in Figure 1.2
While a given unit of an IgG product represents the antibodies produced by many different people, the
biologic origin also means there is substantial batch-to-batch variation in specific antibody content. This variation is most important in the F(ab')2 moiety, as the two “arms” of the IgG molecule carry the specific segments that recognize pathogens and superpathogens. Several IgG actions result from presence of intact and specific F(ab')2 moieties: antiproliferative effects, modulation of apoptosis (cell death) and cell cycle, activation of specific cells, and effects on cell adhesion.2
IgG products also contain natural antibodies that are hard-coded into the human genome. Multiparous
women contain many more anti-idiotype human HLA antigens; these components may be responsible
for the efficacy of IgG products in patients with autoimmune diseases.2
The Fc moiety, while less specific for pathogens than the F(ab')2 arms, is important in the general responses of the immune system to diseases. The general response happens first and protects the body while it reacts to previously encountered antigens by producing specific antibodies. As shown in Figure 1, the Fc fragment has numerous effects on the immunologic response to bacteria and fungi.2
IgG products also contain numerous other substances. These include soluble cytokine inhibitors, soluble CD4, and major histocompatibility complex (MHC) class II. Maltose, sucrose, and other sugars present in the products as stabilizing agents can have immunologic effects.2
Use in Secondary Immunodeficiencies
In the 1980s, HIV was identified as the etiologic agent in the most severe type of SID identified to date, AIDS. Patients died of a multitude of infections as their immune system was compromised by this
retrovirus. IVIG was found to be effective for prophylaxis against other infections in children with HIV.1In addition, high-dose IVIG therapy (1–2 g/kg over 2–5 days) was found to produce increased platelet counts in patients with idiopathic thrombocytopenic purpura (ITP) associated with HIV infection. Researchers also recognized that IVIG therapy could benefit patients living with HIV who developed severe parvovirus B19 or measles infection and in those with autoimmune disorders, in whom high-dose IVIG therapy was efficacious. Products were also developed with high quantities of Ig directed against specific viruses such as hepatitis B or CMV.16
The currently available IVIG products are licensed by FDA as replacement therapy in patients with PID,
adjunctive treatments of patients with certain infections, and/or a limited number of SIDs (Table 2). Medical researchers and clinicians have found the products useful in more than 100 other SIDs, as listed in Table 3. Patients with these conditions have a high risk for infection, and administration of IgG products has been shown to reduce this risk in a wide variety of situations, from specific diseases (e.g., diabetes, uremia, cirrhosis, malnutrition, and disorders of protein loss) to treatments with immunosuppressive drugs, chemotherapy, or radiation.
| Table 2. FDA-Approved Intravenous Immunoglobulin Products |
| Products |
Approved Indications |
| Bivigam (Biotest) |
Primary humoral immunodeficiency |
| Carimune NF, nanofiltered (CSL Behring) |
PID |
| Flebogamma DIF 5% and 10% (Instituto Grifols) |
5%: PID (inherited) in adults and children 2 years of age or older
10%: PID (inherited) and ITP in patients 2 years of age or older |
| Gammagard Liquid (Baxter) |
Primary humoral immunodeficiency in adults and children aged 2 years or older; maintenance therapy in adults with multifocal motor neuropathy |
| Gammagard S/D (Baxter) |
PID in adults and pediatric patients 2 years of age or older; prevention of bacterial infections in hypogammaglobulinemia and/or recurrent bacterial infections associated with B-cell chronic lymphocytic leukemia; prevention and/or control of bleeding in adult chronic ITP; prevention of coronary artery aneurysms associated with Kawasaki syndrome in pediatric patients |
| Gammaplex 5% and 10% (Bio Products) |
5%: primary humoral immunodeficiency in adults and children 2 years of age or older; ITP
10%: primary humoral immunodeficiency in adults; ITP |
| Gamunex-C (Grifols) |
