ADVERTISEMENT

Module 5: Understanding Insulin Therapy for Pharmacy Technicians
IMPORTANT DEFINITIONS
Basal insulin: Basal insulin is insulin that is released in small, consistent increments to maintain stable blood glucose levels throughout the day. Basal insulin therapy mimics the body’s natural production of insulin, which maintains blood glucose stability during periods of fasting, such as overnight.
Bolus insulin: Bolus insulin (often referred to as mealtime insulin) is short-acting or rapid-acting insulin that is used to cover meals or snacks to prevent elevations in glucose related to eating. Bolus insulin therapy mimics the body’s natural production of insulin after eating.
Target blood glucose: Every patient with diabetes has individualized goals for target blood glucose levels that are agreed upon with their health care provider. Normal blood glucose values range broadly, depending on how and when they are measured. Fasting blood glucose is generally the lowest goal number (often 80 to 130 mg/dL), with post-meal blood glucose targets often being less than 180 mg/dL. All goals are individualized on the basis of patient characteristics, risk factors, needs, and preferences.
SMBG: Self-monitoring of blood glucose (SMBG) involves a patient checking his or her own blood glucose level with a home monitor. SMBG is especially important for patients using insulin, because this practice allows patients to calculate insulin doses, monitor for potential adverse effects, and assess how well they are maintaining and achieving their target blood glucose levels. |
BACKGROUND
Over the last decade, diabetes has reached epidemic proportions in the United States (US).1 Since insulin's discovery in the late 1920s, this agent has served as the mainstay of type 1 diabetes (T1D) treatment, in which endogenous insulin-producing beta cells are destroyed by an autoimmune process.2 Conversely, the multiple pathophysiologic defects of type 2 diabetes (T2D), including beta-cell dysfunction and insulin resistance, have led to an explosion of therapy modalities over the last 15 years. Beta-cell function decline and death begin at least 10 years before symptoms of T2D appear. By the time a patient is diagnosed with T2D, only 50% of beta-cell function remains.3 Since this dysfunction progressively deteriorates and is associated with worsening glycemic control, preservation and recovery of beta-cell functional mass is an essential consideration in treating T2D.4 Removing the negative effects of elevated glucose by adding exogenous insulin is one way to preserve beta-cell function.
Approximately 25% to 40% of people with T2D use insulin as part of their therapy to control their disease.5 Based on new data showing that certain agents have benefits on the cardiovascular system and kidneys, management guidelines for patients with T2D have changed significantly.6,7 Unless contraindicated, metformin with lifestyle modifications remain first line therapy. After 2-3 months if A1C is above target, for patients with established atherosclerotic cardiovascular disease (ASCVD) or if chronic kidney disease (CKD) predominates, agents with benefit in these conditions are considered second line therapy (select GLP-1 receptor agonists and SGLT2 inhibitors). Basal insulin is third line therapy (see Table 1).6,7 Basal insulin is also considered as a 3rd or 4th line agent in patients without established ASCVD or CKD.
| Table 1. Summary of Guideline Recommendations6,7 |
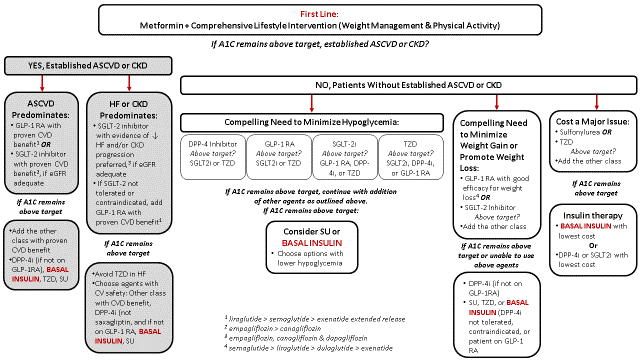 |
Despite the growing number of treatment options, insulin therapy is an important part of therapy. Insulin can be combined with other oral and injectable diabetes treatments that work in different ways to effectively treat patients.2,6,7 The main take-home message of this program is that using insulin therapy is not difficult; it produces a rapid, measurable response to therapy and can be used safely to help patients with diabetes achieve their personalized glycemic goals.
