ADVERTISEMENT

Fungal Frontiers: Expert Decision Making in Medical Mycology
SECTION 1: INTRODUCTION
Welcome to the case-based mycology CME activity, Fungal Frontiers: Expert Decision Making in Medical Mycology. By using challenging cases at the frontiers of mycology, this initiative is designed to assist you in evaluating your decision-making process/benchmarking your approach against that of ID and pharmacy mycology experts. We will present 3 topical case discussions across the management spectrum in this multimedia, interactive activity. We hope you find these cases helpful in evaluating your approach to these challenging case studies to support optimal care of your patients with IFD.
SECTION 2: ANTIFUNGAL PROPHYLAXIS IN AN HSCT TRANSPLANT PATIENT
Case Presentation
A 59-year-old white man with a history of AML has been evaluated, and the team is planning a matched unrelated stem cell transplant. Key features of his history include that he has AML, originally diagnosed in 2016. He was NPM1 negative but FLT3-ITD positive, placing him in a poor-risk category. His past medical history was significant only for an enlarged prostate and mild hypertension, which has been controlled.
He received initial induction chemotherapy with cytarabine plus daunorubicin in a 7+3 regimen. His Day 14 bone marrow biopsy showed a low level of residual leukemia that was markedly hypocellular, and his bone marrow showed rare CD34-positive blasts. He had a repeat bone marrow biopsy at Day 30, which demonstrated normal cellular bone marrow and no significant increase in blasts.
The patient went on to receive 2 cycles of consolidation high-dose cytarabine. His echocardiogram showed an ejection fraction of more than 55%. He had a baseline ECG and chest x-ray that were within normal limits. His weight is 72 kilograms. His estimated creatinine clearance is 85 mL per minute. The patient has no known drug allergies.
The patient was admitted and received a conditioning regimen consisting of busulfan, cyclophosphamide, and rATG, with total body radiation followed by matched sibling stem cell transplant. The donor was CMV negative.
Clinical Considerations: What would be a typical initial prophylactic regimen for this patient?
- A. Ciprofloxacin (500 mg po BID), acyclovir (400 mg po BID), voriconazole (200 mg po BID)
- B. Acyclovir (400 mg po BID), amphotericin B deoxycholate (70 mg IV daily)
- C. Penicillin (750 mg po BID), liposomal amphotericin B (350 mg IV daily)
- D. Levofloxacin (500 mg QD), acyclovir (400 mg BID), fluconazole (400 mg po QD)
Rationale for Answer
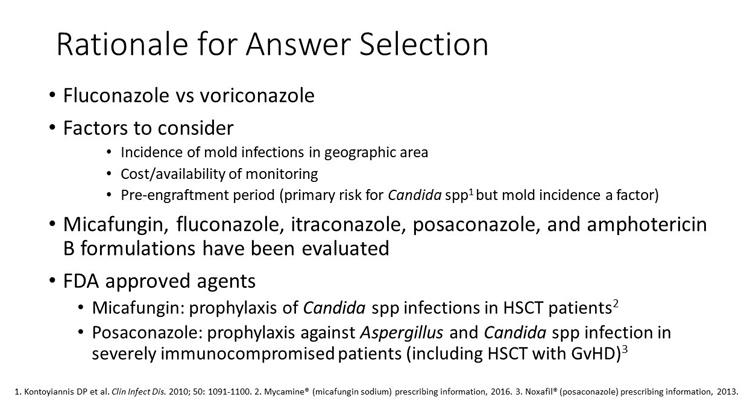
There are 2 possible correct answers here: either the voriconazole regimen or the fluconazole-based regimen. The choice of prophylactic agent may depend on institutional factors such as the incidence of mold infections and the geographic area of interest as well as the cost and availability of therapeutic drug monitoring.
Since this is the pre-engraftment period, the primary risk of fungal infection is Candida spp. However, in some centers, molds are also a consideration. Several agents have been evaluated as prophylaxis in the setting of HCT: micafungin, fluconazole, itraconazole, voriconazole, posaconazole, and amphotericin B formulations. Of these, only micafungin has an FDA indication for "global" prophylaxis of Candida infections in SCT patients. Posaconazole has an FDA indication for prophylaxis of Aspergillus and Candida infections in immunocompromised patients such as HCT recipients with graft versus host disease.
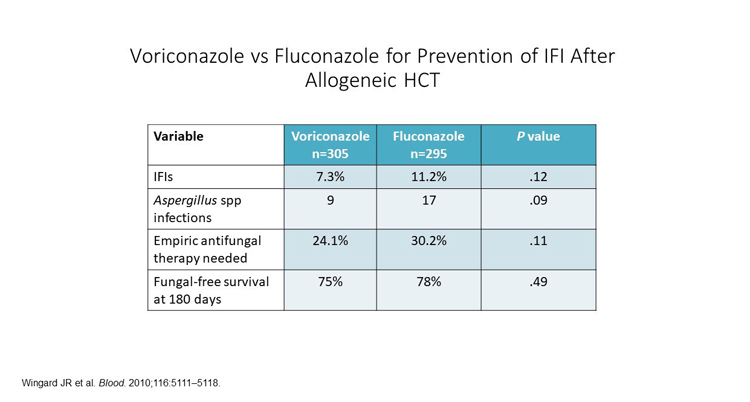
Voriconazole and fluconazole were compared head-to-head in a multi-center open-label randomized trial as prophylaxis in SCT recipients. Prophylaxis was initiated at conditioning (Day 1) and continued until Day 100 or beyond. The end point was tolerability of prophylaxis for at least 100 days and fungal-free survival until 180 days.
As shown in the above figure, the 2 drugs had similar outcomes overall; about 7% of voriconazole and 11% of fluconazole-prophylaxed patients experienced proven or probable invasive fungal infections (IFIs). This was not statistically different. There was a trend toward more Aspergillus infection in the fluconazole arm, but again this was not statistically significant.