Primary humoral immunodeficiency, ITP, and chronic inflammatory demyelinating polyneuropathy |
| Octagam (Octapharma) |
5% product approved for PID; 10% product approved for ITP in adults |
| Privigen (CSL Behring) |
Primary humoral immunodeficiency, ITP in patients 15 years or older, and chronic inflammatory demyelinating polyneuropathy in adults |
| Abbreviations used: Idiopathic thrombocytopenia purpura (ITP); primary immunodeficiencies, PID. |
| Table 3. Off-Label Uses of Intravenous Immunoglobulins |
| First-Line Uses: literature-based evidence, controlled trials, or significant case-based evidence |
Second-Line Uses: Limited literature-based evidence in open-label trials and case series |
Third-Line Uses: Small trials or case reports; IVIG may be considered when all standard therapies have failed |
Fourth-Line Uses: literature does not support use |
| Guillain-Barré syndrome |
Mucocutaneous blistering diseases
• Pemphigus vulgaris
• Pemphigus foliaceus
Mucous membrane pemphigoid (cicatrical pemphigoid)
• Epidermolysis bullosa acquisita |
Parvovirus-associated disease |
West Nile virus |
| Panautonomic polyneuropathy |
Myasthenia gravis syndrome |
Reactive macrophage activation syndromes |
Infectious disease treatment* |
| Miller Fisher syndrome |
Lambert Eaton syndrome |
Hemophagocytic lymphohistiocytosis |
Chronic fatigue of unknown cause |
| Cross-match-positive solid organ transplantation |
Multiple sclerosis |
Acute humoral rejection with solid organ transplantation |
Congenital heart block |
| Multiple myeloma |
Inflammatory myopathies |
Hypereosinophilia syndrome |
Cystic fibrosis |
| Hemolytic disease of the newborn |
Dermatomyositis |
Still’s disease |
Spontaneous abortion of unknown cause |
| |
Polymyositis |
Severe asthma |
Congestive heart failure |
| |
Idiopathic progressive polyneuropathy |
Cystic fibrosis |
Thrombotic thrombocytopenic purpura |
| |
Intractable epilepsy |
Nephrotic/nephritic syndrome |
Diabetes mellitus |
| |
Acute cardiomyopathy |
Von Willebrands disease |
Acute cerebellar ataxia |
| |
Pure red cell aplasia |
Spontaneous abortion with antiphospholipid antibodies |
|
| |
Hemolytic anemia |
Systemic lupus erythematosus |
|
| |
Refractory platelet transfusions |
Evans syndrome |
|
| |
Fetal autoimmune thrombocytopenia |
Blackfan diamond anemia |
|
| |
Stiff person syndrome |
Acquired factor VIII Inhibitors |
|
| |
Human immunodeficiency virus–associated thrombocytopenia |
Rheumatoid arthritis |
|
| |
|
Inclusion body myositis |
|
| |
|
Staphylococcal toxic shock syndrome |
|
| |
|
Invasive group A streptococcal fasciitis and septicemia |
|
| |
|
Infectious disease prophylaxis** |
|
| |
|
Sjogren syndrome |
|
| |
|
Landau–Kleffner syndrome |
|
| |
|
Ocular pemphigoid |
|
| |
|
Birdshot retinochoroidopathy |
|
| |
|
Toxic epidermal necrolysis (Lyell syndrome) |
|
| |
|
Stevens–Johnson syndrome |
|
* In patients with trauma, high-risk neonates, and those with burns.
** In high-risk neonates, recipients of solid-organ transplants, and those with high-risk human immunodeficiency virus infections. |
The burden of SID in these patients is considerable. Up to 85% of patients with CLL can develop SID, and death from subsequent infections is estimated to account for up to 50% of deaths related to CLL and
22% of deaths related to multiple myeloma.12,17
None of the commercially available IVIG products is approved for all indications, and the products differ considerably in their methods of production. Because of these differences, IVIG products should not be considered commodities that can interchanged freely, as clinicians need to match products with patients based on indications, other evidence of efficacy and safety for specific conditions, and comorbidities; pharmacists must consider these important differences in selecting the optimal products to make available in health systems and managed care organizations.15,18
When selecting an IgG product for treatment, both the product characteristics and the patient
comorbidities need to be considered. The IgG products are not all the same and should not be
considered generic equivalents. Patients’ comorbidities — especially renal function, diabetes, cardiac
status, weight, and age — are important factors in the selection of the best IgG product, dose, and rate
of administration.
