ADVERTISEMENT

New Concepts in Patient Blood Management:
How Hospital-Based Pharmacists Can Manage Anemia and Reduce Transfusion Rates
Introduction
Many procedures and medical conditions in the hospital setting are associated with blood loss that may ultimately require a transfusion. Transfusions are costly, medically risky, and tap the precious resource of human blood. The concept of patient blood management (PBM) has evolved to reduce the need for blood transfusions by utilizing alternative strategies and preventive approaches.1-3 PBM is defined as "a multidisciplinary process to promote the optimal use of blood products throughout the hospital." Blood management encompasses the goal of safe and efficient use of various blood-related resources and therapies. Key concepts of BPM are shown in Figure 1.4-6
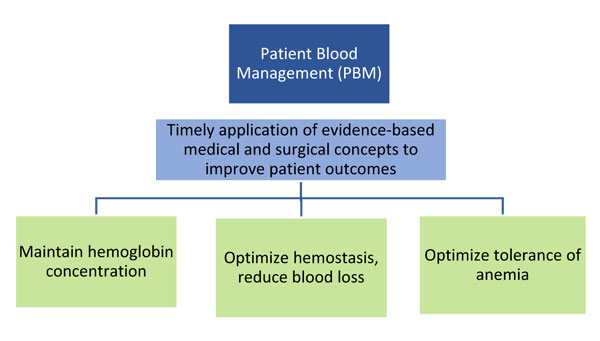 |
| Figure 1. Key Concepts of Patient Blood Management4-6 |
Successful blood management requires multidisciplinary involvement.7 Hospital pharmacists are an essential part of this team, with responsibilities related to blood management protocols, anemia screening and prevention, administration of erythropoietin-stimulating agents (ESAs), and other medication-related roles.8-10
|
"The key question is no longer whether the patient requires a transfusion, but what else can be done...to reduce the likelihood of needing a transfusion and achieving better clinical outcomes. This situation provides several opportunities for pharmacists to fulfill their critical role in contributing to improving the quality of care and well-being of their patients."8
—Shander A, et al. Patient blood management: A role for pharmacists.
Am J Health Syst Pharm. 2017;74(1):e83-e89.
|
Blood Management in the Hospital Setting
Blood transfusion has become one of the most common procedures performed in hospitalized patients in the U.S., with an estimated 13.1 million units of blood transfused in 2013.11 This life-saving procedure is medically necessary in some clinical situations.12 However, blood is an increasingly precious and costly resource for a number of reasons, including fewer eligible donors and the heightened need for testing and monitoring of blood products.13,14 Blood demand has been steadily increasing by 2% to 3% per year in the U.S.15 Increasing transfusion rates in American hospitals are putting severe demand on the blood supply and intensifying the need for greater awareness of PBM concepts. There is growing recognition of the urgent need to conserve blood at many levels. Some key ways to conserve blood include monitoring the sampling of blood (limiting blood wastage or excessive draws), conserving blood transfusion resources, and preventing anemia in patients who are at risk—especially in the perioperative and postoperative periods of certain surgical procedures.14,16
The need to practice strategies for conserving the blood supply applies to all clinical staff, including pharmacists, who often handle responsibilities such as development of procedures or protocols, oversight, and monitoring of medications used to reduce the need for transfused blood.9,17
With the PBM approach, allogeneic (donated) blood is not viewed as the treatment of default for anemic or at-risk patients, but one of several treatment modalities that should be determined based on the needs of the individual patient.5 In fact, the current philosophical shift is to recognize blood transfusion not just as an infusion of fluids into the patient, but more like a tissue transplant or "liquid organ transplant," with inherent associated risks.18 Complications associated with blood products include infection risk, anaphylaxis, and transfusion-associated circulatory overload or lung injuries.18 Another concern is that of "storage lesions," due to destabilization of stored blood, which could affect the recipient over long-term periods.19
|
"Allogeneic blood transfusion is not [just] a simple IV therapy but a de facto tissue transplant, with a host of complexities and complications not to be taken lightly." 8
—Shander A, et al. Patient blood management: A role for pharmacists.
Am J Health Syst Pharm. 2017;74(1):e83-e89.
|
Hospitals and medical organizations are placing greater emphasis on the importance of blood conservation.20 This need is driven by multiple factors, including economics, supply and demand of blood products, and patient safety. Blood transfusions are:
- Costly: Indirect and direct costs of red blood cell transfusions have been estimated from $522 and $1,183 per red blood cell (RBC) unit and continue to climb.21
- Unsustainable: There is an imbalance between the increasing demand on the supply of human blood for transfusion and the dwindling reserves of blood from eligible donors.13
- Labor-intensive: Associated costs that are often overlooked include testing patients before transfusion, storing blood, dispensing from blood bank, and the labor involved in transfusion procedures.22
- High Risk: Safety issues may include elevated infection risk (e.g. pneumonia, hospital-acquired infections), need for ICU stay, risk of antibody or allergic reactions, and mortality risk.23
- Often unnecessary. In a study cited at the International Conference on Transfusion Outcomes on 450 transfusions from a variety of clinical scenarios, between 52% and 69% of transfusions were shown to be inappropriate, while the necessity of another 22% to 35% was uncertain.24
The Role of Anemia in Patient Blood Management
According to the Society for the Advancement of Blood Management (SABM), anemia is a widely under-recognized condition that is often "normalized" by medical professionals—that is, viewed as a relatively harmless problem.25 In fact, anemia is associated with poor medical and surgical outcomes and is an independent risk factor for morbidity and mortality in both the hospital and outpatient surgical ettings.25
Anemia is common in older patients and those with chronic diseases. Unexplained anemia (hemoglobin less than 12 g/dL) occurs in about 24% of adults over age 65 and increases substantially among patients in their 70s and 80s.26,27 Anemia is a common complication of chronic diseases, due in part to impaired iron absorption and metabolism.28 As illustrated in Figure 2, about 40% to 60% of adults with chronic health conditions such as chronic kidney disease (CKD) have anemia.25 Significant proportions of hospitalized patients have either absolute iron deficiency anemia, or functional iron deficiency, meaning they have adequate iron stores but have insufficient iron mobilization to adequately support metabolic needs.25,29 Anemia may be hospital-acquired, with a significant impact on length of stay, mortality risk, and cost of care.30,31
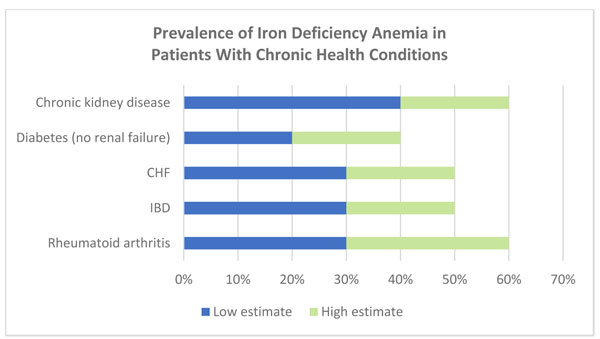 |
Figure 2. Prevalence of Iron Deficiency Anemia in Patients With Chronic Health Conditions
CHF, chronic heart failure; IBD, inflammatory bowel disease.