MIMICKING NATURE: PHYSIOLOGIC CONCEPT OF BASAL-BOLUS INSULIN
In people without diabetes, release of endogenous insulin made in the body is changes in response to increased glucose and decreased glucose levels.8,9 Produced by the pancreatic beta cells, insulin is an anabolic hormone that plays an important role in glucose, protein and fat metabolism as well as in facilitating glucose metabolism and stimulating glucose storage into muscle and liver cells in the form of glycogen. It also reduces glucose made by the liver by inhibiting the secretion of the counter-regulatory hormone glucagon when plasma glucose concentrations rise. In addition, insulin converts excess glucose into fatty acids and triglycerides and promotes storage into adipose tissues. Insulin enhances the incorporation of amino acids into proteins as well.8,9
The concept behind basal-bolus insulin replacing or attempting to mimic natural insulin secretion is illustrated in Figure 2.10 In a basal-bolus model each component represents about half of the patient’s daily insulin needs. The main function of basal insulin is to suppress glucose production in the liver between meals and overnight in addition to stimulating lipid and protein synthesis.11 Long-acting and intermediate insulins are used as replacements to mimic the body’s basal insulin secretion. Typically, in a basal-bolus model, basal insulin accounts for approximately 50% of the total daily insulin needs. The overall percentage of basal insulin that contributes to the total daily dose (TDD) of insulin, however, can vary from patient to patient.
The primary function of bolus insulin is to control hyperglycemia following food consumption by storing nutrients. Rapid-acting or regular insulin is used to cover bolus insulin needs. In a typical basal-bolus model, each bolus insulin dosage replaces approximately 10% to 20% of daily insulin requirements before each meal, depending on how many carbohydrates are in the meal. Balancing carbohydrate consumption with bolus insulin replacement to avoid blood glucose that is too high or too low is the goal of bolus replacement, particularly in a patient with T1D. This usually requires multiple self-testing of blood glucose (BG) throughout the day, before meals, and 1 to 2 hours after meals or exercise and at bedtime to properly assess insulin efficacy. In patients with T1D without functioning beta cells and T2D with dysfunctional beta cells, exogenous insulin may be used as a replacement, yet the manner in which it is done is quite different.7 By the nature of their disease, those with T1D have to have full basal and bolus replacement at initiation, whereas patients with T2D may only require once-daily basal insulin injections for several years before requiring any bolus insulin.12 The dosing of insulin is individualized in both type 1 and type 2 patients based on weight, insulin resistance, etc. A more detailed dosing strategy is discussed later in this module for T2D patients.
| Figure 2. Mimicking Nature's Insulin: The Basal-Bolus Concept |
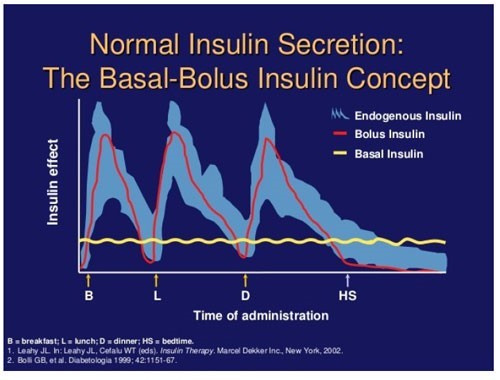 |
Pharmacokinetic and Pharmacodynamic Differences of Bolus and Basal Insulins
Understanding the differences between the pharmacokinetic (PK) and pharmacodynamic (PD) profiles of insulin products helps the provider to understand treatment rationale and dosing and how to safely monitor BG as well as how to implement pattern management.9,13,14 Minor modifications to the insulin protein have resulted in significant differences in PK and PD parameters of formulated insulin products used to assist patients in controlling hyperglycemia. Table 2 illustrates the insulin products dividing them into bolus insulin products (shown in orange) and basal insulin products (shown in purple). Figure 3 illustrates how different insulin products work compared with the body’s own insulin.