Intravenous vs Subcutaneous Administration
Over the past two decades, further refinements in manufacturing and pharmaceutical elegance of IVIG
products led researchers to explore additional ways to make immunoglobulin products more useful in
practice. This led to development of subcutaneous immunoglobulins (SCIG), which many patients could
self-administer and thereby avoid the need for frequent medical appointments to receive IVIG infusions.
This route of administration has improved patient quality of life and also proved useful in patients with
limited IV access. The SCIG products were more effective in some situations, as they provide a depot
release of immunoglobulins over time that simulates the body’s natural functions more closely than the
relatively rapid infusion of these agents intravenously (Table 4).19
| Table 4. Comparison of Intravenous Versus Subcutaneous Immunoglobulin Administration |
| Features |
SCIG |
IVIG |
| Pharmacokinetics |
Stable serum trough IgG level |
Variability in serum IgG level between peak and trough |
| Efficacy |
Clinical efficacy demonstrated in primary immunodeficiencies (noninferior when compared with IVIG) |
Clinical efficacy demonstrated in primary immunodeficiencies |
| Systemic adverse effects |
Infrequent |
Frequent |
| Local site reactions |
Common |
Infrequent |
| Administration |
Self-administration; patient autonomy |
Infusion center/home setting with nursing support for venous access |
| Average length of infusion |
1–2 hours |
2–4 hours |
| Dosing interval |
Weekly |
Variable — every 2–4 weeks |
| Most common adverse events |
Local reactions, headache, vomiting, pain |
Headache, chills, fever, myalgias, fatigue, nausea |
| Premedication |
Typically not used |
Acetaminophen, steroids, antihistamines (oral or intravenous) as needed |
| Patient satisfaction |
Flexibility of infusion frequency, site, patient autonomy, increased flexibility and independence |
Need for venous access, desire for administration in an outpatient setting, need for nursing support staff |
| Associated costs |
Cost of immunoglobulin, self-administration supply costs |
Cost of immunoglobulin, nursing and facility costs, equipment costs, related infusion costs (i.e., premedication, hydration) |
Information from reference 19.
Abbreviations: IgG, immunoglobulin G; IVIG, intravenous immunoglobulin; SCIG, subcutaneous immunoglobulin. |
Currently, all of the FDA-licensed SCIG products are indicated only for PID (Table 5). As with IVIGs, considerable off-label use occurs, with SCIGs beneficial in patients with chronic neuropathies such as chronic inflammatory demyelinating polyradiculoneuropathy and multifocal motor neuropathy.16 SCIG products have also been used in SID disorders, such as hypogammaglobinemia in patients with CLL, living with HIV, or undergoing bone marrow transplantation.12
| Table 5. Currently Approved Subcutaneous Immunoglobulin Products* |
| Cuvitru 20% Immune Globulin Subcutaneous (human) |
| Gammagard 10% Liquid Immune Globulin for IV and Subcutaneous Use (human) |
| Gammaked 10% Immune Globulin for IV and Subcutaneous Use (human) |
| Gamunex-C 10% Immune Globulin for IV and Subcutaneous Use (human) |
| Hizentra 20% Immune Globulin Subcutaneous (human) |
Abbreviation: IV, intravenous.
*All products are approved by the Food and Drug Administration for one indication — primary immunodefiency disorders. |
Safety Concerns Related to IgG Product Characteristics
The many potential uses of immunoglobulin products combined with two very different routes of
administration makes knowledge of product characteristics important for pharmacists.20 In addition to
the mechanisms of action and efficacy of IVIG and SCIG products in patients with SID disorders, the
products have differing safety concerns based on production methods, viral inactivation, fractionation,
purification, and factors such as osmolality, pH, stabilizers, and the relative amounts of IgA, sodium, and
sugars.
