ADVERTISEMENT

Identification and Stabilization of Patients with Opioid Use Disorder
INTRODUCTION
The opioid crisis is an encompassing epidemic involving healthcare professionals, social services, the public sector, law enforcement, lawmakers, and many other community advocates across the United States. While most lay press articles focus on the increasing rate of drug overdose deaths, the scope of the problem is much greater, involving millions of Americans in pain, abusing opioids, and dealing with opioid use disorder (OUD).
This module describes the scope of the opioid crisis, the overlap between OUD and chronic opioid use, and the identification and management of opioid withdrawal.
SCOPE OF THE CRISIS
The opioid epidemic is the largest public health crisis in the United States by almost any measure. Some 20 million Americans report chronic pain, more than 10 million report abusing opioids, and the number of Americans with OUD exceeds 2 million. Nearly 600,000 Americans have died from drug overdoses since 1979, and the rate of drug overdose death has followed an exponential curve, doubling approximately every 8 years.1
In 2016, the most recent year with official data, 63,632 Americans died from drug overdose. This represents an increase of more than 20% from 2015, and unofficial estimates suggest this trend continued into 2017. Drug overdose is now the leading cause of death for Americans under 50, the leading cause of injury-related death in the United States, and the primary driver of the reduction in American life expectancy over the past decade. However, provisional data recently released by the Centers for Disease Control and Prevention (CDC) show that drug overdose death rates appear to have plateaued between September 2017 and March 2018 – the most recent time period with available data.2
Drug overdose rates and primary causes of overdose also vary geographically. While the rise of illicit high-potency opioids (e.g., fentanyl, carfentanil) has disproportionately affected overdose rates, the effect is not uniform nationally.1 For example, illicit opioids such as fentanyl and heroin are major drivers of overdose rates in many urban locations and in New England, prescription opioids continue to plague many parts of Appalachia and the Midwest, and methamphetamine is a main contributor to overdose death in the Southwest. The heterogeneous nature of this epidemic has complicated many large-scale efforts aimed at reducing overdose death rates.
Overdose deaths are but one metric used to measure the breadth and impact of this crisis. In 2015, more than 500,000 emergency department visits and more than 300,000 hospitalizations were related to drug poisonings; opioids accounted for 140,000 of these visits and more than 75,000 hospitalizations.3 Fortunately, the rate of nonfatal overdoses decreased approximately 2% in 2017 across states enrolled in the CDC's Enhanced State Opioid Overdose Surveillance (ESOOS) program, which captures fatal and nonfatal overdose data from 32 states and the District of Columbia; however, 5 enrolled states—Illinois, Kentucky, Maine, Virginia, and Wisconsin—reported increases in nonfatal overdoses during this time.
In 2016, more than 48 million Americans older than 12 years of age self-reported misusing prescription or illicit drugs, and 11.5 million (4.3%) misused prescription opioids—nearly 25% of the population for whom opioids were prescribed. Additionally, more than 2.1 million Americans were estimated to have OUD; nearly 90% of these individuals have OUD primarily involving prescription opioids. Unfortunately, of the 7.4 million Americans with any substance use disorder, only 2.1 million received any kind of formalized treatment, with fewer than 1.5 million (19%) receiving treatment at a specialized facility.3
Children are an increasingly vulnerable population as well. Foster care rates had declined annually from 2000 to 2010. From 2013 to 2015, the number of children in foster care increased 7% nationally to 425,000; substance abuse was a factor in nearly 1 in every 3 cases. This too is disproportionate geographically. States hit hardest by the opioid epidemic — such as West Virginia, Ohio, and Kentucky — have had increases in foster care rates of 20% over the past 4 years. Additionally, the number of children referred to child welfare programs (not all of whom end up in foster care) has also dramatically increased. 4
Because of increasing maternal use of opioids during the perinatal period, rates of neonatal abstinence syndrome (NAS) increased by more than 300% between 1999 and 2013. Multiple states now report that upwards of 3% of all newborns are diagnosed with NAS.5
NAS is a constellation of symptoms representative of drug withdrawal that occur when an infant has been exposed to enough of a substance in utero to develop physiologic dependence; opioids are commonly involved in NAS. Symptoms include tremor, twitches, fussiness, poor feeding, diarrhea, trouble sleeping, and runny nose; the condition can progress to seizures. The hospital stay for a newborn with NAS is significantly longer and costs at least 3 times that of an infant born without the condition; in 2012, the estimated cost of NAS exceeded $300 million. The long-term consequences of NAS at this point are unknown.5
An Australian study showed children with a diagnosis of NAS performed significantly worse on standardized testing through seventh grade (the highest grade assessed). In fact, seventh graders with NAS performed worse than fifth graders without NAS in this study.6
A number of infectious complications specifically relate to injection drug use, including transmissible diseases such as human immunodeficiency virus (HIV) and hepatitis B and C viruses (HBV/HCV) as well as infections requiring hospitalization (e.g., endocarditis, osteomyelitis, bacteremia, and skin and soft tissue infections). Approximately 70% of new HCV infections and 1 in 10 HIV new infections occur in people who inject drugs. Between 2004 and 2014, the number of HCV infections in the United States increased by 400%, and HBV infections increased 20% between 2014 and 2015.7 In the decade ending in 2012, hospitalizations for serious infections related to injection drug use nearly doubled, with the largest proportional increase related to cases of septic arthritis and epidural abscesses. Most patients hospitalized with such infections are either discharged home or leave against medical advice. Initiation of treatment for OUD during hospitalization for unrelated diagnoses (e.g., infection) has been shown to significantly increase both outpatient treatment initiation and retention; this is commonly a missed opportunity in American healthcare systems.8