Data adapted from: Society for the Advancement of Blood Management (SABM). Emerging Evidence on Anemia, Transfusion, Intravenous Iron, and Patient Outcomes: The Case for Expanded Coverage of Intravenous Iron. 2019.25 |
Although anemia is considered epidemic, its prevalence tends to be underrecognized among medical professionals.28 An important reason to take anemia seriously is its association with heightened mortality risk. In a sample from a Medicare database over 1 million older patients, those with heart failure (HF) and anemia had a relative risk (RR) of 2-year mortality of 3.8 (vs. 2.86 for HF alone) and those with CKD and anemia had 3.37 RR (vs. 2.05 for CKD alone). The combination of heart failure, CKD, and anemia carried a 2-year mortality RR of 6.07.32
Managing Anemia in Pre- and Postsurgical Patients
Anemia is an underrecognized but modifiable risk factor among presurgical patients. Among patients who are undergoing a planned (non-emergent) surgical procedure, an estimated 33% have potentially treatable anemia.21 Preoperative anemia is the most frequent predictor of the need for perioperative transfusion, as observed in a systematic review of 62 studies.33 In a large proportion of cases, the anemia could have been treated prior to surgery to avoid the need for a transfusion.34 Among the dangers of untreated preoperative anemia is an increased risk of mortality of 40% or higher.35,36 Even mild preoperative anemia (Hb 10–12 g/dL in women or 10–13 g/dL in men) was shown to be independently associated with a 41% increased risk of mortality and a 31% increase in morbidity in patients undergoing major non-cardiac surgery.35 In a cohort of 7,759 patients undergoing non-cardiac surgeries between 2003 and 2006 at a single center, 90-day mortality risk increased from 0.8% for those with no anemia to 2.9% for patients with pre-operative anemia (defined as Hb<12 g/DL for women and <13 g/dL for men) (Figure 3).25
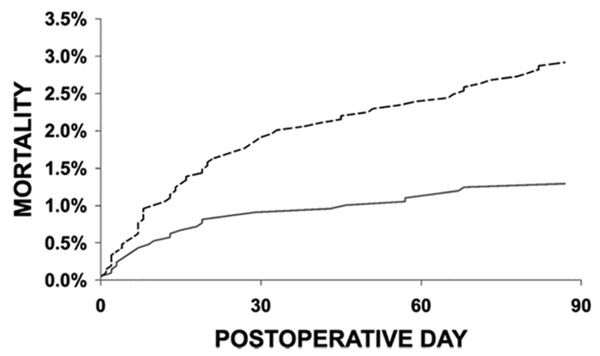 |
Figure 3. Preoperative Anemia Increases Risk of Postoperative Anemia25
Risk-adjusted effect of anemia on postoperative mortality. Time to event comparison for anemic (dashed line) versus non-anemic (solid line) patients. Reprinted with permission from: Beattie WS, et al. Anesthesiology. 2009;110:574-581. |
Screening and diagnosis of iron deficiency and anemia prior to surgery is an important step in improving patient outcomes. Early detection of anemia improves readiness for surgery, reduces risk of transfusion during the perioperative period, and may help to identify comorbid health conditions.37 Recommended laboratory tests to evaluate anemia prior to surgery are listed in Table 1.37
| Table 1. Recommended Laboratory Tests for Detection of Anemia Preoperatively |
| First-tier, general |
| Complete blood count (CBC) |
| Vitamin B12 |
Reticulocyte count
(absolute, reticulocyte hemoglobin content) |
| Folate |
| Serum creatinine |
| First-tier, iron |
| Transferrin saturation |
| Serum ferritin |
| Hemoglobin |
| Iron-binding capacity |
| Second-tier (if diagnosis cannot be made) |
| Thyroid-stimulating hormone (TSH) |
| Direct antiglobulin test (DAT) |
| C-reactive protein (CRP) |
| Soluble transferrin receptor |
| Methyl malonic acid |
| Serum protein electrophoresis |
| Erythropoietin |
| Haptoglobulin |
| Source: Society for the Advancement of Blood Management (SABM)37 |
In its 2019 report, "Anemia in the Pre-Surgical Patient: Recognition, Diagnosis and Management," the SABM presented an algorithm to guide decision-making when patients test positive for anemia in the preoperative stages (Figure 4).37 The authors noted that in cases where ferritin, iron saturation values, or other markers of iron-restricted erythropoiesis are inconclusive, further evaluation may be necessary to rule out iron deficiency or iron sequestration due to inflammation/chronic disease.38 The document further notes that anemia in the setting of decreased transferrin saturation (< 20%) with decreased glomerular filtration rate (GFR < 60) will often respond to intravenous iron.37
| Figure 4. SABM Management Algorithm for Preoperative Anemia37 |
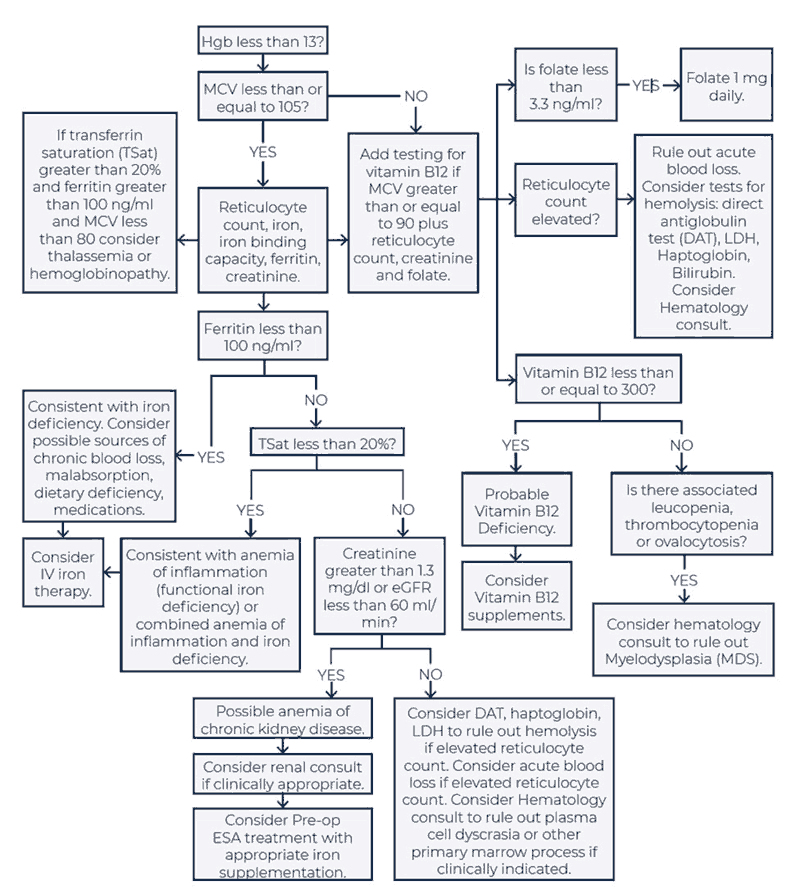 |
Hgb, hemoglobin; MCV, mean corpuscular volume; DAT, direct antiglobulin test; LDH, lactate dehydrogenase; TSat, transferrin saturation; IV, intravenous
Reproduced with permission from the Society for the Advancement of Blood Management (SABM). |
Use of Intravenous Iron to Prevent the Need for Perioperative Blood Transfusions
The presence of severe anemia is one of the most common reasons why patients receive blood transfusions during and after surgery.39 Perioperative transfusion is associated with additional increases in morbidity and mortality risk. There is robust evidence that identification and treatment of preoperative anemia can improve many aspects of patient outcomes and reduce the need for transfusion. In addition to the effect on health for patients with chronic disease, treatment of preoperative anemia:40,41
- Improves readiness for surgery
- Reduces rates of transfusion during perioperative period
- Reduces morbidity and mortality associated with anemia and blood transfusions