Therefore, some centers will use fluconazole, whereas others will use voriconazole because they have more molds locally. If using one of these strategies that does not include mold coverage up front, most centers would include a preemptive monitoring strategy with frequent and regular monitoring of galactomannan. This can potentially alleviate some of the challenges with using a mold-active azole up front, such as drug-drug interactions and toxicity.
Panel Discussion
Dr Ostrosky-Zeichner: This is the case of a stem-cell transplant patient who's at high risk of both bacterial and fungal infections. In this case, I would choose the regimen that includes fluoroquinolone. I would also choose acyclovir as a prophylactic agent for viral diseases. Since this is a high-risk host, I think we need to do anti-mold coverage, so I would choose voriconazole of the options given.
Dr Vazquez: I would agree with Luis. I think we'd do the same thing. We would continue with a quinolone, the acyclovir, and in most situations, we would be using voriconazole. There are still some physicians here, however, who will use fluconazole until there is a problem with the host. That's another alternative or possibility depending on who's practicing and who's been doing this for a while. So, I think alternative of either fluconazole or voriconazole are possibilities here.
Case Presentation Continued
The patient did well clinically initially following transplantation. Tacrolimus was administered twice daily. Approximately 6 weeks posttransplant, the patient engrafted, and ciprofloxacin was discontinued. Acyclovir, trimethoprim/sulfamethoxazole, and fluconazole were continued as prophylaxis. His CMV DNA, galactomannan, and chest x-rays remained negative.
He had some breakthrough reflux and nausea, for which omeprazole was added. He also experienced some peripheral neuropathy, for which gabapentin was given. The patient then developed worsening GI complaints of diarrhea, nausea with occasional vomiting, and fatigue. Ranitidine, milk of magnesia, and sucralfate were added to his regimen in a step-wise fashion, with little relief. Symptoms continued to worsen along with a new rash suggestive of skin GvHD. A flexible sigmoidoscopy/EGD demonstrated moderate GvHD in the stomach and duodenum. Prednisone 2 mg/kg was initiated.
Clinical Considerations: Which agent should the patient receive now for antifungal prophylaxis?
- A. Isavuconazole
- B. Posaconazole
- C. Liposomal amphotericin B
- D. Fluconazole
Rationale for Answer
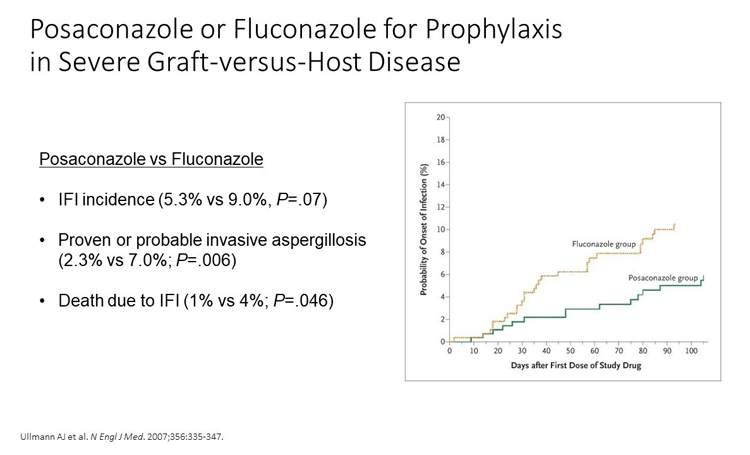
I would choose posaconazole for this patient. In clinical trials, posaconazole was compared with fluconazole in this setting and demonstrated some benefits, especially with regard to the mold activity, as shown in the figure. The use of 2 immunosuppressants in this case (ie, tacrolimus and the corticosteroids for management of the GvHD) renders the patient at risk for invasive mold disease. The patient doesn't seem to have too many drug-drug interactions that would prevent us from using posaconazole here, so I think it's appropriate to initiate posaconazole at this time.
Panel Discussion:
Dr Vazquez: This patient now has developed GvHD. As patients develop GvHD, the incidence of filamentous fungi (or mold) infection increases, specifically Aspergillus spp, which would be number 1, followed possibly by Mucor, and then, later on, by some of the dematiaceous fungi. I would definitely choose something that is easy to deliver and that covers both Aspergillus spp and Mucor. So, I would choose posaconazole.
Dr Ostrosky-Zeichner: I agree with Dr Vazquez. This is the exact type of patient that was enrolled in the posaconazole trials. The risk for mold infections is increasing. The patient has GvHD, so this is a patient where posaconazole has been proven to function well.
Case Continued—QTc Prolongation
Approximately 1 week later, the patient experienced additional diarrhea and fever. He was found to have an E coli bacteremia that was susceptible to cefepime but resistant to quinolones. Cefepime was initiated and other prophylaxis was continued. He was hypotensive and tachycardic, and a repeat ECG demonstrated QTc prolongation, with a QTc interval of 515 ms.
Clinical Considerations: What would be appropriate therapy now?