The economic burden of the opioid epidemic in the United States cannot be understated. The annual healthcare cost alone of treating opioid overdose, abuse, and addiction was nearly $100 billion in 2015. Further, the cost to the criminal justice is nearly $8 billion, and lost productivity among those who do not die of an overdose costs more $20 billion (in 2015 dollars). Using the "value of statistical life" estimate from the Council of Economic Advisors, the estimated total cost of the opioid epidemic in 2015 was $504 billion; 85% of this cost was related to overdose fatalities.9 Altarum, a nonprofit agency evaluating economic health, recently estimated the cumulative cost of the opioid epidemic at $1 trillion since 2001; again, the largest costs were attributed to lost productivity among those who die from drug overdose.10 A recent Brookings report estimated that at least 20% of the decline in labor force participation between 2000 and 2015 was the result of opioid overprescribing. Among prime-age men not in the workforce, nearly half take pain medication on a daily basis—primarily prescription pain medication.11
The current drug crisis in the United States, commonly represented by overdose deaths attributed to illicit and prescription opioids, is a complex, multifaceted epidemic impacting nearly every aspect of American life. Comprehensive solutions addressing prevention, treatment, and harm reduction strategies are desperately needed to reduce the scale and impact of drug abuse. The remainder of this module will focus on one facet of treatment: identification and early stabilization of patients with OUD.
TIME COURSE OF OPIOID USE DISORDER
Approximately 8 in 10 individuals who currently misuse heroin were first exposed to opioids as prescription medications.12 Further, 1 in 4 individuals prescribed long-term opioid therapy go on to abuse the drugs, 1 in 10 develop OUD, and 1 in 20 begin use of heroin.13 These sobering statistics, along with the limited evidence base supporting opioid use for treatment of chronic pain,14 has prompted a number of responses from a variety of agencies to reduce the exposure of the general population to unnecessary long-term opioid therapy.
The most notable of these responses was the CDC guidelines for chronic opioid prescribing, which provided 12 recommendations for providers who initiate or continue long-term opioid therapy.15 The recommendation with the strongest support within the guidelines was for prescribers to offer or arrange evidence-based treatment for OUD. Unfortunately, no single screening tool for OUD is recommended in these guidelines.
Several screening tools are available for patients receiving prescription opioids; these tools can provide insights into various risk factors for development of OUD. Among these tools are the Opioid Risk Tool (ORT), the Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R) and the Brief Risk Interview (BRI). A summary of the elements of these instruments is in Table 1. None of these tools has been shown to be superior to the others, and evaluation by the CDC identified serious or very serious limitations regarding the diagnostic accuracy of all screening criteria. Additionally, all 3 tools were limited by "serious imprecision," and the ORT was noted to have "very serious inconsistency."15 Other tools such as the Pain Medication Questionnaire (PMQ); Diagnosis, Intractability, Risk, Efficacy (DIRE); Prescription Drug Use Questionnaire-Self report (PDUQp); Narcotic Risk Manager (NRM); and Brief Risk Questionnaire (BRQ) have similar limitations. To date, no studies have evaluated the efficacy of using urine drug screening to detect opioid abuse in chronic pain.15
| Table 1. Elements of Selected Screening Tools for Opioid Use Disorder |
| Tools |
Administration Method |
Administration Time |
No. Assessment Items |
Elements Assessed |
| Opioid Risk Tool (ORT) |
Self-report |
<1 minute |
10 |
- Age
- Personal or family history of substance abuse
- History of sexual abuse
- Psychological disease
|
| Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R) |
Paper and pencil questionnaire |
<10 minutes |
24 |
- Personal, family, or friend history of substance abuse
- Emotional volatility, coping skills
- Aspects of opioid use
- Criminality
- History of sexual abuse
|
| Brief Risk Interview (BRI) |
Structured clinical interview |
6–12 minutes |
12 |
- Aspects of opioid use
- Psychological disease
- Personal or family history of substance abuse
- Criminality
- Reading ability
|
| Source: Reference 15. |
Although a highly validated tool to detect opioid misuse in chronic pain does not currently exist, several risk factors for development of OUD among exposed individuals have been identified. These include daily dose of prescribed opioid of more than 100 morphine milligram equivalents (MME), use for longer than 3 months, history of depression, history of substance use disorder, and adolescence.16 Using the socio-ecological model, the Substance Abuse and Mental Health Services Administration (SAMHSA) has identified a variety of potential risk factors for development of OUD. These are organized into individual, relationship, community, and societal levels (Figure 1).17
Figure 1. Socioecological Model of Risk Factors for Development of Opioid Use Disorder
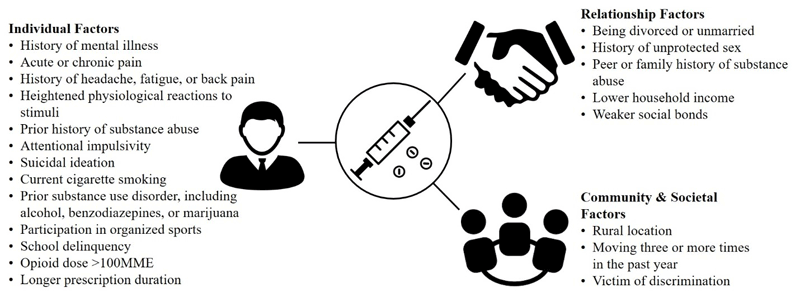 |
| Source: Factors compiled from reference 17. |
Finally, the role of genetic variation or predisposition to development of OUD is unclear at present. Twin studies have documented that genetic variance can explain up to 54% of the liability for OUD; genomewide association studies have also shown that many different alleles may have a small effect in conveying OUD risk, specifically variation at the mu-opioid receptor gene.18 However, most studies are currently limited by small sample size, and there is no available genetic test to predict individual risk of developing OUD.