Effective approaches to treating preoperative anemia may include oral or IV iron, vitamin B12, folate, and ESAs.14,16,42 Oral iron may be sufficient for some patients with anemia. Oral iron supplementation can be considered if the patient is able to tolerate this therapy and if there is sufficient time (e.g., 2 to 4 months) prior to surgery to increase iron stores. However, oral iron may be inadequate for preoperative patients due to its relatively slow rates for iron repletion, high incidence of gastrointestinal (GI) intolerance, and complications in patients with certain diseases.43-46 Available oral iron preparations are summarized in Table 2.47
| Table 2. Commonly Available Oral Iron Formulations47 |
Agent
(% elemental iron) |
Dosage form |
Elemental
irondose |
| Ferrous gluconate (12%) |
325 mg tablet |
38 mg |
| Ferrous sulfate (20%) |
220 mg
5mL oral solution
325 mg immediate-release tablet
160 mg extended-release tablet |
44 mg
65 mg
50 mg |
| Ferrous fumarate (33%) |
325 mg tablet
150 mg extended-release tablet |
107 mg
107 mg |
Polysaccharide iron complex
(100%) |
150 mg capsule
100mg
5mL solution |
150 mg
100 mg |
The preferred approach to managing perioperative anemia is presurgical administration of IV iron.37 Compared with oral iron, IV iron is better tolerated, has a faster onset of action, and is significantly more effective, especially for patients with inflammation.43,48-50 A systematic review and meta-analysis by Litton and colleagues examined 72 studies (encompassing 10,605 patients) to evaluate the efficacy and safety of IV iron for reducing the requirement of allogeneic blood transfusion.51 The meta-analysis confirmed that IV iron effectively reduces the requirement for RBC transfusion, thereby reducing associated risk. This benefit was found to be consistent across many types of diseases and was present whether IV iron was compared with oral iron or with no iron.51 In patients with chronic inflammation, iron absorption may be decreased due to increased hepcidin expression in these patients.52 IV iron preparations are shown in Table 3.53-57
| Table 3. Parenteral Iron Agents, Adult Doses53-57 |
| Iron sucrose injectable solution, 20 mg Fe/mL (Venofer®) |
| Indication |
Dose |
Dosage Notes/Precautions |
| CKD, Non-dialysis |
200 mg IV per dose (over 2-5 min or via infusion over 15 min); 5 doses over 14 days |
Cumulative 1,000 mg in 14 days |
| CKD, Dialysis dependent |
100 mg (over 2–5 min or via infusion over 15 min) per dialysis session |
Do not exceed cumulative total dose of 1,000 mg divided in 3 doses/week |
| CKD, Peritoneal dialysis-dependent |
300 mg IV over 1.5 hr for 2 doses, 14 days apart. Then, 400 mg IV over 2.5 hr 14 days later |
|
| LMW iron dextran injectable solution, 50 mg Fe/mL (INFeD®) |
| Indication |
Dose |
Dosage Notes/Precautions |
| Iron deficiency |
0.0442 (desired Hgb–observed Hgb) x LBW + (0.26 x LBW) |
Give test dose of 0.5 mL and observe patient for 1 hour prior to giving remainder of therapeutic dose
Labeling contains a boxed warning for risk of anaphylactic reactions, including test dose. See product labeling for details. |
| Blood loss |
Total dose (mg) = blood loss (mL) x HCT |
See product labeling for dosage formula used in blood loss |
| Ferric gluconate injectable solution, 12.5 mg Fe/mL (Ferrlecit®) |
| Indication |
Dose |
Dosage Notes/Precautions |
| Iron deficiency/anemia |
125 mg IV infusion over 1 hr |
Most patients require 250 mg/infusion for 8 dialysis sessions
Do not exceed 12.5 mg/min administration rate |
| Ferric carboxymaltose injectable solution, 50 mg Fe/mL (Injectafer®) |
| Indication |
Dose |
Dosage Notes/Precautions |
| Iron deficiency/anemia Patient weight < 50 kg |
2 doses of 15 mg/kg separated by at least 7 days; administer via IV infusion (over at least 15 min) or slow IV push (100mg/min) |
Do not exceed 1,500 mg elemental iron per course |
| Patient weight >/= 50 kg |
2 doses of 750 mg separated by at least 7 days; administer via IV infusion (over at least 15 min) or slow IV push (100 mg/min) |
Do not exceed 1,500 mg per course |
| Ferumoxytol, 30 mg Fe/mL (Feraheme®) |
| Indication |
Dose |
Dosage Notes/Precautions |
| Iron deficiency/anemia |
510 mg IV. over at least 15 minutes, followed by 510 mg IV infusion over at least 15 minutes 3 to 8 days later |
No data in pregnancy |
| Fe=iron; CKD=chronic kidney disease; IV=intravenous; LMW= low molecular weight; Hgb=hemoglobin; LBW= lean body weight; HCT=hematocrit |
Several studies have provided data to support the use of preoperative IV iron for reducing the need for blood transfusions. In a small Australian study of 72 patients with iron deficiency anemia assigned to either IV iron or usual care prior to surgery, iron therapy was associated with a 60% reduction in allogeneic blood transfusion rates (12.5% vs 31.25%). The iron-treated group had significant improvements in secondary endpoints of shorter hospital stay and higher hemoglobin rates 4 weeks post-discharge.58
Treatment with ferric carboxymaltose with or without erythropoietin has been shown to significantly reduce the need for red-cell transfusions in perioperative patients with hip fractures, other major surgeries, and CKD.59-61 A study in Spain evaluated 266 patients with anemia undergoing elective surgery for colon cancer, of whom 111 received ferric carboxymaltose and compared to 155 who had received no iron preoperatively. Compared with no iron, the iron-treated group showed a significantly lower need for red blood cell transfusion (9.9% vs. 38.7%) and significantly shorter hospital stay (P<0.001 for both) as well as improved hemoglobin levels 30 days post-surgery.62