- A. Liposomal amphotericin B
- B. Keep posaconazole, adjust dose
- C. Fluconazole
- D. Switch back to voriconazole
- E. Isavuconazole
- F. Micafungin
Rationale for Answer
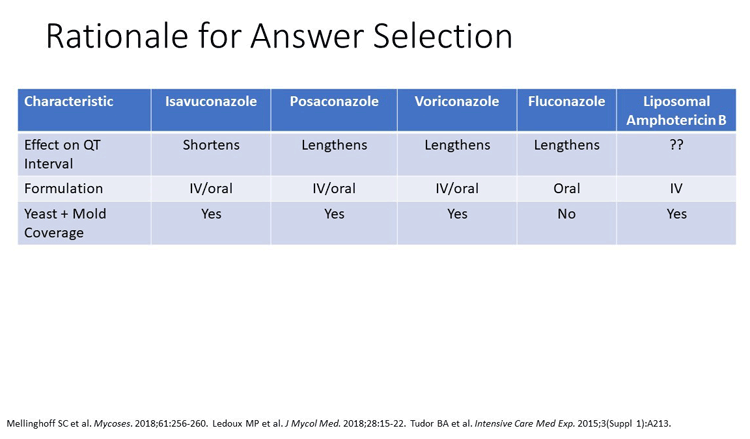
QT prolongation potential (particularly with interacting drugs) has been an issue identified as a limitation of older azoles used in the prophylaxis setting.[Vazquez 2016] Isavuconazole is a good choice here, simply because there have been some data suggesting that it even shortens the QT interval in patients and certainly does not seem to be associated with prolonged QT interval. It is reasonable, although it certainly hasn't had clinical trials data in a randomized trial of prophylaxis.
We know it has both yeast activity and mold activity and can be an attractive agent for use in prophylaxis in this study. I agree that other alternatives would include liposomal amphotericin B and micafungin. Alternatively, fluconazole here, but you're really lacking that mold activity if you choose fluconazole. Therefore, you would be missing the molds that the patient is at high risk for at this point.
Panel Discussion:
Dr Ostrosky-Zeichner: Options in this case would include liposomal amphotericin B or micafungin if we wanted to go to an IV regimen. If we wanted to stick to an oral formulation, we would probably choose isavuconazole.
Dr Vazquez: I would agree. The options are if parenteral, then echinocandin or liposomal amphotericin B. Preferably, if you can de-escalate to oral therapy, then probably the choice would be the isavuconazonium sulfate, because it can be used parenterally, and it can also be de-escalated to oral.
Final Commentary on the Case
Certainly, the drug interaction issue comes up a lot with many of these agents, and the azoles being the biggest offenders with any of the prophylaxis types of settings where you have patients on multiple other agents.[Vazquez 2016] You have some wiggle room in terms of what you're choosing. With regard to chemotherapy agents here, vincristine is frequently contraindicated. We use that a lot in the ALL patient population. That can be difficult and a challenge to deal with prophylaxis in that setting, as well as cyclophosphamide at times. When using these alternate agents, it's important to consider the drug interaction issues with any of the azoles that we're going to be using for prophylaxis.
SECTION 3. INVASIVE PULMONARY ASPERGILLUS FUMIGATUS IN A LUNG TRANSPLANT RECIPIENT IN THE UNITED STATES
Case Presentation
A 56-year-old white man was referred to the ID service for fever and an infiltrate that had been present for approximately 9 months after a bilateral lung transplant. The patient had received the bilateral transplant with a primary CMV mismatch for pulmonary fibrosis.
Since the transplant, however, he has suffered several episodes of rejection and has been on mycophenolate 500 mg q12h, prednisone 20 mg once a day, and tacrolimus 2 mg per day. He was admitted now for increasing dyspnea, and a CT scan of the chest showed multifocal pneumonia and a large bilateral pleural effusion. Bronchoscopy done on admission with lung biopsy reveals invasive Aspergillus fumigatus.
Clinical Consideration: Which antifungal would you select?
- 1. Voriconazole
- 2. Liposomal amphotericin B
- 3. An echinocandin
- 4. Posaconazole
- 5. Isavuconazole
- 6. Combination therapy with an echinocandin plus an azole
Rationale for Answer

So, there are 2 possible options here. Voriconazole is the older option. It's the one that's recommended if you look at the infectious disease guidelines, but those were published in 2016. The other option certainly is isavuconazole or isavuconazonium, and the Maertens study certainly showed that it is not inferior to voriconazole, so either one would be perfect for a patient like this.
Perhaps a year or 2 ago, we would have all used voriconazole. I think many of us now are actually switching to isavuconazole because of the fact that the drug-drug interactions are not as bad. The adverse event profile actually seems pretty good in these patients, especially because these patients are going to be on it for a while.
It is also important to note that this is invasive aspergillosis in a non-neutropenic patient, which is a little bit different of a disease than invasive pulmonary aspergillosis in the bone marrow transplant or neutropenic patient. Along with that, this patient also has a complication. It looks like the patient has an empyema along with the pulmonary aspergillosis, complicating the entire picture.
Panel Discussion:
Dr Johnson: On the basis of the Herbrecht data for invasive aspergillosis treatment, we would generally start with voriconazole for aspergillosis. This is an interesting case, and sometimes you don't always have a solid diagnosis at the time that you have pulmonary opacities or nodules. If we were more uncertain, we might consider something with broader mold activity, such as posaconazole or liposomal amphotericin B.
Given the fact that we have a biopsy here that is suggestive of Aspergillus spp infection, then voriconazole would probably be our first choice. However, we would have to look at all the drug interactions and potential comorbidities in this patient and see if we could actually use voriconazole.
Dr Ostrosky-Zeichner: I think Dr Johnson gives a very good answer. I do want to mention that we do have a clinical trial that compared isavuconazole to voriconazole. In this clinical trial, isavuconazole was not inferior to voriconazole. Therefore, some centers are choosing now to use isavuconazole in this setting given, a slightly better tolerance on adverse event profile as well as a slightly better drug interaction profile.
The question is, always, should we be using combination antifungal therapy up front for invasive aspergillosis? If you go through the Marr data, you would conclude that perhaps the best setting for this combination therapy would be early therapy, early diagnosis of the disease. I think the option of an echinocandin plus an azole is a valid option for this case as well.
Case Continued
The patient was started on voriconazole, initially 300 mg twice a day. As is custom with a lot of the lung transplants, he was also initiated on azithromycin 500 mg once a day. Unfortunately, he was readmitted again with increasing shortness of breath, dyspnea, and reaccumulation of pleural fluid.