OPIOID USE DISORDER
The Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM-5), used commonly to standardize evaluation of signs and symptoms in psychiatry, characterizes OUD as signs and symptoms that represent a problematic pattern of opioid use, leading to clinically significant impairment or distress within a 12-month period. The signs and symptoms represent prolonged and compulsive administration of opioids in greater than prescribed quantities or use for no legitimate medical reason.19
Diagnostic Criteria
The diagnosis of OUD is now a single diagnostic category that combines the separate diagnoses of opioid abuse and opioid dependence from the Diagnostic and Statistical Manual of Mental Disorders 4th Edition (DSM-IV). Many of the diagnostic signs and symptoms remain the same, with some minor changes. A history of legal problems was a diagnostic criterion in the DSM-IV, but this is not included in the DSM-5. Craving, or the intense desire or urge to use the opioid, was added to the DSM-5 diagnostic criteria. Additionally, the severity of OUD was added as a specifier in the DSM-5. Mild OUD is characterized by 2 to 3 symptoms, moderate as 4 to 5 symptoms, and severe as the presence of 6 or more symptoms.19
As with all DSM-5 substance use disorders, diagnostic criteria for OUD are categorized into four groups: impaired control, social impairment, risky use, and pharmacologic criteria.19,20
An individual with OUD exhibits a compulsive pattern of behaviors with impaired control over the use of opioids. Patients often take more opioids than intended or over a longer period of time with unsuccessful efforts to control or decrease use despite a desire to do so. Patients also spend a great deal of time trying to obtain, use, or recover from the opioid. Classical conditioning and activation of the reward system in the brain leads to craving. Craving can occur anytime or anywhere; however, it occurs more frequently in the environments where opioids were used previously or in the presence of individuals with whom opioid use occurred.19
Social, occupational, and interpersonal problems often arise with recurrent opioid use. Work, home, or school obligations may not be fulfilled, and social, occupational, or recreational activities may be reduced or given up because of the opioid use.19
Individuals may use opioids repeatedly in physically hazardous situations and may continue using opioids despite having the knowledge that the opioid may have caused or exacerbated a persistent or recurrent physical or psychological problem. Having such a problem is not diagnostic criteria; the inability to stop use of the opioids is an element in diagnsos.19
Among the pharmacologic criteria, tolerance—defined as either the need for more opioids to achieve a desired effect or decreased effect with the same quantity of use—can occur in individuals with OUD. The development of tolerance depends on the individual and the substance being used. Withdrawal can also occur in individuals with OUD. Opioid withdrawal symptoms occur when there is a decline in blood or tissue opioid concentrations below a specific threshold in an individual with a physiologic opioid dependence. Opioids may subsequently be used by the individual to relieve or prevent withdrawal symptoms.19 Tolerance and withdrawal can occur in individuals who chronically take prescribed opioids for a legitimate medical reason; for this reason, those individuals are excluded from the diagnostic criteria. Additionally, it is important to realize that physical dependence on a substance is not necessary for the OUD diagnosis.