Production Methods
Available IgG products are derived from whole blood plasma or plasmapheresis obtained from donors at
U.S. sites, most of them registered with FDA. A variety of techniques are used to separate the IgG from
other plasma components, including cold ethanol fractionation (Cohn-Oncley is the original method;
Kistler–Nitschmann is an alternative method used by one manufacturer), nanofiltration, ultrafiltration,
ion-exchange chromatography, anion-exchange chromatography, and low pH incubation. Products differ
in their finished forms (liquid or lyophilized) and whether they have been solvent/detergent (S/D)
treated.18
While the amount of IgG in finished products is stated on product labeling (e.g., 5%, 10%) the amounts
of subclasses of IgG (i.e., IgG1, IgG2, IgG3, IgG4) is different among the various brands. Amounts of other plasma components also differ (e.g., IgA, IgM, albumin, sodium); these variances can be important
based on patient factors and diseases being treated.18
Some IgG brands must be refrigerated, others can be stored at ambient temperatures up to 30° C, and
expiratory dates of some products differ based on whether they were stored under refrigeration or at ambient room temperatures. These factors can be important considerations for pharmacies with limited
refrigerated space. None of the products should be frozen.18
Viral inactivation and removal of transmissible spongiform encephalopathies (TSEs, or prions) is another very important step in IgG product manufacturing. In the 1990s, the transmission of hepatitis C virus through the administration of IVIG was noted with some products.21 The immediate withdrawal of these products was warranted, and they were replaced with new formulations that are safe from transmission of this virus.
Since the 1990s, many different methods of viral inactivation/removal and TSE removal have been used,
including solvent/detergent treatment, inactivation of the viruses with lipid coats through use of fatty
acids or enzymes, heat treatment, acid treatment, nanofiltration, depth filtration, and alcohol
precipitation (Tables 6 and 7).22–24 No one method has been deemed superior over the others, and there have not been any other reports of transmission of microbial infections by IVIG since these processes were put in place. Of interest, there has never been a report of transmission of HIV with the administration of any IVIG product.
| Table 6. Viral Inactivation Methods |
| Solvent detergent |
Chemical treatment to inactivate lipid coated viruses |
| Caprylate |
Eight-chain fatty acid of plant origin that inactivates lipid coated viruses |
| Pasteurization |
Heat treatment method to inactivate all viruses |
| Low pH |
Acid condition incompatible with survival of virus |
| Pepsin |
Enzymes to digest lipid coat of virus |
| Nanofiltration2 |
Removes all viruses through use of size 20- and 35-nanometer filters |
| Depth filtration |
Removes virus |
| Alcohol precipitation |
Removes virus |
| Sources: References 22 (solvent detergent and pasteurization), 23 (caprylate), and 24 (nanofiltration). |
| Table 7. Pharmaceutical Aspects of Intravenous Immunoglobulin: Viral Inactivation Methods |
| Products |
Methods of Viral Inactivation |
| Flebogamma DIF (liquid, 5%/10%) |
Solvent detergent; nanofiltration (35 + 20 nm); pasteurization; low pH; PEG precipitation; fraction II and III alcohol incubation; fraction I precipitation |
| Gammagard S/D (lyophilized) |
Solvent detergent |
| Gammagard Liquid 10% |
Solvent detergent; low pH; nanofiltration 35 nm |
| Gamunex-C (liquid, 10%) |
Caprylate precipitation/incubation; low pH |
| Privigen (liquid, 10%) |
Depth filtration; nanofiltration 20 nm; low pH |
| Carimune NF (lyophilized, 6% after reconstitution) |
Nanofiltration; low pH/pepsin |
| Gammaplex 5% |
Solvent detergent |
| Octagam (liquid, 5%, 10%) |
Solvent detergent; low pH |
| Abbreviation: PEG, polyethylene glycol. |
Because of the possibility of viral mutations and identification of new viruses or prions, use of multiple methods of inactivation and screening of donors is imperative in IgG production.