Clinical Considerations: What would be the best option to evaluate or reevaluate this patient?
- A. Serum galactomannan
- B. Bronchoscopy with BAL and send for fungal, AFB, and viral staining
- C. Serum Fungitell® assay
- D. Fungal blood cultures
Rationale for Answer
Certainly, I see the value of bronchoscopy with BAL, sending off for fungal stains, AFB stains, and viral stains is definitely the key here. The galactomannan in patients like this is not very helpful, considering galactomannan really is much more specific in the neutropenic patient with Aspergillus spp. The β-D-glucan (Fungitell®) assay may be positive, but it has not been studied in these types of patients with invasive pulmonary aspergillosis. Fungal blood cultures would be done on any patient, although it would be rare to find a mold or any other fungal infection in such a patient. He's really been on broad-spectrum antifungal therapy.
My best guess for the etiology here would be a fungal Aspergillus spp infection that is recalcitrant to therapy, or there is a mycobacterium, or a viral infection going on at the same time.
Panel Discussion:
Dr Ostrosky-Zeichner: This patient is getting more complicated. Again, a high-risk host with invasive aspergillosis. We started ideal treatment, and the patient is not responding. In this case we need to be very aggressive and try to pursue additional infections in this patient. I would choose to do a bronchoscopy with BAL, and send for fungal AFB, viral stains, and cultures. I think we need to address the pleural effusion as well with a pleural tap.
Dr Johnson: I agree, this patient is not responding to first-line therapy. At this point we have to consider a lot of different things. Whether or not we have the correct fungus that we're targeting, it could be a broader spectrum. Some of the tests listed here like Fungitell, or fungal blood cultures, etc., aren't necessarily going to be positive in a mold patient. That's important to consider. AFB stains and viral stains as well might be helpful to get a solid diagnosis in this patient.
We really need to back up, and rethink, and make sure that we have the right diagnosis, as well as maybe think about drug concentrations. Voriconazole can have variable pharmacokinetics, and it might be good to make sure that we have adequate levels on board in this patient. Certainly, the dosing that he's on seems adequate, but we've seen drug interactions or metabolic derangements in patients that can lead to undetectable levels. That might be helpful as well.

Case Continued
The patient undergoes a repeat bronchoscopy with another bronchoalveolar lavage, and all the
specimens are sent to the lab. The repeat cultures again reveal Aspergillus fumigatus. A repeat thoracentesis also reveals Aspergillus fumigatus. In addition, because of these persistent effusions, bilateral chest tubes have now been inserted to drain the aspergillosis empyema that has developed.
Voriconazole, in the meantime, is continued for 2 months. Afterwards, he's readmitted again in 2 months with diffuse interstitial infiltrates. This time, a repeat bronchoscopy reveals not only Aspergillus fumigatus, but also cytomegalovirus pneumonia. He's got a combination of a viral and a fungal infection.
Clinical Considerations: What would you recommend now?
- 1. Repeat serum galactomannan
- 2. Send isolates for in vitro susceptibility testing
- 3. Lung biopsy
- 4. Switch to another antifungal
- 5. Therapeutic drug monitoring
- 6. 2, 4, 5
Rationale for Answer

I think, in this complicated, complex patient certainly, after lung transplant, there are several things that need to be done simultaneously instead of taking it step-wise. One, I think doing therapeutic drug monitoring is certainly very important, making sure we have the adequate level. Second is switching to another antifungal considering that the patient has already been on voriconazole for several months. It's a concern it's not working, or it's not getting to the site of infection, or there is resistance. Third is sending the isolates out for in vitro susceptibility testing. Although resistance to azoles in the United States is rare, and it was initially described back in 1998 in the Netherlands, it's spread throughout Europe. It's seen now in Asia. There actually have been several cases in the United States with the same types of mutations. I think it would be important in this patient to basically go ahead and do several things simultaneously with the hope that we can improve the morbidity and the outcome of this patient.
Panel Discussion:
Dr Johnson: Being the pharmacist in the group, I would probably go with number five, getting therapeutic drug monitoring and ensuring that we have adequate levels. Of course, I think we have to consider other options here, as long as we do have adequate drug levels, and think about drug resistance.
There is some circulating resistance to fumigatus that has been reported. A lot of this is in Europe, but it has been seen here. That would be an important factor as well. I think shoring up all of those things would be important, and perhaps switching to another antifungal agent if this patient is no longer responding and worsening on therapy.
Dr Ostrosky-Zeichner: I agree with Dr Johnson. Causes for antifungal failure in this setting will include resistance, which is, as Dr Johnson mentioned, rare in the United States but something we need to consider. Another possibility is inadequate drug levels. We need to investigate those 2, but I think in the meantime, we probably need to do an antifungal switch. In this case, the guidelines tell us to go to liposomal amphotericin B.
Case Continued
The patient was started on ganciclovir. Voriconazole and azithromycin were continued; however, a repeat bronchoscopy and BAL continued to reveal Aspergillus fumigatus. In vitro susceptibility studies that were done 1 week later now reveal Aspergillus fumigatus resistant to voriconazole, with an MIC of greater than 16 mcg/mL. However, A fumigatus remains susceptible to amphotericin B with an MIC of 0.5 mcg/mL, to 2 echinocandins with an MEC of 0.03 mcg/mL, and to posaconazole and isavuconazole at 0.5 mcg/mL.
Clinical Consideration: What would be your antifungal option after reviewing the in vitro susceptibility results?