| Table 2. Insulin Pharmacokinetics & Pharmacodynamics1 |
| |
Insulin Type |
Start of Action |
Duration of Action |
| Bolus Insulin |
Very Fast-Acting
Fiasp (faster aspart) |
0-6 min |
3-4 hr |
Rapid-Acting
Lispro, Aspart & Glulisine
Inhaled insulin |
5-15 min
Within 5 min |
3-4 hr
3 hr |
Short-Acting
Regular U-100 |
½-1 hr |
6-8 hr |
| Basal Insulin |
Intermediate-Acting
NPH
Regular U-500 |
1-2 hr
½ hr |
10-20 hr
Up to 24 hr |
Long-Acting2
Detemir
Glargine U-100
Glargine U-300
Degludec U-100 & U-200 |
1½ -2 hr
1-2 hr
1-2 hr
½-1½ hr |
12-24 hr3
20-24 hr
26 hr
~30-42 hr |
| Figure 3. Approximations of Insulin Action Profiles of Insulins |
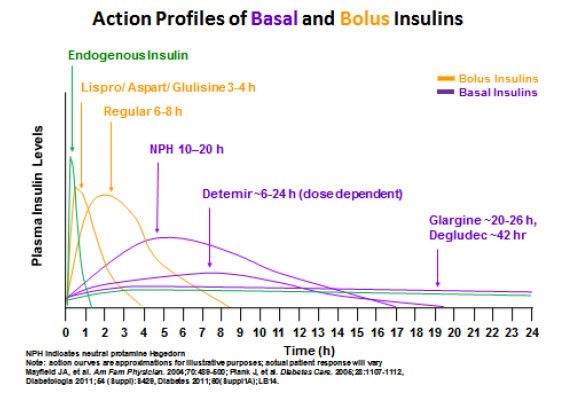 |
Bolus Insulin
Bolus injectable replacement insulin falls into two main categories: rapid-acting and short-acting insulins. Injectable rapid-acting insulin analogs include insulin lispro (Humalog), aspart (Novolog, Fiasp) and glulisine (Apidra).15-17,22 Because these drugs begin to work in only a few minutes, patients should inject these insulins right before the meal or within 15 minutes of eating the meal. With rapid-acting insulin durations of 3 to 4 hours, usually the insulin is cleared before the next meal, which reduces the opportunity for insulin stacking (accumulation) and hypoglycemic events. This is an advantage over regular or short-acting insulin (Humulin R or Novolin R),18,19 which has a longer onset and is ideally administered 30 minutes before a meal, and with a longer duration that can increase between-meal hypoglycemic risk. All currently injectable bolus insulin products can be mixed with intermediate-acting insulin (Humulin N or Novolin N).20,21
Fast-acting insulin aspart (Fiasp) is the most recently approved bolus insulin. It contains niacinamide which leads to a more rapid absorption into the bloodstream compared to conventional aspart..22 In patients with T2D inadequately controlled with basal insulin and oral agents, faster aspart was found noninferior to conventional aspart as part of a basal-bolus regimen but showed an increase in post-meal hypoglycemia.23 Faster aspart can be dosed at the start of a meal or within 20 minutes after starting a meal. The product is available as 100 U/mL multidose vials or FlexTouch pens.22
In mid-December 2017, a “follow on” product called Admelog24 (insulin lispro U-100) became available in the US. This agent has the identical amino acid sequence as the existing Humalog15 (insulin lispro U-100). The medication is not categorized as a biosimilar drug by the U.S. Food and Drug Administration (FDA). Rather, the FDA refers to it as a "follow-on" product.24 This is mainly semantics since biosimilar products must have a "reference product" to be proposed as a biosimilar, and currently no insulin lispro products are licensed under the Public Health Service Act. Admelog is available in a vial and prefilled Solostar pen.24
Inhaled insulin (Afrezza) is a bolus inhaled technosphere insulin powder product.25 This agent is only used as mealtime insulin with fixed doses in increments of 4, 8, or 12 units, which can be inhaled separately or added together to the appropriate dosage, as shown in Figure 4. Baseline pulmonary function tests are required before using this product, which should not be taken by patients with asthma, chronic obstructive pulmonary disease, or who currently smoke. The most common adverse events are hypoglycemia, cough, and throat pain or irritation.25
Basal Insulin
The two main types of basal insulin are intermediate-acting and long-acting. NPH, the only intermediate-acting insulin currently available, is relatively inexpensive and usually dosed once or twice daily. NPH insulin is cloudy white in color due to the zinc additive designed to increase the duration of action. NPH is the only insulin that can be mixed in the same syringe with all currently available bolus injectable insulin products. When combining insulins, the bolus insulin should be drawn up in the syringe first, followed by NPH. This order (clear before cloudy) helps preserve the PK/PD profile of the bolus insulin.20,21
Currently, three long-acting insulin analog products exist: detemir (Levemir),26 glargine (Lantus U-100; Toujeo U-300; Basaglar)27,28 and degludec (Tresiba U-100 or U-200).29 They all have a long onset and a relatively long duration of action. Detemir's peak and duration are shorter, and both increase as the dose of detemir is increased. Both glargine products and degludec are considered relatively "peakless" insulins meaning they provide relatively stable insulin coverage over at least 24 hours without a significant peak (see Figure 3), although this can vary from patient to patient. Degludec's duration of action is almost twice that of U-100 glargine, and the two of them have more predictable PK/PD profiles compared with detemir and NPH. The concentrated U-300 insulin glargine (Toujeo) is also available in a SoloStar insulin pen, for people who require higher daily dosages of insulin glargine.