Patient Risk Factors: Product Osmolarity, pH, and Stabilizer, IgA, and Sodium Content
When considering product selection for the individual patient, both comorbidities and IVIG product
characteristics should be considered. Table 8 lists some of the most important characteristics to
consider in product selection for patients with risk factors.25,26
| Table 8. Patient Risk Factors as Related to Immunoglobulin Product Characteristics |
| Patient Risk Factors |
Product Characteristics
|
| |
Volume Load
|
Sugar Content
|
Sodium Content
|
Osmolality
|
pH
|
IgA Content
|
| Cardiac impairment |
•
|
|
•
|
•
|
|
|
| Renal dysfunction |
•
|
•
|
•
|
•
|
|
|
| Anti-IgA antibodies |
|
|
|
|
|
•
|
| Thrombotic risk |
•
|
|
•
|
•
|
|
|
| Diabetic risk |
|
•
|
|
|
|
|
| Advanced age |
•
|
•
|
•
|
•
|
|
|
| Young age (neonatal/pediatric) |
•
|
|
•
|
•
|
•
|
|
| Sources of information: References 25 and 26. |
Volume load for the patient is a very important factor for patients who have cardiac impairment, renal
dysfunction, or any type of thrombotic risk, in those patients who have diabetes or prediabetes, and
those at the ends of the lifespan (neonates and those aged 65 years or older). For these patients, IVIG
products at the 10% concentration and those that are also iso-osmolar should be used to minimize the
risk of fluid overload.
Stabilizers are used in IVIG products to decrease the formation of end-to-end dimers between IgG
molecules. Stabilizers also limit fragments, polymers, and discoloration of the protein solution; only
when such problems were solved through the use of stabilizers was the development of stable IgG
solutions possible.
Products that use sugars instead of amino acids as stabilizers present greater risk to patients of
advanced age and those with diabetes or renal disease (Table 9). Low- or no-sodium IVIG products are better choices for patients with cardiac, renal, or thrombotic risk factors and in neonates and older
adults so that complications related to hyperosmolality are avoided.
| Table 9. Considerations Regarding Stabilizers Used in Immunoglobulin Products |
| Stabilizers |
Concerns |
Present in Which Products |
| D-Sorbitol |
Sorbitol is used as a stabilizer agent in some products. Even though it may not increase the glucose level in the blood, it is metabolized to fructose. Caution needs to be exercised with patients with heredity fructose intolerance. |
Flebogamma DIF (liquid, 5%/10%); Gammaplex 5%; 10% (also contains glycine) |
| Maltose (10%) |
Potential for false-positive glucose readings. This problem occurs only with the monitoring method that uses the enzyme GDH-PQQ. This method is employed in some home and point-of-care glucose monitoring devices used by patients with diabetes.
Caution should be exercised when administering products containing maltose. |
Octagam (liquid, 5%; 10%) |
| L-Proline-based |
Proline shields hydrophobic domains of IgG molecules from interfering with one another and thus minimizes IgG dimer formation.
A few patients may have hyperprolinemia and should not receive products stabilized with L-proline. Patient populations more likely to have hyperprolinemia are those with DeGeorges syndrome and schizophrenia. |
Privigen (liquid, 10%) |
| Sucrose (10%) |
Sucrose as a stabilizer does not increase glucose levels or the need for insulin in patents with diabetes. However, there has been a disproportionate increase of adverse events involving renal insufficiency associated with sucrose-containing products. Sucrose adds to the osmolality of the IVIG product final solution and viscosity. |
Carimmune NF (lyophilized) |
| Glucose (2%) |
Glucose contributes to hyperglycemia in patients with diabetes. Insulin may need to be administered to account for rise in glucose level. |
Gammagard 5% S/D (lyophilized) |
| Glycine-based |
Amino acid stabilizer not associated with adverse comorbid conditions or glucose levels. |
Gammagard Liquid (liquid, 10%); Gamunex-C (liquid, 10%); Gammaked 10% (liquid); Gammaplex 5%/10% (also contains sorbitol) |
Product IgA content is a concern only in patients with anti-IgA antibodies. Low IgA levels can indicate
such antibodies, but many other causes could be involved. When patients have anti-IgA antibodies,
severe adverse reactions or anaphylaxis can result from treatment with IgG products containing IgA.