A UK study compared IV iron therapy (ferrous carboxymaltose) with oral iron (ferrous sulphate) in 116 patients with anemia treated 2 weeks prior to surgery. Transfusion rates were similar in both groups. Hemoglobin, ferritin, and transferrin saturation levels were higher in the IV-iron treated group immediately prior to surgery and following surgery.63
Concomitant use of ESAs may further reduce the need for blood transfusions. These agents stimulate the bone marrow to produce red blood cells.64 This category includes:65
- erythropoietin (EPO)
- epoetin alfa
- epoetin beta
- darbepoetin alfa
- methoxy polyethylene glycol-epoetin beta
- epoetin alfa epbx (biosimilar)
Patients treated with ESAs have a rapid, supraphysiologic rate of RBC production, but this outstrips the availability of transferrin-bound circulating iron faster than it can be replenished from iron stores.66 Using an ESA in combination with supplemental iron may optimize pre-surgical red blood cell production and minimize the functional iron deficiency that can be induced by ESAs. Use of ESA is standard therapy patients with chronic renal failure.67 ESA use is usually recommended at the lowest possible dose and shortest administration time. These agents typically add to overall cost of therapy.68
During Surgery: Topical Hemostatic Agents, Massive Transfusion Protocols
Topical hemostatic agents are used during the surgical procedure when hemostasis is inadequately controlled via standard surgical techniques such as electrocautery, or as an adjunct to electrocautery.69 Hospital-based pharmacists may be called upon to oversee the storage and distribution of topical hemostatic agents. According to some PBM experts, topical agents should be handled in a manner similar to that of other medications that are evaluated by the pharmacy and therapeutics committee—rather than as devices or surgical equipment.69 The two main categories of topical hemostatic agents are mechanical agents, which promote hemostasis using a passive substrate, and biologically active agents, which enhance coagulation at the bleeding site (Table 4).70
| Table 4. Categories of Topical Hemostatic Agents70 |
| Type |
Categories |
Issues, Pros/Cons |
Mechanical Barrier
Forms barrier to block blood flow and create a thrombogenic surface |
· Porcine gelatin
· Bovine collagen
· Oxidized cellulose
· Polysaccharide spheres |
Requires intact coagulation system
Used for minimal bleeding
Low cost, easy to use, biodegradable |
Biologically Active
Contains thrombin |
· Bovine
· Pooled human
· Recombinant |
Requires intact fibrinogen but does not rely on patient's intrinsic clotting system |
Flowable
Combines thrombin-containing and mechanical hemostat |
· Collagen or gelatin plus thrombin |
Forms flowable paste
Delivered via syringe |
Fibrin Sealants
Combines fibrinogen with thrombin |
Combines human fibrinogen and bovine, human or recombinant thrombin
May contain antifibrinolytic agent and calcium to stabilize clot
Used for local or diffuse moderate bleeding |
Forms hemostatic plug
Dual components delivered via syringe
Effective in patients with defects in other parts of their coagulation pathways. |
| Data adapted from: Ferraris VA. Hemostasis Symposium: Impact of Topical Hemostatic Agents70 |
Hospital pharmacists should be included in transfusion committee meetings and may have a role in developing massive transfusion protocols. Massive transfusion protocols are an approach to improve outcomes in patients with massive blood loss due to trauma, major surgery, GI bleed, and other causes of hemorrhage.71,72 These protocols are tailored for specific scenarios, such as obstetric complications leading to hemorrhage.73 In some centers, pharmacists have an important role in developing and participating in massive transfusion protocols. Pharmacists may be involved in coordinating with the blood bank for distribution of blood products, and in advising on agents such as antifibrinolytic drugs that are used to reduce bleeding and utilization of blood products. Antifibrinolytic agents include tranexamic acid and epsilon aminocaproic acid, which are synthetic lysine analogs that inhibit fibrinolysis by displacing plasminogen from fibrin. Another inhibitor of fibrinolysis, aprotinin, was withdrawn from the market due to bleeding concerns.74
Patient Blood Management in Chronic Kidney Disease
Iron deficiency and anemia are common complications of CKD, regardless of whether or not patients are dependent on dialysis. Anemia in CKD stems from reduced production of erythropoietin from the kidneys.65,75 Iron deficiency is an important factor, and a large proportion of patients with CKD are iron-deficient and thus are candidates for iron supplementation.76 As CKD progresses, many patients eventually require ESAs to manage anemia, or adjunctive iron in addition to ESAs. Management of anemia in CKD is a complex process that involves optimizing hemoglobin and transferrin saturation levels while minimizing potential safety concerns, including infections, adverse reactions, and cardiovascular complications.46,77,78