When switching from one basal insulin product to another, dosage adjustments are usually necessary prevent hypoglycemia. When converting twice-a-day NPH to insulin glargine U-100 or U-300, the recommended starting dose is 80% of the total daily NPH dose administered once daily at the same time of day.27,28 When converting between glargine and detemir, it has been found that the conversion is not one-to-one as previously thought.30 Insulin glargine (U-100) appears to be between 20% and 39% stronger than detemir. A conservative approach is to increase the once-daily glargine dose used by 10% when converting from insulin glargine (U-100) to detemir. Adjustments are typically needed when converted from U-100 glargine to U-300 glargine.28 For patients controlled on insulin glargine U-100 being converted to concentrated insulin glargine U-300, it is expected that the total daily dose will be higher by at least 10%.28
In December 2016, Basaglar (insulin glargine U-100, which has an identical amino acid sequence as Lantus) was the first “follow on” insulin to become available in the US.31 Like Admelog, Basaglar is not categorized as a biosimilar drug by the FDA, yet the PK profile, efficacy, and safety are similar to Lantus. Basaglar is available in a prefilled KwikPen device.31
All bolus and basal insulins are manufactured as physiologic pH solutions with the exception of insulin glargine (both U-100 and U-300 formulations), which has a pH of 4. This acidic pH is necessary for glargine’s prolonged mechanism of action and may theoretically cause injection-site burning. This is not usually a concern, however.
Lastly, a concentrated U-500 formulation of regular insulin can be used for patients who require larger doses of daily insulin, but this is then considered a basal formulation.32 U-500 regular insulin is usually reserved for very insulin-resistant patients who require large volumes of insulin (200 units per day). A dedicated U-500 syringe for the use with Humulin U-500 insulin is available.33 Previously, patients had to calculate dosing using a conversion method when drawing the U-500 insulin into U-100 syringes or tuberculin syringes. This was a cause for concern due to potential errors in dosing calculations.
Insulin Mixtures34-39
Fixed-mixed-dose insulin products can be used twice daily in some patients with the benefits being ease of use and no mixing required. However, adjustment in fixed-mixed insulin dosages change both basal and bolus components with each dosage modification. In general, fixed-mixed insulins are not preferred if insulin components (either basal or bolus) require frequent dose changes. Table 3 lists currently available insulin mixtures.