IVIG products that are IgA-depleted are available for such patients.
Establishing Dosing and Monitoring Regimens
Dosing and administration of IVIG and SCIG products is specified in product labeling for approved
indications of the commercially available products. These should be followed when IgG products are
used for replacement therapy as either IVIG or SCIG except in patients with morbid obesity, as detailed below. When used as immunotherapy, IVIG/SCIG products often need to be titrated to achieve the
desired clinical effects or changes in monitoring parameters (e.g., serum IgG levels).
While actual body weight has historically been used for dosing of IgG products, the optimal dose in
patients with obesity is poorly studied and has long been controversial. IVIG has very little distribution into fat, but patients with obesity have an increased volume of distribution because of extra body fluids. One approach to dosing IVIG developed at The Ohio State University that accounts for the extra body fluids uses actual body weight (ABW) for patients weighing 100 kg or less and an adjusted
body weight for those weighing more than 100 kg. Thus, for patients weighing less than 100 kg,
the ABW is multiplied times the per-kg dose recommended in product labeling. For patients weighing
more than 100 kg, the dosing weight is calculated using the ideal body weight (IBW) as follows27:
Dosing weight (in kg) = [(ABW – IBW)/2] + IBW
For this equation, the IBW is calculated as it is for the Cockcroft-Gault equation [50 kg for men or 45 kgfor women + 2.3 X (height in inches – 60)].
Other authors have proposed using the Cockcroft-Gault IBW for dosing IgG, but this approach does not account for the increased volume of distribution in patients with obesity. It is an effective cost-saving measure. In one institution, use of IBW lowered the usage of IgG products by 20%, and pharmacist-driven stewardship efforts have ensured prescriber compliance with such guidelines.28,29 However, the clinical impact of this approach remains unknown.
For IVIG products on the U.S. market (with concentrations of 5% or 10%), refer to the manufacturers’
guidelines for infusion, but note that the dosing guidelines are written in mg/kg/min. The infusion
pumps are set in mL/h. Start with an initial rate that allows for observation and monitoring over a 15–30
minute period. Increase the rate as specified and then successively escalate the rate by one third to
determine the patient’s maximum tolerated rate. Adverse events can occur at any time; the rate should
be slowed until they subside. If the symptoms do not subside, the IVIG infusion should be stopped and
symptomatic treatments administered if warranted. For subsequent infusions in patients who have had
adverse reactions, premedications (e.g., acetaminophen, steroids, oral or intravenous antihistamines)
can be be administered to improve tolerability.
The comorbidities of patients must be taken into consideration when determining the rate of
administration. Rapid infusion rates are not recommended in those with renal dysfunction or a history
of thromboembolic events, other cardiac impairment, or other serious adverse events to IVIG therapy.
While most IVIG infusions require 2–4 hours for administration (given every 2 to 4 weeks), doses of SCIG are commonly infused weekly in a 1- to 2-hour period. The rapid infusion creates prominent “goose
bumps” in the subcutaneous tissue that go down in a few hours. Injection site swelling and mild
inflammation are normal reactions to SCIG administration. Headache, vomiting, and pain can also occur
and can be managed symptomatically; premedication is not usually used before SCIG infusions.
More serious adverse events have been reported rarely with SCIG. These include allergic, neurologic
(e.g., more severe headaches accompanied by other symptoms), renal or circulatory, urinary, and
cardiologic adverse effects, as listed in product labeling.
When converting patients from IVIG to SCIG therapy, a 1.37:1 dose conversion is used for most
products; for Cuvitru, the conversion is 1.30:1, and for Hyqvia, the conversion is 1:1. For example, for a patient receiving IVIG 40 g monthly, the conversion would be 1.37 X 40 = 54.8 g divided weekly, which
would be about 14 g weekly of a standard SCIG product, 13 g of Cuvitru weekly or 26 grams biweekly, or
40 grams of Hyqvia monthly. Remember to round doses to the nearest vial size and adjust the number
of injection sites to the patient’s body type and level of tolerance. Administered subcutaneously, these
amounts provide a relatively constant serum IgG level with a low peak and higher trough over a dosing interval; doses administered intravenously yield more of a peak-and-trough effect in serum
concentrations.