- 1. Continue voriconazole
- 2. Switch to LAmB
- 3. Switch to an echinocandin
- 4. Switch to posaconazole
- 5. Switch to isavuconazole
- 6. Consider combination therapy with echinocandin and LAmB
Rationale for Answer
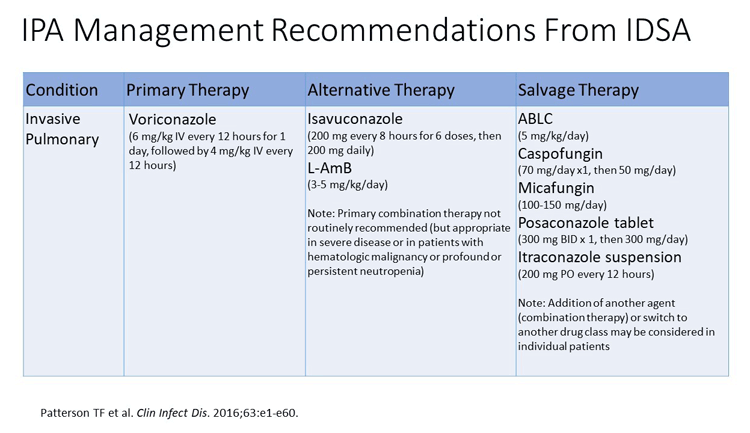
First, a look at the guidelines reveals that if the patient is failing or has refractory disease, switching classes to either a liposomal amphotericin B or an echinocandin would be your best option. Switching classes—I think in this case, to liposomal amphotericin B plus an echinocandin—may be an option.
Second—and this can't be stressed enough when the patient is on immunosuppression—diminishing the degree of immune suppression also improves the outcome tremendously. The third option (as with this patient), is drainage and eliminating the source or source control, which in this situation happens to be the Aspergillus empyema. A combination of those 3 things, I think, would be the optimal therapy for this type of patient.
Panel Discussion:
Dr Ostrosky-Zeichner: The antifungal susceptibility testing in this case shows us resistance to voriconazole with an MIC greater than 16 mcg/mL. While the posaconazole and isavuconazole MICs appear to be in the treatable range, I would be very nervous because of the possibility of cross resistance. In this case, I would follow the guidelines and switch to another therapeutic class. In this case, I would probably choose liposomal amphotericin B or an echinocandin.
Dr Johnson: I would agree. This is a little bit of uncharted territory. We don't have a lot of data to guide us about what to do with a resistant case, and what is appropriate in terms of cross-resistant azoles, and how they might perform in this setting. They look to be susceptible. I would also be a little uncomfortable with that knowing that often, if there are elevated MICs to voriconazole for Aspergillus, they may also be elevated to isavuconazole or posaconazole.
I think alternative agents such as liposomal amphotericin B would be appropriate in this setting for this patient. Alternatively, if you think the patient can't tolerate liposomal amphotericin B, you could consider an echinocandin, although as single agents in Aspergillus, they might not be as effective.
Case Continued
The patient was switched to LAmB at 5 mg/kg daily. Three months later, however, the patient again is readmitted with new multifocal pulmonary infiltrates. Bronchoscopy again reveals Aspergillus fumigatus. This time, the Aspergillus spp is accompanied by Pseudomonas aeruginosa.
The patient eventually develops ARDS and septic shock and expires. On autopsy, interestingly enough, Aspergillus fumigatus was cultured from the respiratory tract despite the patient's being on antifungals, azoles, and amphotericin B for approximately 9 months. In addition, both lungs and pleural cavity showed fibrotic changes with fibrinous exudate. This case illustrates how difficult it is to eradicate aspergillosis in an immunosuppressed patient with problems with source control—in this case the empyema Aspergillus. Probably for that reason, we see the generation of secondary resistance to voriconazole because of the high load that the patient's on.
Final Thoughts
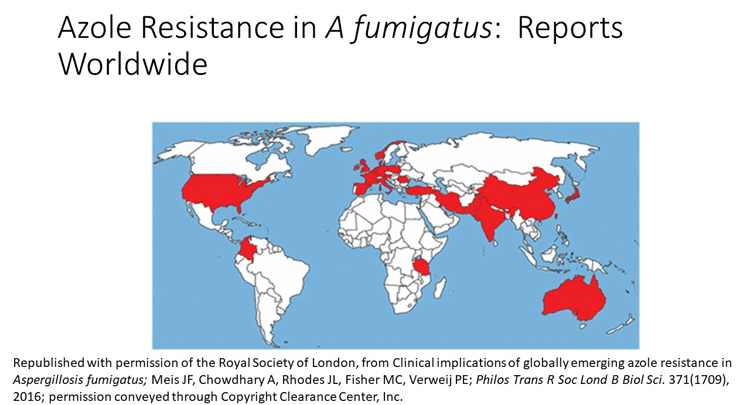
As mentioned previously, triazole resistance among environmental and clinical isolates of Aspergillus fumigatus is a major problem in regions of Europe, particularly the UK, France, Spain, Belgium, and the Netherlands. Recently, cases have been reported more broadly as well, as shown in the image.
Major azole resistance mechanisms in Aspergillus fumigatus involve point mutations in the cyp51A gene encoding for the 14α sterol demethylase. This results in amino acid alteration in these critical regions of the cyp51 protein A. Recent analysis and available data on target modification-dependent azole resistance in Aspergillus fumigatus have identified at least 18 mutations on 14 different loci. The amino acid residues often involved in triazole resistance include G54, L98, G138, P216, the F219, M220, and E427. There are also non-cyp51 type mutations as well.[Chowdhary 2017]
It's important to note we have multiple mechanisms of resistance. As a matter of fact, in this patient here the point mutations were identical to the point mutations that have been described in the Netherlands, in Spain, and in the UK, even though this patient had never been to Europe.