| Table 3. Insulin Product Information and Storage15-22, 24-28 ,31, 32, 34-39 |
| Rapid-Acting Insulins |
| Brand |
Generic |
Product Availability |
Stability at Room Temperature (In Use) |
| Humalog/Admelog |
Lispro |
100 U/mL in vial, cartridge, or KwikPen, 200 U/mL in KwikPen |
All products: 28 d |
| Novolog |
Aspart |
100 U/mL vial, cartridge, FlexPen, or FlexTouch |
All products: 28 d |
| Apidra |
Glulisine |
100 U/mL vial or SoloStar pen |
All products: 28 d |
| Fiasp |
Aspart |
100 U/ml vial or FlexTouch Pen |
All products: 28 d |
| Short-Acting Insulins |
| Brand |
Generic |
Product Availability |
Stability at Room Temperature (In Use) |
| Humulin R |
Regular |
100 U/mL vial |
Vial: 31 d |
| Humulin R U-500 |
Regular |
500 U/mL vial or KwikPen |
Vial: 40 d KwikPen: 28 d |
| Novolin R |
Regular |
100 U/mL vial |
Vial: 42 d |
| Intermediate-Acting Insulins |
| Brand |
Generic |
Product Availability |
Stability at Room Temperature (In Use) |
| Humulin N |
NPH |
100 U/mL vial or KwikPen |
Vial: 31 d KwikPen: 14 d |
| Novolin N |
NPH |
100 U/mL vial |
Vial: 42 d |
| Long-Acting Insulins |
| Brand |
Generic |
Product Availability |
Stability at Room Temperature (In Use) |
| Lantus |
Glargine |
100 U/mL vial or SoloStar pen |
All products: 28 d |
| Toujeo |
Glargine |
300 U/mL SoloStar pen, Max SoloStar pen |
SoloStar: 42 d |
| Levemir |
Detemir |
100 U/mL vial and FlexTouch pen |
All products: 42 d |
| Basaglar |
Glargine |
100 U/mL KwikPen |
28 d |
| Tresiba |
Degludec |
100 U/mL FlexTouch pen/vial, 200 U/mL FlexTouch pen |
All products: 56 d |
| Insulin Mixtures |
| Brand |
Generic |
Product Availability |
Stability at Room Temperature (In Use) |
| Ryzodeg |
70% degludec, 30% aspart |
100 U/mL FlexTouch pen |
FlexTouch: 28 d |
| NovoLog 70/30 |
70% aspart protamine suspension, 30% aspart |
100 U/mL vial or FlexPen |
Vial: 28 d FlexPen: 14 d |
| Humalog 75/25 |
75% lispro protamine suspension, 25% lispro |
100 U/mL vial or KwikPen |
Vial: 28 d KwikPen: 10 d |
| Humalog 50/50 |
50% lispro protamine suspension, 50% lispro |
100 U/mL vial or KwikPen |
Vial: 28 d KwikPen: 10 d |
| Humulin 70/30 |
70% NPH, 30% regular |
100 U/mL vial and KwikPen |
Vial: 31 d KwikPen: 10 d |
| Novolin 70/30 |
70% NPH, 30% regular |
100 U/mL vial |
Vial: 42 d |
| Inhaled Insulin |
| Brand |
Generic |
Product Availability |
Stability at Room Temperature (In Use) |
| Afrezza |
Regular |
4-U, 8-U, and 12-U single-use cartridge |
Opened strips: 3 d Unopened strips: 10 d Inhaler: 15 d |
Table modified from that developed by: Emily Smith, PharmD Candidate, and Lynn Fletcher, PharmD, Clinical Pharmacist, Health Linc, Purdue University, IN., February 28, 2016.
d = days; min = minutes; SD = standard deviation; U = units |
| Figure 4. Inhaled powdered insulin Dose Conversion Chart25 |
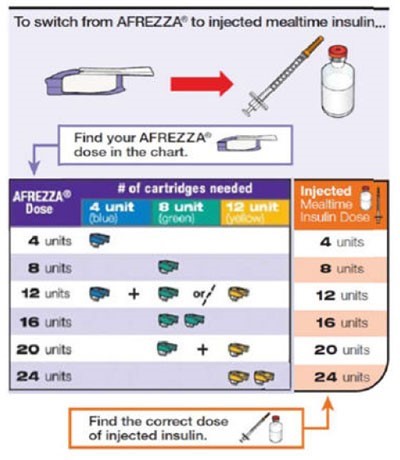 |
PRACTICAL APPROACH TO INITIATING INSULIN AND DOSING ADJUSTMENTS
Patients with T1D Using the Basal-Bolus Method
Patients with T1D usually begin insulin using the basal-bolus method, with very conservative dosing and loose glycemic goals until the individual's response is determined. Empirically, the total daily insulin dose is 0.4 to 0.5 units/kg; however, lower doses may initially be needed during the honeymoon phase when the pancreas may still have some function remaining.40,41 In general, the daily dose is divided into 2 parts, with 50% as basal insulin (long-acting insulin or intermediate-acting NPH) and 50% as bolus insulin (mealtime insulin). The bolus insulin will be divided among 3 meals. Bolus doses may need adjustment based on the carbohydrate content of each meal. Very small adjustments are usually made one at a time until target goals are met. SMBG requirements are usually 4 to 8 times per day, if not more, especially as the individualized dose response is determined.