Monitoring of IgG therapy differs based on the condition being treated. Serum IgG levels can be measured just before the next doses to determine the trough concentration, with doses and frequency adjusted as needed. Clinical response is the most important monitoring parameter for most conditions, with avoidance of infections and improvement in target symptoms the best indicators of IgG efficacy.
Summary
The use of Ig for prevention of infections dates back to the 1940s and for agammaglobulinemia to 1951.
IVIG was not available until 1979 when carbohydrate and amino acid stabilizers were used to decrease
dimerization and aggregate formation. This innovation allowed for larger dose administration and
therapeutic levels to effectively treat primary immune deficiencies.
The use of IVIG in a patient with ITP led to the serendipitous discovery that IVIG modulated the immune system to block the attack of antibodies on platelets by blocking the Fc receptor sites. This discovery led to the use of IgG products as immunotherapy for neurologic disorders. Before development of
antiretroviral products in the 1990s, IVIG products were important interventions in patients with HIV,
especially children. For patients with CLL or multiple myeloma, the protection from pathogens provided
by IgG products is critical, as most deaths from these conditions are associated with infections. Similarly, immunodeficiencies induced by medications such as rituximab can be managed with IVIG or SCIG
treatment.
SIDs differ from PIDs in the fact that the cause of the deficiency may be temporary and therefore the
IVIG treatment may be short term as well. Often the cause of the SID is iatrogenic and could resolve
spontaneously. The approach to SID should start with identification of the most likely cause (Table 1).10–12 Independent of the cause, the treatment plan is similar to PID and monitoring IgG levels can improve the reduction of infections related to the SID.
SCIG products were developed over the past two decades to provide IgG therapies on a more
convenient basis for patients. They also have the advantage of ensuring a more consistent blood level of
IgG over each weekly dosing interval.
By applying the information provided in this article, pharmacists can contribute to the health care team during management of patients with PID or any of the numerous SID conditions that occur commonly.
IgG products are complex, but with the requisite knowledge and skills, they can be used effectively and
safely to improve the lives of millions of patients.
Resources
American Academy of Allergy, Asthma & Immunology: Provides consumers with information about
primary and secondary immunodeficiencies. https://www.aaaai.org/conditions-and-treatments
IG Living: magazine for the immune globulin community, including patients with Ig-treatable chronic illness and their caregivers. www.igliving.com
Immune Deficiency Foundation: Organization providing information and advocacy for those with
primary immunodeficiencies. https://primaryimmune.org
REFERENCES
- Detrick B. Immunology. In: Carroll KC, Hobden JA, Miller S, Morse SA, Mietzner TA, Detrick B, Mitchell TG, McKerrow JH, Sakanari JA. eds. Jawetz, Melnick, & Adelberg’s Medical Microbiology, 27e New York, NY: McGraw-Hill; http://accesspharmacy.mhmedical.com/content.aspx?bookid=1551§ionid=94105968. Accessed November 16, 2017.
- Jolles S, Sewell WAC, Misbah SA. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol. 2005(Oct);142:1–11.
- Harvey RD III. Introduction. Pharmacotherapy. 2005;25(11 Pt 2):71S–72S.
- Orange JS. Clinical update in immunoglobulin therapy for primary immunodeficiency diseases. In: Clinical Focus On Primary Immunodeficiencies. Towson, MD: Immune Deficiency Foundation; 2011.
- Lingman-Framme J, Fasth A. Subcutaneous immunoglobulin for primary and secondary immunodeficiencies: an evidence-based review. Drugs. 2013;73(12):1307-1319.
- Orange JS, Hossny EM, Weiler CR, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117(4 Suppl):S525-553.
- Oscier D, Dearden C, Eren E, et al. Guidelines on the diagnosis, investigation and management of chronic lymphocytic leukaemia. Br J Haematol. 2012;159(5):541-564.