SECTION 4. A 51-YEAR-OLD WOMAN WITH ABDOMINAL SEPSIS
Case Presentation
The patient is a 51-year-old woman with history of travel to India for an elective abdominal liposuction. The procedure was complicated by hollow viscus perforation with subsequent development of septic shock and abdominal sepsis. The patient is transferred to your hospital after 10 days abroad for a higher level of care.
On admission, the patient is hemodynamically unstable, requiring vasopressors, has a temperature of 38.7°C, has diffuse bilateral crackles and rales, and the abdomen is rigid with guarding. A CT of the chest and abdomen shows changes compatible with ARDS and multiple large rim-enhancing collections through the abdomen. Her white blood cell count is 27,000/mm3, and her hemoglobin in 7.0 g/dL. Blood gases show a pH of 7.2 and an elevated lactate. Interventional radiology has placed a pigtail catheter in the largest collection, and the procedure report mentions a large volume of purulent fluid that was drained successfully. Fluid and blood cultures are pending.
Clinical Consideration: What empiric antimicrobials would you use in this case?
- A. Meropenem, daptomcyin, and fluconazole
- B. Cefepime and metronidazole
- C. Meropenem, vancomycin, and micafungin
- D. Ampicillin-sulbactam and vancomycin
- E. No empiric treatment is warranted
Rationale for Answer

Viewing country-specific epidemiology, one should be concerned about multidrug-resistant organisms. Empiric coverage with broad spectrum antimicrobials is warranted while culture results are pending. In this case, we chose meropenem for Gram-negative pathogens including Pseudomonas and ESBL producers, as well as Enterobacter spp. Vancomycin would be used to cover for the possibility of Enterococcus, and micafungin over fluconazole as a fungicidal agent in this case where invasive candidiasis is also a possibility.
Panel Discussion:
Dr Johnson: This is an interesting case of a woman who had recent travel to India and abdominal surgery with a perforation. We're thinking about potentially multidrug-resistant Gram-negative organisms as well as abdominal content such as Enterococci (including vancomycin-resistant Enterococci) and yeast. When I think of the abdomen and yeast, I think of non-albicans Candida as well as albicans, so that's a consideration.
Thinking about all this together, of the choices given, I would say that meropenem plus vancomycin and micafungin would be the best choice, with culture data pending to ensure that we're actually going to get the Gram-negative organisms that she's at risk for, particularly the multidrug-resistant Gram-negatives as well as non-albicans Candida with your micafungin.
Dr Vazquez: This is a case of a complicated intra-abdominal infection. In that situation, it's going to be a polymicrobial infection, including not only the anaerobes, the Gram-negatives, and in this situation, because she had the surgery in India, potentially a multidrug-resistant Gram-negative. It's certainly a possibility. Because she's in shock, I usually tend to add an anti-Candida agent. I would use Candida directed empiric therapy or prophylaxis in this situation. I would agree with the meropenem, the vancomycin, and the micafungin in this setting.
Case Continued
After 72 hours, the patient continues to be febrile and hemodynamically unstable with high ventilator requirements. Blood cultures are growing multidrug-resistant Acinetobacter spp, and abdominal fluid is growing the same organism as well as Candida haemulonii.
Clinical Consideration: In addition to pursuing further surgical drainage, what antimicrobial changes would you make at this point?
- a. Continue meropenem and micafungin, stop vancomycin
- b. Continue meropenem and vancomycin, de-escalate micafungin to fluconazole
- c. Discontinue all, start ceftazidime-avibactam and liposomal amphotericin B
- d. Discontinue all, start ceftazidime-avibactam and fluconazole
- e. Discontinue meropenem and vancomycin, add ceftazidime-avibactam and metronidazole
Rationale for Answer

The patient has polymicrobial infection of abdominal origin. Acinetobacter in this region may harbor extensive resistant mechanisms, including carbapenemase. Therefore, it is appropriate to escalate meropenem to the newer combination product ceftazidime-avibactam for Acinetobacter spp with carbapenemases. Continued anaerobic coverage is necessary, hence the need to add metronidazole. Alternatively, meropenem-vaborbactam could be used, since this newer combination product also has activity against carbapenemases. Cultures are not reporting Gram-positive, so it is appropriate to discontinue vancomycin.
The report of Candida haemulonii from this region of the world should make us very suspicious for Candida auris. The isolate needs to be referred for MALDI-TOF for re-identification.
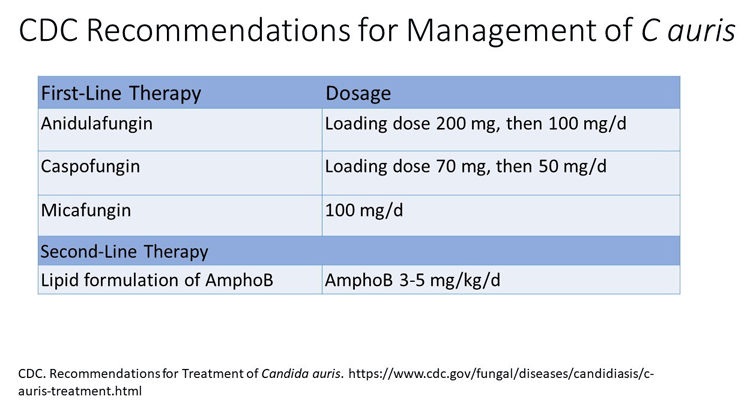
The appropriate empirical therapy for this agent is still echinocandin, as these organisms are still often resistant to azoles.
Panel Discussion:
Dr Vazquez: I think in this situation here, now that we know we have a Enterobacteriaceae that could have multidrug resistance, we may have to make sure there's no carbapenemase activity. I think the discontinuation of meropenem would definitely be feasible and the addition then of a drug that's stable in carbapenemase resistance, so ceftazidime-avibactam could be one of them. Because it does not have anaerobic activity, you're going to have to add metronidazole to it. I would go ahead, and I would agree and continue with the ceftazidime-avibactam as one of the options with metronidazole.