Specifiers
As mentioned above, the diagnosis of OUD may include specifiers to determine the patient's current severity. Specifiers for remission status are also included. Remission status can be characterized by the duration an individual no longer meets diagnostic criteria for OUD. Early remission is defined as no longer meeting criteria for at least 3 months but less than 12 months. Sustained remission is no longer meeting criteria for OUD for 12 months or longer. Individuals may still have craving; this symptom is considered an exception during the period of remission.19
Screening
As discussed previously, patients prescribed long-term opioids should be screened for OUD.15 Because of the limitations of established screening tools, asking about opioid misuse can be a natural progression after asking about alcohol use. Screening for OUD, especially in a setting where an individual may abruptly stop taking opioids (e.g., inpatient hospitalization, admission to a rehabilitation facility, or detention in a correctional facility) can also help determine those at risk for opioid withdrawal.21
SIGNS AND SYMPTOMS OF OPIOID WITHDRAWAL
Opioid withdrawal occurs after a reduction in or abrupt cessation of opioid use in a person who is physiologically dependent on opioids.22 Spontaneous withdrawal can occur in individuals who take opioids as prescribed for a legitimate medical reason or in patients with OUD. Precipitated withdrawal occurs when an opioid antagonist or partial agonist is given to an opioid-dependent individual who has a full opioid agonist such as heroin or methadone in their system, thus causing displacement of the full opioid agonist from opioid receptor.22 Both spontaneous and precipitated withdrawal produce signs and symptoms opposite to that of acute intoxication of opioids.22
Clinical manifestations of opioid withdrawal include both subjective and objective symptoms. Subjective symptoms include feelings of restlessness, anxiety, irritability, "achiness," and sensitivity to pain.23 Diagnostic criteria for opioid withdrawal in the DSM- 5 include 3 or more of the following signs and symptoms: dysphoric mood, nausea or vomiting, muscle aches, lacrimation or rhinorrhea, pupillary dilation, piloerection or sweating, diarrhea, yawning, fever, and/or insomnia.19 Other objective symptoms of withdrawal may include tremor and elevated blood pressure.23
The severity and onset of withdrawal symptoms are influenced by the individual's level of dependence and the half-life of the opioid last used. Short-acting opioids such as heroin produce withdrawal symptoms within hours, and these last 7 to 14 days. Conversely, withdrawal symptoms produced by long-acting opioids such as methadone may not have an onset of symptoms for days and produce withdrawal symptoms for several weeks (Table 2).24 Protracted withdrawal symptoms—such as fatigue, anhedonia, poor appetite, insomnia, and depression—can occur after the acute symptoms and may last for several weeks. Patients may also be at risk for suicide.19
| Table 2. Opioid Withdrawal Symptom Timeline |
| Opioids |
Onset (h) |
Peak (h) |
Duration (d) |
| Fentanyl |
3–5 |
8–12 |
4–5 |
Heroin
Oxycodone
Hydrocodone
Codeine
Morphine |
8–12 |
36–72 |
7–14 |
Methadone
Buprenorphine |
24–72 |
4–6 |
14–21 |
| Source: Reference 24. |
Assessment
A thorough evaluation of patients at risk for opioid withdrawal should be completed as well as a medical and psychiatric history and physical examination.25
The Clinical Opiate Withdrawal Scale (COWS) is a validated, clinician-rated tool for assessing the acute opioid withdrawal severity.22 COWS includes 11 objective and subjective signs and symptoms of opioid withdrawal that are each scored from 0 to 4 or 5 based on the sign and symptom severity.25 A COWS score up to 12 is considered mild withdrawal, 13 to 24 is moderate withdrawal, 25 to 36 is moderately severe withdrawal, and scores greater than 36 are considered severe withdrawal.22 This scale can be used for monitoring in the setting of acute opioid withdrawal or monitoring of precipitated withdrawal when buprenorphine (partial opioid agonist) or naltrexone (opioid antagonist) is being started.
PHARMACOLOGIC TREATMENT OF ACUTE OPIOID WITHDRAWAL
Medically supervised opioid withdrawal, often referred to as detoxification, uses alternative medications to reduce the symptoms of withdrawal as the offending opioids are stopped.23,24 Although detoxification can help patients feel better and facilitate participation in treatment decision making and rehabilitation, it usually does not produce long-term abstinence for patients with OUD, and it is not the standard of care for pregnant women with OUD.24,26 Detoxification reduces tolerance, which can increase the risk of overdose and death if a person relapses and begins using the same amount of opioids as before detoxification. Unmedicated opioid withdrawal is rarely life threatening.27
Medications can help control the rate of opioid cessation and/or relieve symptoms of withdrawal.23 Use of long-acting alternative opioids is the most effective treatment to relieve symptoms in patients in acute opioid withdrawal.28 However, these agents can be initiated, prescribed, and administered only under specific conditions as discussed below.29 Therefore, nonopioid medications are also used to assist with symptom control, although less effectively.24,26,30 Ultrarapid opioid detoxification (UROD) can be performed to precipitate withdrawal while patient is under general anesthesia; however, this is not recommended by the American Society of Addiction Medicine (ASAM) and is not discussed in this program.31
Methadone