Using Insulin to Treat Patients with T2D
Compared with the intensive insulin regimens required by patients with T1D, initiating insulin in those with T2D is much more straightforward. The patient’s A1C can help guide initial insulin doses. The A1C, a clinical marker used to measure glycemic control, is a weighted mean of glucose concentrations from the previous 2 to 3 months. The A1C accounts for fasting plasma glucose (FPG) and postprandial glucose (PPG) concentrations. Figure 5 illustrates that not all A1C values are made up of the same FPG and PPG proportions.11 At higher A1C values (greater than 9%), FPG concentration contributes more to the overall A1C than PPG. For this reason, basal insulin is often used first when treating patients with T2D. If A1C remains above goal and FPG are controlled, bolus insulin can be added to cover postprandial glucose excursions.
| Figure 5 |
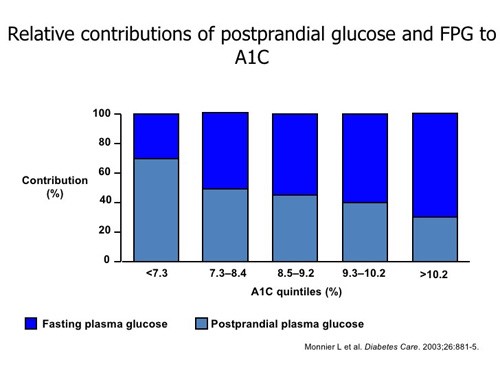 |
The American Diabetes Association (ADA) and European Association for the Study of Diabetes insulin-use guidelines demonstrate the use of the relative contributions of FPG and PPG of the A1C to guide initiation of insulin (see Table 4).6,7 In general, insulin is considered third line therapy; however, insulin should be considered as the first injectable initiated if A1C is very high (> 11%); the patient is experiencing weight loss, polyuria, or polydipsia which suggest insulin deficiency; or if T1D is a possibility. A combination of a GLP-1 receptor agonist and basal insulin or basal bolus insulin should be considered if the A1C is over 10% or the patients current A1C is 2% above target goal. To improve adherence and reduce daily injections, GLP-1 receptor agonist and long acting insulin mixtures are available.
| Table 4. Combination Injectable Therapy for T2D6,7 |
| If A1C above target goal, despite dual/triple therapy: 3 Options |
| OPTION 1 |
OPTION 2 |
OPTION 3 |
GLP-1 RA is preferred option in most cases. |
Basal insulin IF A1C very high (≥11%), or symptoms/evidence of catabolism (weight loss, polyuria, polydipsia) or T1D is a possibility. |
Consider combination injection therapy (GLP1 + basal insulin or basal-bolus insulin)
IF A1C ≥10% and/or above 2% above target. |
| ↓ |
↓ |
↓ |
Initiate GLP-1 RA; gradual titration to maintenance dose. |
Initiate basal Insulin; titrate until glucose goals are met. |
Initiate based on individual treatments or use combination pen. |
If A1C above target goal: |
| ↓ |
↓ |
↓ |
Add basal insulin; titrate until glucose goals are met. |
Add GLP1 RA; titrate as indicated,
OR
Add 1 rapid-acting insulin injection before largest meal. |
Add additional basal insulina
OR
If FPG at target, add rapid-acting insulin before largest meal. |
If A1C above target goal but FBG at target: |
| ↓ |
↓ |
↓ |
| Add rapid-acting insulin before largest meal. Initiate stepwise addition of premeal insulin to largest meals every 2-3 months if post prandial and A1C goals not met. (Basal-bolus). If A1C remains above target, consider additional Diabetes Self-Management Education and Support (DSMES). |
| a. Although not the preferred method, premixed insulin BID may be substituted for basal-bolus, and titrated to TID dosing if A1C targets not met with BID dosing. |
Another, quite practical way to view and approach insulin use is featured in Figure 6. The main goal with insulin use is that patients see the positive effects on their daily SMBG, and experience little or no hypoglycemia. For these reasons, start off with a low dose basal insulin and consistently titrate the daily dose upward until morning FPG values are between 80 and 130 mg/dL in most patients. The target glycemic goals (A1C and SMBG) can be set and personalized for each patient based on his or her individual factors (e.g., age, duration of diabetes, complications), and some patients, especial older ones, may require less stringent goals.7,43
Practical Approach to Using Insulin in T2D
A rather practical method of initiation is to begin basal insulin at 10 units once daily, in the morning or at bedtime, and then increase the daily dose by 3 units every 3 days until morning FPG goals are achieved (Figure 6). Typically, goals are met within a few weeks to the first month, and patients experience reduced symptoms of hyperglycemia without low glucose events. A1C should be checked within 2 to 3 months to assess therapy. If FBG goals are met but the A1C remains above goal, additional mealtime coverage may be required. Patients should check their BG before each meal for 2 to 3 days and use these values and the pattern management in Table 5 to determine which meal contributes the most to high PPG. Bolus insulin can be added prior to that meal and adjusted as necessary until BG goals are met. Usually the post-prandial blood glucose goal is < 180mg/dL.