Dr Johnson: I agree. I'm also a little bit concerned about the diagnosis of haemulonii because sometimes that can be misidentified. That's a relatively uncommon organism in our institution, and so that would trigger me to be very concerned about the yeast that this woman has and request further workup with the lab.
Case Continued
The patient was started on ceftazidime-avibactam and metronidazole. Micafungin was continued, and the rest of the antibiotics were discontinued. After 48 hours, the patient was weaned off the vasopressors, but she continued to have low grade fevers and her white count remained at 15,000/mm3. The fungal isolate was identified as Candida auris by MALDI-TOF, and the micafungin MIC was 2 mcg/mL, whereas the fluconazole MIC was 16 mcg/mL.
Clinical Consideration: What antifungal changes would you make at this point?
- a. De-escalate micafungin to high-dose fluconazole
- b. Discontinue micafungin and start liposomal amphotericin B
- c. Discontinue micafungin and start voriconazole
- d. Continue micafungin
- e. Stop antifungals
Rationale for Answer
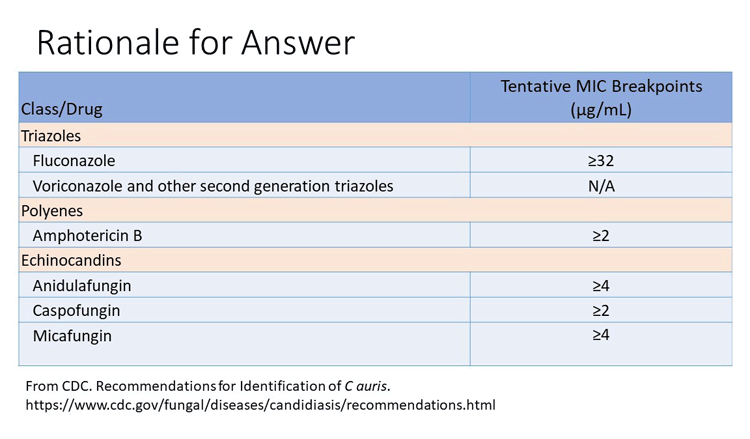
The patient has improved but continues to have fever and leukocytosis. Both the fluconazole and micafungin MICs are close to the suggested tentative breakpoint. Candida auris should be assumed to be fluconazole resistant and has been known to develop echinocandin resistance as well. Voriconazole is not recommended when fluconazole resistance may be present.

The best option at this point, where the patient may be experiencing a microbiological failure, is to start liposomal amphotericin B, as resistance has been reported but is still rare.
Panel Discussion:
Dr Vazquez: I think there's a couple of possibilities. Because this patient has really been treated and has surgically improved and medically improved, with an MIC of 2 to micafungin, switching to liposomal amphotericin B is a possibility. However, the other possibility may be just continuing the micafungin to finish off since the patient improves, although the patient continues to have low grade fevers and a white count of 15,000/mm3. I think that would be another option, is just to continue it through and finish off the course.
Dr Johnson: Patients with intra-abdominal infections can be particularly challenging. This woman has multiple organisms isolated, which makes it even more so. It might not be somewhat surprising that she continued to have some symptoms for a while.
I agree that you could take a few different approaches here and try to treat her for generally longer duration, or switch therapy because you're worried about this thing persisting over time. Discontinuing micafungin and starting liposomal amphotericin B is certainly an option for her. Fluconazole would really not be optimal in this case given that MIC was 16 mcg/mL, and so I think other agents should be considered.
List of Acronyms
|
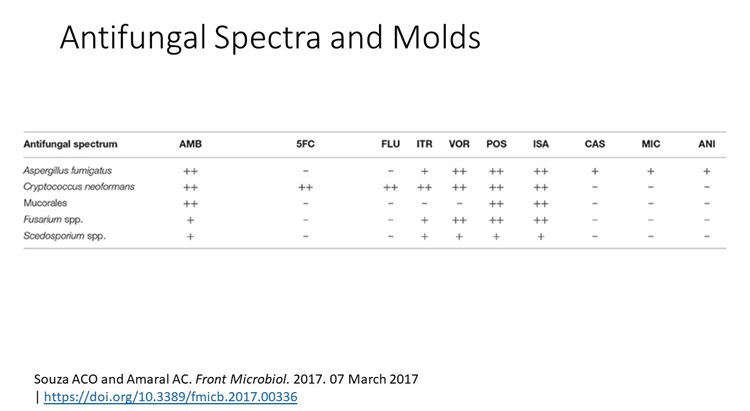
5FC flucytosine
ABLC amphotericin B lipid complex
AFB acid-fast bacteria
ALL acute lymphoblastic leukemia; acute lymphocytic leukemia
AMB amphotericin B
AML acute myelogenous leukemia
ARDS acute respiratory distress syndrome
BAL bronchoalveolar lavage
BID twice daily
CAS caspofungin
CMV cytomegalovirus
CT computed tomography
EGD esophagogastroduodenoscopy
ESBL extended spectrum beta lactamase
FDA US Food and Drug Administration
FLU fluconazole
GERD gastroesophageal reflux disease
GvHD graft versus host disease
HCT hematocrit
Hgb hemoglobin
HiDAC high-dose cytarabine
|
HSCT hematopoietic stem cell transplant
ID infectious disease
IDSA Infectious Diseases Society of America
IFD invasive fungal disease
IFI invasive fungal infection
IPA invasive pulmonary aspergillosis
ISA isavuconazole
ITR itraconazole
L-AmB liposomal amphotericin B
MALDI-TOF matrix assisted laser desorption ionization–time of flight
MIC micafungin; minimum inhibitory concentration
NPM1 nucleophosmin 1
PMH past medical history
PO orally; by mouth
POS posaconazole
qd once daily
rATG rabbit antithymocyte globulin
SCT stem cell transplant; stem cell transplantation
TDM therapeutic drug monitoring
VOR voriconazole
WBC white blood count
|
References
- Alanio A, Bretagne S. Pneumocystis jirovecii detection in asymptomatic patients: what does its natural history tell us? [version 1; referees: 3 approved]. F1000Research 2017, 6(F1000 Faculty Rev):739. (doi: 10.12688/f1000research.10619.1)
- Bassetti M, Righi E, De Pascale G, et al. How to manage aspergillosis in non-neutropenic intensive care unit patients. Crit Care. 2014;18:458.