Methadone is a long-acting, full mu-opioid agonist that is approved by FDA for detoxification from opioids, treatment of OUD, and pain.32 When used for OUD or opioid withdrawal, methadone can be administered and dispensed only in a SAMHSA-certified and Drug Enforcement Administration (DEA)–registered opioid treatment program (OTP).33 However, the Controlled Substances Act does allow exceptions to this. The so- called "3-day rule" allows practitioners to administer but not prescribe methadone (or buprenorphine) to a patient in acute opioid withdrawal for up to 72 hours while arrangements are being made for ongoing care (maintenance treatment or detoxification). This 72-hour limit does not apply when withdrawal or maintenance is being managed during an admission for medical or surgical conditions other than opioid dependency (e.g., following a motor vehicle accident or myocardial infarction) and transfer would complicate treatment. In addition, a Drug Addiction Treatment Act of 2000 (DATA 2000) waiver is not required for practitioners to administer buprenorphine or methadone in such circumstances, although they should consult with the patient's substance misuse treatment provider as needed.20
When treating opioid withdrawal, a tapered dose of methadone replaces the illicit opioid. Significant variability exists in the duration of methadone tapers, and the taper regimen should be tailored specifically to each individual. Short-term detoxification can be completed over 3 to 4 weeks, but detoxification using a maintenance dose of methadone may require months of treatment.23,32 Methadone tapering can occur in the inpatient or outpatient setting. In the outpatient setting, OTPs provide directly observed therapy in which the patient is supervised during his or her self-administration of daily doses of methadone.25
An initial methadone dose of 20 mg to 30 mg daily can be administered under supervision to assist in mitigating withdrawal symptoms. Peak levels generally occur in 2 to 4 hours; dose adjustments should be made after reassessment at that time. The maximum dose on the first day generally should not exceed 40 mg. After stabilization at a dose that adequately alleviates withdrawal symptoms, the dose should be maintained for 2 to 3 days, at which point the dose can be gradually decreased every 1 to 2 days. For short-term detoxification, a 10%–20% daily dose reduction is usually tolerated. Patients requesting detoxification from long-term methadone maintenance treatment for OUD usually require dose reductions of less than 10% at intervals of 10 to 14 days.32
The most frequent adverse effects of methadone include lightheadedness, dizziness, sedation, nausea, vomiting, and sweating. As a full mu-opioid agonist, methadone can produce respiratory depression and death, necessitating careful monitoring during treatment initiation and dose titration.32 Methadone also carries the risk of QT-interval prolongation and torsades de pointes through inhibition of cardiac potassium channels. This risk has been typically associated with higher doses and multiple daily doses, but it has also been reported with typical dosing. Monitoring is recommended for individuals with a history of cardiac conduction abnormalities, those concomitantly using medications that affect cardiac conduction, or those at increased risk of dysrhythmias.32,34
Methadone undergoes hepatic metabolism through multiple cytochrome P (CYP) 450 enzymes including CYP3A4, CYP2B6, CYP2C19, CYP2C9, and CYP2D6; therefore, inducers and inhibitors of these enzymes can affect the pharmacokinetic and pharmacodynamics of methadone. The antiretrovirals efavirenz, nelfinavir, nevirapine, ritonavir, and lopinavir/ritonavir and the CYP3A4 inducers rifampin, phenytoin, phenobarbital, carbamazepine, and St. John's wort have been shown to reduce the plasma levels of methadone. Methadone clearance is decreased by CYP450 inhibitors, such as azole antifungals, macrolide antibiotics, and some selective serotonin reuptake inhibitors (sertraline and fluvoxamine). Careful monitoring of drug interactions and their effects is recommended.32
Buprenorphine
Buprenorphine is a long-acting, partial mu-opioid agonist that is approved by FDA for the treatment of OUD. It is also often used during management of opioid withdrawal.24 Buprenorphine can be prescribed or dispensed by healthcare practitioners (physicians, nurse practitioners, and physician assistants) who have a DATA 2000 waiver or by OTPs without a waiver. With this waiver, certification, or DEA registration, the prescriber is given a DEA license number that begins with the letter "X". Practitioners with this "X- license" can provide office-based opioid treatment in an outpatient setting using prescriptions for buprenorphine or buprenorphine/naloxone. Limits are set to treatment of 30 patients at one time during the first year of practice and 100 patients thereafter. Through reapplication, physicians can treat up to 275 patients beginning in the third year.20
Buprenorphine is available alone or in combination with naloxone. Naloxone, a mu- opioid receptor antagonist, is added to prevent misuse of buprenorphine. When taken as directed, naloxone has poor bioavailability and therefore does not exert its antagonistic effect. However, if buprenorphine/naloxone is crushed or dissolved and used intravenously or intranasally, the naloxone antagonism takes effect and prevents the binding of buprenorphine to the opioid receptor.35 For the purpose of this discussion, buprenorphine, unless otherwise specified, will refer to both the buprenorphine alone and buprenorphine/naloxone combination product.