For patients with CKD, the occurrence of renal anemia greatly increases the need for transfusions and increases rates of morbidity and mortality from serious organ damage, including heart failure.32,79 The need for multiple transfusions delays potential transplant and increases transplant complications.80 The clinical decision-making process in managing anemia in CKD involves many complex factors, including whether the patient is on dialysis and how to balance optimal target hemoglobin/transferrin saturation levels with potential elevated cardiovascular risk, which was seen in clinical trials when patients achieved target levels.65,81,82 At the same time, the clinician must be aware that patients may be unresponsive or hyporesponsive to ESAs.77 Supplemental iron has been shown to reduce the need for and dosage of ESAs.83
A number of factors must be considered when selecting the formulation and dosage of iron supplementation for patients with CKD, including diagnosis, medical comorbidities such as cancer, comorbid conditions such as bleeding disorders, and concomitant medications.46,81,84 Total dose infusion (TDI) is one method of providing high doses of iron on an outpatient basis, but the best approach to administration must be determined according to individual patient and clinical criteria.85
Treatment Recommendations for Iron Supplementation in CKD
Disadvantages of oral iron supplementation for CKD include GI toxicity, poor absorption from the GI tract, and high rates of nonadherence.86,87 IV iron supplementation overcomes most of these issues.86 Knowledge of preparation and administration of IV iron is essential for pharmacists involved in the care of CKD. This includes awareness of the timing of the infusion, need for bolus doses, potential for infusion-related adverse effects (e.g., hypotension, nausea, low back pain) and awareness of the pharmacokinetics of different agents.
Important considerations for IV iron dosing include the need to maintain hemoglobin levels, the potential for hypersensitivity to some IV iron formulations, and the need to reduce cardiovascular risk and monitor for hypotension. Anaphylactic reactions are rare with low-molecular weight (LMW) IV iron dextran, but a test dose is required as per a boxed warning in the labeling.45
Pharmacist intervention can have a positive impact on outcomes of patients with CKD.88 Among the barriers preventing optimal care in CKD are complexity of care, patient nonadherence to treatment, and providers’ lack of familiarity with treatment recommendations.79 In a comprehensive review of clinical practice guidelines from the National Kidney Foundation and the U.S. Renal Data System, Bacchus et al noted, “Pharmacists are ideally positioned to help select therapy, influence formulary use, educate healthcare professionals and patients, develop and implement dosing and monitoring protocols, and possibly promote quality improvement.”79
The Pharmacists’ Role in Blood Management
The role of hospital pharmacists as integral members of the care team has expanded in recent years, with pharmacists taking on more responsibilities in the clinical management of patients. This shift has occurred in conjunction with increasing complexity of health problems in the aging population and an expanding number of treatment options for those conditions.9 Pharmacists' roles related to PBM may include:17
- Screening for anemia and monitoring change
- Preparing and administering IV ESAs, iron products, or blood products
- Institutional decision-making regarding surgical risk management and/or blood management
- Implementing transfusion/blood management guidelines and protocol development
- Developing guidelines and algorithms for selection and use of therapies
- Providing patient education
Economics is an important aspect of managing anemia in hospitalized/preoperative patients. Cost to payers and healthcare institutions derive from blood acquisition costs (with little opportunity for pass-through by hospitals), staff time and labor related to transfusions (monitoring, testing, storage, materials, and dispensing of blood products), and medical treatment for complications of anemia. Blood prices continue to rise as collection costs increase. The true cost of transfusion is much higher than the cost of each unit of blood. A study by Shander and colleagues showed the average per-patient cost of red cell transfusion in the surgical setting was $3,433 when counting indirect overhead, transfusion process costs, blood acquisition cost, and direct overhead cost per unit.89
Some hospitals may not place sufficient emphasis on lowering the financial burden of anemia treatment and blood transfusions. Staff may have misperceptions about the actual cost of blood and why blood needs to be conserved. Hospitals lack educational efforts and financial incentives for staff to implement blood management measures. Hospital pharmacists are an important part of the healthcare team when it comes to implementing policies for cost containment.90
Recommendations for Patient Education
A recent shift in the philosophy of PBM involves greater emphasis on a patient-centered approach, rather than a procedural or product-centered approach.5,7 This patient-centered shift is consistent with the ongoing shift in pharmacists' roles, from a product- or medication-oriented focus to an overall focus on optimizing patient care by overseeing and managing medication therapy.9