- Brüggemann RJ, Alffenaar JW, Blijlevens NM, et al. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis. 2009;48:1441-1458.
- CDC. Recommendations for Treatment of Candida auris. https://www.cdc.gov/fungal/diseases/candidiasis/c-auris-treatment.html Accessed May 3, 2018.
- CDC. Recommendations for Identification of C auris. https://www.cdc.gov/fungal/diseases/candidiasis/recommendations.html Accessed May 3, 2018.
- Chowdhary A, Sharma C, Meis JF. Azole-resistant aspergillosis: epidemiology, molecular mechanisms, and treatment. J Infect Dis. 2017;216(suppl 3):S436-S444.
- Hsu LY, Apisarnthanarak A, Khan E, Suwantarat N, Ghafur A, Tambyah PA. Carbapenem-resistant Acinetobacter baumannii and Enterobacteriaceae in South and Southeast Asia. Clin Microbiol Rev. 2017;30:1-22.
- Kakkar M, Walia K, Vong S, Chatterjee P, Sharma A. Antibiotic resistance and its containment in India. BMJ. 2017 Sep 5;358:j2687. doi: 10.1136/bmj.j2687
- Kathuria S, Singh PK, Sharma C, et al. Multidrug-resistant Candida auris misidentified as Candida haemulonii: characterization by matrix-assisted laser desorption ionization-time of flight mass spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CLSI broth microdilution, and Etest method. J Clin Microbiol. 2015;53:1823-1830.
- Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091-1100.
- Labricciosa FM, Sartelli M, Abbo LM, et al. Epidemiology and risk factors for isolation of multi-drug-resistant organisms in patients with complicated intra-abdominal infections. Surg Infect (Larchmt). 2018;19:264-272.
- Lagacé-Wiens P, Walkty A, Karlowsky JA. Ceftazidime-avibactam: an evidence-based review of its pharmacology and potential use in the treatment of Gram-negative bacterial infections. Core Evid. 2014;9:13-25.
- Ledoux MP, Denis J, Nivoix Y, Herbrecht R. Isavuconazole: a new broad-spectrum azole. Part 2: pharmacokinetics and clinical activity. J Mycol Med. 2018;28:15-22.
- Levesque E, Rizk F, Noorah Z, et al. Detection of (1,3)-β-d-glucan for the diagnosis of invasive fungal infection in liver transplant recipients. Int J Mol Sci. 2017;18(4). doi: 10.3390/ijms18040862
- Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760-769.
- Marchetti O, Lamoth F, Mikulska M, et al; European Conference on Infections in Leukemia (ECIL) Laboratory Working Groups. ECIL recommendations for the use of biological markers for the diagnosis of invasive fungal diseases in leukemic patients and hematopoietic SCT recipients. Bone Marrow Transplant. 2012;47:846-854.
- Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162:81-89.
- Meis JF, Chowdhary A, Rhodes JL, Fisher MC, Verweij PE. Clinical implications of globally emerging azole resistance in Aspergillus fumigatus. Philos Trans R Soc Lond B Biol Sci. 2016 Dec 5;371(1709). pii: 20150460. doi: 10.1098/rstb.2015.0460
- Mellinghoff SC, Bassetti M, Dörfel D, et al. Isavuconazole shortens the QTc interval. Mycoses. 2018;61:256-260.
- Mycamine® (micafungin sodium) prescribing information. Northbrook, IL: Astellas Pharma Inc; 2016.
- Noxafil® (posaconazole) prescribing information. Whitehouse Station, NJ: Merck & Co., Inc; 2013.
- Pascual A, Csajka C, Buclin T, et al. Challenging recommended oral and intravenous voriconazole doses for improved efficacy and safety: population pharmacokinetics-based analysis of adult patients with invasive fungal infections. Clin Infect Dis. 2012;55:381-390.
- Patel TS, Pogue JM, Mills JP, Kaye KS Meropenem-vaborbactam: a new weapon in the war against infections due to resistant Gram-negative bacteria. Future Microbiol. 2018 Apr 25. doi: 10.2217/fmb-2018-0054
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1-e60. doi: 10.1093/cid/ciw326
- Sears D, Schwartz BS. Candida auris: an emerging multidrug-resistant pathogen. Int J Infect Dis. 2017;63:95-98.
- Souza AC, Amaral AC. Antifungal therapy for systemic mycosis and the nanobiotechnology era: improving efficacy, biodistribution and toxicity. Front Microbiol. 2017;8:336. doi: 10.3389/fmicb.2017.00336
- Tudor B-A, Slama A, Roth G, Krenn C-G. Amphotericin B® treatment causes QT prolongation in lung transplant-patients. Intensive Care Med Exp. 2015;3(Suppl 1):A213.
- Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335-347.
- van Duin D, Lok JJ, Earley M, et al. Antibacterial Resistance Leadership Group. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis. 2018;66:163-171.
- Vazquez L. Antifungal prophylaxis in immunocompromised patients. Mediterr J Hematol Infect Dis. 2016;8:1-9.
- Wingard JR, Carter SL, Walsh TJ, et al; Blood and Marrow Transplant Clinical Trials Network. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116:5111-5118.
Back Top