Buprenorphine is not approved by FDA for treatment of opioid withdrawal. However, buprenorphine is comparable to methadone in reduction of opioid withdrawal symptoms.24 As a mu-opioid receptor partial agonist, buprenorphine acts as an agonist when no other mu-opioid agonists are present, but as an antagonist when mu-opioid agonists, such as heroin or methadone, are bound to the opioid receptor or taken while buprenorphine is present. Therefore, precipitated withdrawal can occur if recent use has occurred. To reduce the risk of precipitated withdrawal, opioid-dependent patients should be experiencing mild-to-moderate withdrawal symptoms before starting buprenorphine. The ASAM recommends waiting to start buprenorphine 12 to 18 hours after a short-acting opioid is used, and 24 to 48 hours after a long-acting opioid is used. A COWS score can be assessed to determine the severity of withdrawal, with a score of 11 to 12 or greater being safe for buprenorphine administration. COWS can also be used to monitor for precipitated withdrawal after buprenorphine administration.31
Starting doses of buprenorphine are generally 2mg to 4 mg with observation for 1 hour after administration. If there is symptom improvement, an additional 2 to 4 mg can be taken 2 more times throughout the day. Buprenorphine 4 to 16 mg daily is generally enough to mitigate withdrawal effects; the dose of buprenorphine is tapered over several days to weeks by 10% to 20% every 1 to 2 days. No specific duration of tapering is supported by an adequate body of evidence; tapering should be tailored to each individual's needs. If a patient wishes to withdraw from treatment with buprenorphine maintenance for OUD, then tapering over several months may be necessary. A reduction in the starting dose by one-half and a slower dose titration is recommended for patients with severe hepatic impairment.36
Adverse effects of buprenorphine include headache, anxiety, constipation, perspiration, fluid retention, urinary hesitancy, and sleep disturbance. As a partial agonist, buprenorphine has a lower risk of respiratory depression than methadone; however, the risk increases with concomitant use with other CNS depressants. Buprenorphine also does not carry the risk of QT-interval prolongation that is seen with methadone. Buprenorphine is metabolized by the cytochrome CYP3A4; therefore, drug interactions with inducers and inhibitors of this enzyme should be monitored.36
Nonopioid Medications
Multiple nonopioid medications have been used to help alleviate the signs and symptoms of opioid withdrawal. The best studied medications are the alpha-2 adrenergic agonists, which include clonidine and the recently FDA-approved lofexidine. The alpha-2 agonists can be used alone or in combination with opioid agonists to help reduce anxiety, piloerection, and signs or symptoms of autonomic overactivity secondary to noradrenergic rebound that occurs during opioid detoxification.28,35,37 Buprenorphine is more effective at relieving withdrawal symptoms than alpha-2 agonists.24 However, because of the limitations placed on prescribing and administration of the opioid agonists for OUD and withdrawal, alpha-2 agonists may be the only option for some prescribers.31
Although not approved by FDA for treatment of opioid withdrawal, clonidine has been used historically. Doses of 0.1 mg to 0.3 mg every 6 to 8 hours are generally used up to a maximum daily dose of 1.2 mg. The dose is then tapered by 0.1 mg per dose every 1 to 2 days. Hypotension and bradycardia limit the ability to use clonidine, and blood pressure and heart rate should be routinely monitored.31 Other common adverse effects include drowsiness, headache, dizziness, dry mouth, and abdominal pain.38
Lofexidine, an alpha-2 agonist, was recently approved for the treatment of opioid withdrawal symptoms in the United States; it was approved in the United Kingdom in 1992. In clinical trials, lofexidine demonstrated greater efficacy than placebo in reduction of opioid withdrawal symptoms with fewer hypotensive adverse effects.39
The usual starting lofexidine dose is 3 of the 0.18-mg tablets taken 4 times a day during the peak of opioid withdrawal symptoms, with a maximum daily dose of 2.88 mg (16 tablets) and a single dose not to exceed 0.72 mg (4 tablets). The dose is then tapered over 2 to 4 days by reducing the dosage by 1 tablet per dose every 1 to 2 days. Dosing is guided by relief of withdrawal symptoms and can be continued for up to 14 days. Dose reductions are recommended for hepatic impairment; product labeling recommends reduction to 2.16 mg/d for Child–Pugh scores of 5–6, 1.44 mg/d for scores of 7–9, and 0.72 mg/d for those with scores of 10 or greater.40
Common adverse effects of lofexidine include orthostatic hypotension, bradycardia, dizziness, somnolence, sedation, and dry mouth. Patients being treated in the ambulatory setting should be instructed on self-monitoring for hypotension, orthostasis, and bradycardia. QT-interval prolongation can occur; electrocardiographic monitoring is recommended for at-risk patients (those with congestive heart failure, bradyarrhythmias, hepatic impairment, or renal impairment) or in patients taking this agent in combination with methadone. Lofexidine is metabolized by cytochrome CYP2D6, and the CYP2D6 inhibitor paroxetine has been shown to increase the risk of adverse effects.40
Other nonopioid medications used for symptomatic relief include loperamide for diarrhea, prochlorperazine or ondansetron for nausea and vomiting, and acetaminophen or nonsteroidal anti-inflammatory drugs for pain. Benzodiazepines can be used to treat anxiety, but these should generally be avoided in patients receiving opioids (including methadone and buprenorphine) due to an increased risk of respiratory depression.31
Comparison of Medications for Opioid Withdrawal
Key characteristics of medications used in opioid withdrawal are summarized in Table 3. Buprenorphine and methadone are considered equally effective for opioid withdrawal, but buprenorphine may resolve symptoms more quickly. Further studies are recommended to confirm this observation.24,26 The alpha-2 agonists, clonidine and lofexidine, have been shown to be less effective than buprenorphine in reducing the severity and duration of opioid withdrawal as well as facilitating treatment completion.24,35 Compared with methadone, the alpha-2 agonists showed similar opioid withdrawal outcomes but had higher rates of adverse effects. Methadone treatment duration was also significantly longer than treatment with alpha-2 agonists.37 Further studies are recommended.