Clinical pharmacists' interventions have been shown to improve patient outcomes in iron deficiency anemia."9 In a study investigating the role of clinical pharmacists in managing patients with iron deficiency anemia, patients receiving clinical pharmacist management achieved their target hemoglobin concentrations in an average of 28 days compared to 41 days for usual care, and had fewer hospital visits related to anemia or ESA therapy.9
In most institutions patient consent is needed before a procedure that may involve transfusion. This may necessitate education by the pharmacist about the benefits and risks of transfusion, what situations might warrant the need for transfusion, and the types of therapies used to manage hemorrhage.91 For patients receiving oral or IV iron supplementation, the pharmacist may be called upon to discuss risks and adverse effects of this therapy.9,17 On its website, the SABM offers a free, downloadable patient brochure that can be used to educate patients about PBM.92
Conclusion
An approach to PBM recommended by the SABM (Figure 5)18 emphasizes multidisciplinary steps for blood conservation, but many components of this strategy are consistent with pharmacists’ duties in the hospital setting. Hospital pharmacists and other healthcare professionals involved in administration of blood products, need education about the urgent needs for blood conservation and its impact on quality of patient care and outcomes, blood supply concerns, and economic variables.
| Figure 5. Patient Blood Management Principles |
 |
| Reproduced with permission from the Society for Advancement of Blood Management (SABM).18 |
References
- Auerbach M, Goodnough LT, Picard D, et al. The role of intravenous iron in anemia management and transfusion avoidance. Transfusion. 2008;48(5):988-1000.
- Sazama K. The ethics of blood management. Vox sanguinis. 2007;92(2):95-102.
- Butcher A, Richards T. Cornerstones of patient blood management in surgery. Transfus Med. 2018;28(2):150-157.
- Filipescu D, Banateanu R, Beuran M, et al. Perioperative Patient Blood Management Programme. Multidisciplinary recommendations from the Patient Blood Management Initiative Group. Rom J Anaesth Intensive Care. 2017;24(2):139-157.
- Shander A, Javidroozi M, Lobel G. Patient Blood Management in the Intensive Care Unit. Transfus Med Rev. 2017;31(4):264-271.
- Meybohm P, Fischer D, Schnitzbauer A, et al. [Patient blood management: Current state of the literature]. Chirurg. 2016;87(1):40-46.
- Choorapoikayil S, Zacharowski K, Meybohm P. Patient blood management: is it worth to be employed? Curr Opin Anaesthesiol. 2016;29(2):186-191.
- Tahaineh LM, Khasawneh AH. A randomised control trial to evaluate the clinical pharmacist's role in managing iron deficiency anaemia patients. Int J Pharm Pract. 2018;26(1):55-62.
- Shander A, Nemeth J, Cruz JE, et al. Patient blood management: A role for pharmacists. Am J Health Syst Pharm. 2017;74(1):e83-e89.
- Debenito JM, Billups SJ, Tran TS, et al. Impact of a clinical pharmacy anemia management service on adherence to monitoring guidelines, clinical outcomes, and medication utilization. J Manag Care Spec Pharm. 2014;20(7):715-720.
- Rahman MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103(2):495-504.
- Zacharowski K, Spahn DR. Patient blood management equals patient safety. Best Pract Res Clin Anaesthesiol. 2016;30(2):159-169.
- Hofmann A, Farmer S, Shander A. Five drivers shifting the paradigm from product-focused transfusion practice to patient blood management. Oncologist. 2011;16 Suppl 3:3-11.
- Ansari S, Szallasi A. Blood management by transfusion triggers: when less is more. Blood Transfus. 2012;10(1):28-33.
- Riley W, Schwei M, McCullough J. The United States' potential blood donor pool: estimating the prevalence of donor-exclusion factors on the pool of potential donors. Transfusion. 2007;47(7):1180-1188.
- Leahy MF, Mukhtar SA. From blood transfusion to patient blood management: a new paradigm for patient care and cost assessment of blood transfusion practice. Int Med J. 2012;42(3):332-338.
- Gilmartin C. Pharmacist's role in managing anemia in patients with chronic kidney disease: potential clinical and economic benefits. Am J Health Syst Pharm. 2007;64(13 Suppl 8):S15-22; quiz S23-15.
- Society for the Advancement of Blood Management (SABM). Patient Blood Management: A Professional Guide.
- Qi Z, Roback JD, Voit EO. Effects of Storage Time on Glycolysis in Donated Human Blood Units. Metabolites. 2017;7(2).
- Likosky DS, FitzGerald DC, Groom RC, et al. Effect of the perioperative blood transfusion and blood conservation in cardiac surgery clinical practice guidelines of the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists upon clinical practices. Anesth Analg. 2010;111(2):316-323.
- Shander A, Knight K, Thurer R, et al. Prevalence and outcomes of anemia in surgery: a systematic review of the literature. Am J Med. 2004;116 Suppl 7A:58S-69S.
- Shander A, Hofmann A, Ozawa S, et al. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753-765.
- Shander A, Fink A, Javidroozi M, et al. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev. 2011;25(3):232-246 e253.
- Sweeney JD. Control of blood utilization. Transfus Apheresis Sci. 2008;39(2):139-144.