- Immune Deficiency Foundation. Primary immunodeficiency diseases in America: 2007. The third national survey of patients.https://primaryimmune.org/wp-content/uploads/2011/04/Primary-Immunodeficiency-Diseases-in-America-2007The-Third-National-Survey-of-Patients.pdf. Accessed December 28, 2017.
- Boyle ML, Scalchunes C. Impact of intravenous immunoglobulin (IVIG) treatment among patients with primary immunodeficiency diseases. Pharm Policy Law. 2008;10:133–146.
- Fernandez J. Overview of immunodeficiency disorders. In: Merck Manual, Professional Version. 2016. http://www.merckmanuals.com/professional/immunology-allergic-disorders/immunodeficiency-disorders/overview-of-immunodeficiency-disorders. Accessed December 28, 2017.
- Kelleher P, Misbah SA. What is Good’s syndrome? Immunological abnormalities in patients with thymoma. J Clin Pathol. 2003;56:12–16.
- Compagno N, Malipiero G, Cinetto F, Agostini C. Immunoglobulin replacement therapy in secondary hypogammaglobulinemia. Front Immunol. 2014;5(626):1–6.
- Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leukemia. 2013;13(2):106–111.
- Kelesidis T, Daikos G, Boumpas D, Tsiodras S. Does rituximab increase the incidence of infectious complications? A narrative review. Int J Infect Dis. 2001;15(1):e2–e16.
- Siegel J. The product: all intravenous immunoglobulins are not equivalent. Pharmacotherapy. 2005;25(11 Pt 2):78S–84S.
- Hadden RDM, Marreno F. Switch from intravenous to subcutaneous immunoglobulin in CIDP and MMN: improved tolerability and patient satisfaction. Ther Adv Neurol Disord. 2015;8(1):14–19.
- Dhalla F, Misbah SA. Secondary antibody deficiencies. Curr Opin Allergy Clin Immunol. 2015;15(6):505–513.
- Siegel J. Intravenous immune globulins: therapeutic, pharmaceutical, administration, and cost considerations. Pharm Pract News. 2015(SE2015):67–74.
- Berger M. Subcutaneous immunoglobulin replacement in primary immunodeficiencies. Clin Immunol. 2004;112(1):1–7.
- Tichy EM, Vaughan L. Selecting a polyclonal immune globulin treatment for a patient with primary immune deficiency disease: role of the clinical pharmacist. Am J Health Syst Pharm. 2016;73(8):533–46.
- Flora K, Schiele M, Benner K, et al. An outbreak of hepatitis C among recipients of intravenous immunoglobulin. Ann Allergy Asthma Immunol. 1996;76(2):160–2.
- Chang CE, Eo HG, Lee YS, et al. Human intravenous immunoglobulin preparation and virus inactivation by pasteurization and solvent detergent treatment. Prep Biochem Biotechnol. 2000;30(3):177–197.
- Hughes RA, Donofrio P, Bril V, et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol. 2008;7(2):136–144.
- Boschetti N, Stucki M, Peter S, Kempf C. Virus safety of intravenous immunoglobulin: future challenges. Clin Rev Allergy Immunol. 2005;29:333-344.
- Gelfand EW. Differences between IGIV products: impact on clinical outcome. Int Immunopharmacol. 2006;6(4):592–599.
- Sorensen R. Expert opinion regarding clinical and other outcome considerations in the formulary review of immune globulin. J Manag Care Pharm. 2007;13(3):278–283.
- Siegel J. Immunoglobulins and obesity. Pharm Pract News. 2010(Jan 7). http://www.pharmacypracticenews.com/Opinion/Article/01-10/Immunoglobulins-and-Obesity/14546. Accessed December 27, 2017.
- Rocchio MA, Hussey AP, Southard RA, Szumita PM. Impact of ideal body weight dosing for all inpatient i.v. immune globulin indications. Am J Health Syst Pharm. 2013;70(9):751–752.
- Rocchio MA, Schurr JW, Hussey AP, Szumita PM. Intravenous immune globulin stewardship program at a tertiary academic medical center. Ann Pharmacother. 2017;51(2):135–139.
Back Top