| Table 3. Medications Used for Opioid Withdrawal |
| Medications |
Initial Target Dose |
Tapering Schedule |
Adverse Effects |
| Methadone |
20–30 mg/d |
- Individualized based on patient's withdrawal symptoms
- Short-term detoxification: 10%–20% decrease in daily dose
- Detoxification from methadone maintenance: <10% decrease in dose every 10–14 days
|
- Lightheadedness
- Sedation
- Nausea/vomiting
- Respiratory depression
- QT-interval prolongation
|
| Buprenorphine |
4–16 mg/d |
- Individualized based on patient's withdrawal symptoms
- Short-term detoxification: 10%–20% decrease in daily dose
- Detoxification from buprenorphine maintenance: <10% decrease in dose every 10–14 days
|
- Respiratory depression
- Headache
- Anxiety
- Constipation
- Perspiration
- Fluid retention
- Urinary hesitancy
- Sleep disturbance
|
| Lofexidine |
0.54 mg 4 times a day |
- Individualized based on patient's withdrawal symptoms
- Reduce by 1 tablet per dose every 1 to 2 days
|
- Orthostatic hypotension
- Bradycardia
- Dizziness
- Somnolence
- Sedation
- Dry mouth
- QT-interval prolongation
|
| Clonidine |
0.1–0.3 mg every 6–8 h |
- Individualized based on patient's withdrawal symptoms
- 0.1 mg per dose every 1–2 days
|
- Hypotension
- Bradycardia
- Drowsiness
- Headache
- Dizziness
- Dry mouth
- Abdominal pain
|
| Source: Compiled from references 15 and 17. |
SUMMARY
The opioid crisis and OUD affect a substantial number of Americans. Because of the lack of reliable predictors of opioid misuse and OUD, every patient prescribed opioids for chronic pain should be screened for potential OUD before and throughout treatment. Patients with OUD or who use opioids chronically are at risk for opioid withdrawal; monitoring signs and symptoms of withdrawal with the COWS tool can help tailor treatment with methadone, buprenorphine, or nonopioid treatment options, including the alpha-2 agonist lofexidine. Referral should be made for further treatment of OUD after detoxification to prevent relapse.
Pearls
- Screening for OUD should occur in any patient receiving long-term opioids, but most screening tools have substantial limitations.
- Established risk factors for development of OUD are grouped into individual, relationship, community, and societal levels. Most are individual.
- The DSM-5 provides diagnostic criteria for OUD based on history of impaired control, social impairment, risk use, and pharmacologic criteria.
- Opioid withdrawal occurs after reduction in or abrupt cessation of opioid use in a person who is physiologically dependent on opioids.
- Acute signs and symptoms of opioid withdrawal include dysphoric mood, nausea or vomiting, muscle aches, lacrimation or rhinorrhea, pupillary dilation, piloerection or sweating, diarrhea, yawning, fever, and insomnia.
- The Clinical Opioid Withdrawal Scale (COWS) is used to assess acute opioid withdrawal severity.
- Methadone is approved by FDA for opioid detoxification and is dispensed and administered through opioid treatment programs (OTPs). However, practitioners can administer but not prescribe methadone (or buprenorphine) to a patient in acute opioid withdrawal for up to 72 hours while arrangements are being made for ongoing care. When unrelated procedures require management for longer time periods (following myocardial infarction or motor vehicle accident, for example), these practitioners can continue methadone or buprenorphine detoxification without being in an OTP or having a DATA 2000 waiver.
- Buprenorphine can be prescribed or dispensed by healthcare practitioners who have a DATA 2000 waiver or through OTPs without a waiver for opioid detoxification.
- Lofexidine, an alpha-2 agonist, was recently approved by FDA for treatment of opioid withdrawal and can be prescribed without restrictions.
- Significant variability exists in the duration of tapers of each medication used for opioid withdrawal. The taper regimen should be tailored specifically to each individual.
- Medication-assisted therapy is the standard of care in management of OUD. Detoxification, while sometimes used in nonpregnant patients, reduces tolerance and places individuals at high risk for overdose and death; because of this, referral should be made for further treatment of OUD after detoxification to prevent relapse.
|
References
- Jalal H, Buchanich JM, Roberts MS, et al. Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science. 2018;361(6408).
- Ahmad FB, Rossen LM, Spencer MR, et al. Provisional drug overdose death counts. National Center for Health Statistics. 2018. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Accessed November 1, 2018.
- Centers for Disease Control and Prevention. 2018 Annual Surveillance Report of Drug-Related Risks and Outcomes — United States. Surveillance Special Report. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. https://www.cdc.gov/drugoverdose/pdf/pubs/2018-cdc-drug-surveillance-report.pdf. Published August 31, 2018. Accessed November 1, 2018.
- Collier L. Young victims of the opioid crisis. Monitor Psychol. 2018;49(1):18.
- Ko JY, Patrick SW, Tong VT, et al. Incidence of neonatal abstinence syndrome — 28 states, 1999-2013. MMWR Morb Mortal Wkly Rep. 2016;65(31):799-802.
- Oei JL, Melhuish E, Uebel H, et al. Neonatal abstinence syndrome and high school performance. Pediatrics. 2017;139(2).
- Zibbell JE, Asher AK, Patel RC, et al. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health. 2018;108(2):175-181.
- Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376.
- The Council of Economic Advisors. The underestimated cost of the opioid crisis. https://www.whitehouse.gov/sites/whitehouse.gov/files/images/The%20Underestimated%20Cost%20of%20the%20Opioid%20Crisis.pdf. Published November 2017. Accessed November 1, 2018.