- Society for the Advancement of Blood Management (SABM). Emerging Evidence on Anemia, Transfusion, Intravenous Iron, and Patient Outcomes: The Case for Expanded Coverage of Intravenous Iron. 2019. Available at: https://www.sabm.org/wp-content/uploads/2019/02/Emerging-Evidence-on-Anemia-V6-Final.pdf.
- Goodnough LT, Schrier SL. Evaluation and management of anemia in the elderly. Am J Hematol. 2014;89(1):88-96.
- Le CH. The Prevalence of Anemia and Moderate-Severe Anemia in the US Population (NHANES 2003-2012). PLoS One. 2016;11(11):e0166635.
- Goel S, Miller A, Agarwal C, et al. Imaging Modalities to Identity Inflammation in an Atherosclerotic Plaque. Radiol Res Pract. 2015;2015:410967.
- Thomas DW, Hinchliffe RF, Briggs C, et al. Guideline for the laboratory diagnosis of functional iron deficiency. Br J Haematol. 2013;161(5):639-648.
- Koch CG, Li L, Sun Z, et al. Hospital-acquired anemia: prevalence, outcomes, and healthcare implications. J Hosp Med. 2013;8(9):506-512.
- Makam AN, Nguyen OK, Clark C, et al. Incidence, Predictors, and Outcomes of Hospital-Acquired Anemia. J Hosp Med. 2017;12(5):317-322.
- Herzog CA, Muster HA, Li S, et al. Impact of congestive heart failure, chronic kidney disease, and anemia on survival in the Medicare population. J Card Fail. 2004;10(6):467-472.
- Khanna MP, Hebert PC, Fergusson DA. Review of the clinical practice literature on patient characteristics associated with perioperative allogeneic red blood cell transfusion. Transfus Med Rev. 2003;17(2):110-119.
- Peyrin-Biroulet L, Williet N, Cacoub P. Guidelines on the diagnosis and treatment of iron deficiency across indications: a systematic review. Am J Clin Nutr. 2015;102(6):1585-1594.
- Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet. 2011;378(9800):1396-1407.
- Beattie WS, Karkouti K, Wijeysundera DN, et al. Risk associated with preoperative anemia in noncardiac surgery: a single-center cohort study. Anesthesiology. 2009;110(3):574-581.
- Society for the Advancement of Blood Management (SABM). Anemia in the Pre-Surgical Patient: Recognition, Diagnosis, and Management. New Insights and Concepts for the Primary Care Provider. 2019. Available at: https://www.sabm.org/wp-content/uploads/2019/02/Anemia-Recognition-Diagnosis-Management-in-the-Pre-surgical-Patient-Final.pdf.
- Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106(1):13-22.
- Elhenawy AM, Meyer SR, Bagshaw SM, et al. Role of preoperative intravenous iron therapy to correct anemia before major surgery: study protocol for systematic review and meta-analysis. Syst Rev. 2015;4:29.
- Cuenca J, Garcia-Erce JA, Munoz M. Efficacy of intravenous iron sucrose administration for correcting preoperative anemia in patients scheduled for major orthopedic surgery. Anesthesiology. 2008;109(1):151-152; author reply 152.
- Yoo YC, Shim JK, Kim JC, et al. Effect of single recombinant human erythropoietin injection on transfusion requirements in preoperatively anemic patients undergoing valvular heart surgery. Anesthesiology. 2011;115(5):929-937.
- Theusinger OM, Felix C, Spahn DR. Strategies to reduce the use of blood products: a European perspective. Curr Opin Anaesthesiol. 2012;25(1):59-65.
- Bonovas S, Fiorino G, Allocca M, et al. Intravenous Versus Oral Iron for the Treatment of Anemia in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore). 2016;95(2):e2308.
- Tolkien Z, Stecher L, Mander AP, et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10(2):e0117383.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91(1):31-38.
- Auerbach M, Ballard H. Clinical use of intravenous iron: administration, efficacy, and safety. Hematology Am Soc Hematol Educ Program. 2010;2010:338-347.
- Iron Preparations, Oral. Drugs.com. Available at: https://www.drugs.com/monograph/iron-preparations-oral.html.
- Adkinson NF, Strauss WE, Bernard K, et al. Comparative safety of intravenous Ferumoxytol versus Ferric Carboxymaltose for the Treatment of Iron Deficiency Anemia: rationale and study design of a randomized double-blind study with a focus on acute hypersensitivity reactions. J Blood Med. 2017;8:155-163.
- Fleming RE, Ponka P. Iron overload in human disease. N Engl J Med. 2012;366(4):348-359.
- Toblli JE, Angerosa M. Optimizing iron delivery in the management of anemia: patient considerations and the role of ferric carboxymaltose. Drug Des Devel Ther. 2014;8:2475-2491.
- Litton E, Xiao J, Ho KM. Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials. BMJ. 2013;347:f4822.
- D'Angelo G. Role of hepcidin in the pathophysiology and diagnosis of anemia. Blood Res. 2013;48(1):10-15.
- Venofer (iron sucrose) injection [package insert]. Shirley, NY: American Regent, 2019.
- InFed (iron iron dextran) injection [package insert]. Madison, NJ: Allergan, 2018.
- Ferrlecit (ferric gluconate complex in sucrose) injection [package insert]. Bridgewater, NJ: Sanofi-Aventis, 2015.
- Injectafer (ferric carboxymaltose) injection [package insert]. Shirley, NY: American Regent, 2018.
- Feraheme (ferumoxytol) injection [package insert]. Waltham, MA: Amag Pharmaceuticals, 2018.
- Froessler B, Palm P, Weber I, et al. The Important Role for Intravenous Iron in Perioperative Patient Blood Management in Major Abdominal Surgery: A Randomized Controlled Trial. Ann Surg. 2016;264(1):41-46.