- Altarum. Economic toll of the opioid crisis in U.S. exceeded $1 trillion since 2001. https://altarum.org/news/economic-toll-opioid-crisis-us-exceeded-1-trillion-2001. Published February 13, 2018. Accessed November 1, 2018.
- Krueger A. Where have all the workers gone? An inquiry into the decline of U.S. labor force participation rate. Brookings Papers on Economic Activity. 2017.
- Muhuri P, Gfroerer J, Davies M. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Review. https://www.samhsa.gov/data/sites/default/files/DR006/DR006/nonmedical-pain-reliever-use-2013.htm. Published August2013. Accessed November 1, 2018.
- Carlson RG, Nahhas RW, Martins SS, et al. Predictors of transition to heroin use among initially non-opioid dependent illicit pharmaceutical opioid users: a natural history study. Drug Alcohol Depend. 2016;160:127-134.
- Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA. 2018;319(9):872-882.
- Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645.
- Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253-1263.
- Substance Abuse and Mental Health Services Administration. Preventing prescription drug misuse: understanding who is at risk. https://www.samhsa.gov/capt/sites/default/files/resources/preventing-prescription-drug-misuse-understanding.pdf. Published May 2016. Accessed November 1, 2018.
- Berrettini W. A brief review of the genetics and pharmacogenetics of opioid use disorders. Dialogues Clin Neurosci. 2017;19(3):229-236.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American Psychiatric Association; 2013.
- Substance Abuse and Mental Health Services Administration. Detoxification and Substance Abuse Treatment. Treatment Improvement Protocol (TIP) Series 45 HHS Publication No (SMA) 15-4131. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2015. https://store.samhsa.gov/shin/content//SMA13-4131/SMA13-4131.pdf. Accessed October 24, 2018.
- U.S. Department of Health and Human Services (HHS) Office of the Surgeon General. Facing Addiction in America: The Surgeon General's Spotlight on Opioids. Washington, DC: U.S. Department of Health and Human Services, 2018. https://addiction.surgeongeneral.gov/sites/default/files/Spotlight-on-Opioids_09192018.pdf. Accessed October 24, 2018.
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35(2):253-259.
- Amato L, Davoli M, Minozzi S, et al. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst Rev. 2013(2):CD003409.
- Gowing L, Ali R, White JM, Mbewe D. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017;2:CD002025.
- Fareed A, Patil D, Scheinberg K, et al. Comparison of QTc interval prolongation for patients in methadone versus buprenorphine maintenance treatment: a 5-year follow-up. J Addict Dis. 2013;32(3):244-251.
- Amato L, Davoli M, Ferri M, et al. Effectiveness of interventions on opiate withdrawal treatment: an overview of systematic reviews. Drug Alcohol Depend. 2004;73(3):219-226.
- Farrell M. Opiate withdrawal. Addiction. 1994;89(11):1471-1475.
- Meader N. A comparison of methadone, buprenorphine and alpha(2) adrenergic agonists for opioid detoxification: a mixed treatment comparison meta-analysis. Drug Alcohol Depend. 2010;108(1-2):110-114.
- Sullivan LE, Fiellin DA. Narrative review: buprenorphine for opioid-dependent patients in office practice. Ann Intern Med. 2008;148(9):662-670.
- Gowing L, Farrell M, Ali R, White JM. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2009(2):CD002024.
- American Society of Addiction Medicine. The ASAM: National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. Chevy Chase, MD: American Society of Addiction Medicine; 2015:1-60. https://www.asam.org/docs/default-source/practice-support/guidelines-and-consensus-docs/asam-national-practice-guideline-supplement.pdf?sfvrsn=24. Accessed October 24, 2018.
- Dolophine Hydrochloride (methadone hydrochloride) [package insert]. Roxane Laboratories, Inc., Columbus, OH, 2006. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/006134s028lbl.pdf. Accessed October 24, 2018.
- Day E, Ison J, Strang J. Inpatient versus other settings for detoxification for opioid dependence. Cochrane Database Syst Rev. 2005(2):CD004580.
- Mujtaba S, Romero J, Taub CC. Methadone, QTc prolongation and torsades de pointes: current concepts, management and a hidden twist in the tale? J Cardiovasc Dis Res. 2013;4(4):229-235.
- Diaper AM, Law FD, Melichar JK. Pharmacological strategies for detoxification. Br J Clin Pharmacol. 2014;77(2):302-314.
- Suboxone (buprenorphine/naloxone) [package insert]. Richmond, VA: Reckitt Benckiser Pharmaceuticals Inc.; 2011. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020733s007s008lbl.pdf. Accessed November 5, 2018.
- Gowing L, Farrell MF, Ali R, White JM. Alpha-2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2014(3):CD002024.
- Catapres (clonidine hydrochloride) [package insert]. Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT. 2009. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/017407s034lbl.pdf. Accessed November 5, 2018.
- Strang J, Bearn J, Gossop M. Lofexidine for opiate detoxification: review of recent randomised and open controlled trials. Am J Addict. 1999;8(4):337-348.
- Lucemrya (lofexidine) [package insert]. US WorldMeds, LLC, Louisville, KY. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209229s000lbl.pdf. Accessed October 24, 2018.
Back to Top