- Richards T, Clevenger B, Keidan J, et al. PREVENTT: preoperative intravenous iron to treat anaemia in major surgery: study protocol for a randomised controlled trial. Trials. 2015;16:254.
- Litton E, Baker S, Erber W, et al. The IRONMAN trial: a protocol for a multicentre randomised placebo-controlled trial of intravenous iron in intensive care unit patients with anaemia. Crit Care Resusc. 2014;16(4):285-290.
- Bernabeu-Wittel M, Aparicio R, Romero M, et al. Ferric carboxymaltose with or without erythropoietin for the prevention of red-cell transfusions in the perioperative period of osteoporotic hip fractures: a randomized contolled trial. The PAHFRAC-01 project. BMC. 2012;13:27.
- Calleja JL, Delgado S, del Val A, et al. Ferric carboxymaltose reduces transfusions and hospital stay in patients with colon cancer and anemia. Int J Colorectal Dis. 2016;31(3):543-551.
- Keeler BD, Simpson JA, Ng O, et al. Randomized clinical trial of preoperative oral versus intravenous iron in anaemic patients with colorectal cancer. Br J Surg. 2017;104(3):214-221.
- Hayat A, Haria D, Salifu MO. Erythropoietin stimulating agents in the management of anemia of chronic kidney disease. Patient Prefer Adherence. 2008;2:195-200.
- Locatelli F, Del Vecchio L. Erythropoiesis-stimulating agents in renal medicine. Oncologist. 2011;16 Suppl 3:19-24.
- Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol. 2006;1 Suppl 1:S4-8.
- Hingorani AD, Sofat R, Morris RW, et al. Is it important to measure or reduce C-reactive protein in people at risk of cardiovascular disease? Eur Heart J. 2012;33(18):2258-2264.
- Gebara SN, Moubayed H. The role of the pharmacist in optimizing the use of erythropoietin stimulating agents. J Oncol Pharm Pract. 2010;16(1):33-37.
- Shander A, Kaplan LJ, Harris MT, et al. Topical hemostatic therapy in surgery: bridging the knowledge and practice gap. J Am Coll Surg. 2014;219(3):570-579.e574.
- Ferraris VA. Hemostasis Symposium: Impact of Topical Hemostatic Agents. 2014. Available at: https://www.slideserve.com/walda/hemostasis-symposium-impact-of-topical-hemostatic-agents
- Foster JC, Sappenfield JW, Smith RS, et al. Initiation and Termination of Massive Transfusion Protocols: Current Strategies and Future Prospects. Anesth Analg. 2017;125(6):2045-2055.
- Patil V, Shetmahajan M. Massive transfusion and massive transfusion protocol. Indian J Anaesth. 2014;58(5):590-595.
- Neb H, Zacharowski K, Meybohm P. Strategies to reduce blood product utilization in obstetric practice. Curr Opin Anaesthesiol. 2017;30(3):294-299.
- Levy JH, Koster A, Quinones QJ, et al. Antifibrinolytic Therapy and Perioperative Considerations. Anesthesiology. 2018;128(3):657-670.
- Del Vecchio L, Cavalli A, Tucci B, et al. Chronic kidney disease-associated anemia: new remedies. Curr Opin Investig Drugs. 2010;11(9):1030-1038.
- Hsu CY, McCulloch CE, Curhan GC. Iron status and hemoglobin level in chronic renal insufficiency. J am Soc Nephrol. 2002;13(11):2783-2786.
- Agarwal R. Individualizing decision-making--resurrecting the doctor-patient relationship in the anemia debate. Clin J Am Soc Nephrol. 2010;5(7):1340-1346.
- Stompor T, Olszewski A, Kierzkowska I. Can we prolong life of patients with advanced chronic kidney disease: what is the clinical evidence? Pol Arch Med Wewn. 2011;121(3):88-93.
- Bacchus S, O'Mara N, Manley H, et al. Meeting new challenges in the management of anemia of chronic kidney disease through collaborative care with pharmacists. Ann Pharmacother. 2009;43(11):1857-1866.
- Foley RN. Treatment of anemia in chronic kidney disease: known, unknown, and both. J Blood Med. 2011;2:103-112.
- Coyne DW. It's time to compare anemia management strategies in hemodialysis. Clin J Am Soc Nephrol. 2010;5(4):740-742.
- Nichols B, Shrestha RP, Horowitz J, et al. Simplification of an erythropoiesis model for design of anemia management protocols in end stage renal disease. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:83-86.
- Fishbane S, Frei GL, Maesaka J. Reduction in recombinant human erythropoietin doses by the use of chronic intravenous iron supplementation. Am J Kidney Dis. 1995;26(1):41-46.
- Macdougall IC. New anemia therapies: translating novel strategies from bench to bedside. Am J Kidney Dis. 2011.
- Gilreath JA, Stenehjem DD, Rodgers GM. Total dose iron dextran infusion in cancer patients: is it SaFe2+? J Natl Compr Canc Netw. 2012;10(5):669-676.
- Gafter-Gvili A, Schechter A, Rozen-Zvi B. Iron Deficiency Anemia in Chronic Kidney Disease. Acta Haematol. 2019;142(1):44-50.
- Henry DH, Dahl NV, Auerbach M, et al. Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist. 2007;12(2):231-242.
- Salgado TM, Moles R, Benrimoj SI, et al. Pharmacists' interventions in the management of patients with chronic kidney disease: a systematic review. Nephrol Dial Transplant. 2012;27(1):276-292.
- Shander A, Hofmann A, Ozawa S, Javidroozi M. The true cost of red blood cell transfusion in surgical patients. Blood. 2008;112:3045.
- Gebara SN, Moubayed H. The role of the pharmacist in optimizing the use of erythropoietin stimulating agents. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2010;16(1):33-37.
- Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166(2):199-207 e115.
- Society for the Advancement of Blood Management (SABM). A Patient’s Guide to Patient Blood Management. 2019. Available at: https://www.sabm.org/publications/.
Back Top