An alternative and preferred method to adding bolus insulin would be to consider a trial of a GLP-1 receptor agonist if the patient is not already on one. This option is useful, especially for overweight patients, as GLP-1 RAs may result in improved glycemic control without hypoglycemia and with the added benefit of weight loss. Another option is to switch the basal insulin to a fixed-mixed-insulin product administered twice a day. As described earlier, there are inherent limitations to using fixed dosing insulin. (Figure 6)
| Table 5. Pattern Management:* General Insulin Dose Adjustments |
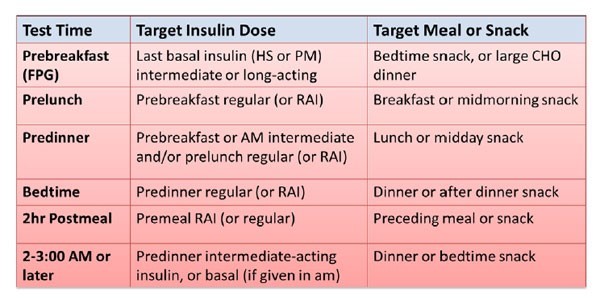 |
*Assumes proper insulin administration, timing, regular meals, etc.
CHO = carbohydrate; FPG = fasting plasma glucose; HS = at bedtime; RAI = rapid acting insulin
Kroon LA, Williams C. Table 53-15.In: Alldredge BK, Corelli RL, Ernst ME, et al, eds. Koda-Kimble and
Young's Applied Therapeutics: The Clinical Use of Drugs.10th ed. Philadelphia, PA: Lippincott
Williams & Wilkins; 2013:1223-1300. |
HELPING PATIENTS WITH T2D TO MANAGE THEIR OWN INSULIN REGIMEN
Performing SMBG and understanding the meaning of the glucose values helps patients understand how their medication, exercise, and food choices impact their glucose control. Therefore, testing BG at home is essential. Because basal insulin is generally initiated first, patients should test at least once each morning before they eat. When patients have reached their glucose goals, they can potentially switch to twice-weekly testing. Figure 7 illustrates varied dosing strategies using 2 to 4 daily injections of basal and bolus injections. No matter which insulin regimen a patient is prescribed, using Table 5 as a tool to identify which insulin, meal, or snack may be causing highs or lows during different times of the day is helpful. Understanding the PK and PD of the type(s) of insulin can help when making dosage adjustments. This works for the majority of patients; however, there are some PK/PD differences to consider in some special populations.14,42
Recommendations for Pattern Management to Guide Insulin Dose Adjustments
Clinicians can use the SMBG log to have patients track glucose values, times of day, and insulin dose as well as food and activity levels each day. At least 7 days of values should be used to help identify whether a BG pattern can be established. Additional information from the patient that may be important includes: 1. How many doses of insulin have you missed in the last week? 2. Have you noticed any foods that make your glucose readings higher? 3. Have you had any changes in meals or activities in the last week? If no significant changes are identified, then a patient may need to be referred to their provider for a insulin dose adjustment. Patient’s may need to be reminded of their glycemic targets and goal range, such as morning glucose values of 80 to 130 mg/dL, to be able to identify out-of-goal range values. Using the pattern management tool shown in Table 5 helps to identify where the insulin change may need to be made. Adjustments are made one at a time in small (approximately 10%) increments, starting with either the earliest time of high blood glucose in the day or the most crucial time, such as hypoglycemic events.
| Figure 7. Examples of Insulin Dosing Regimens |
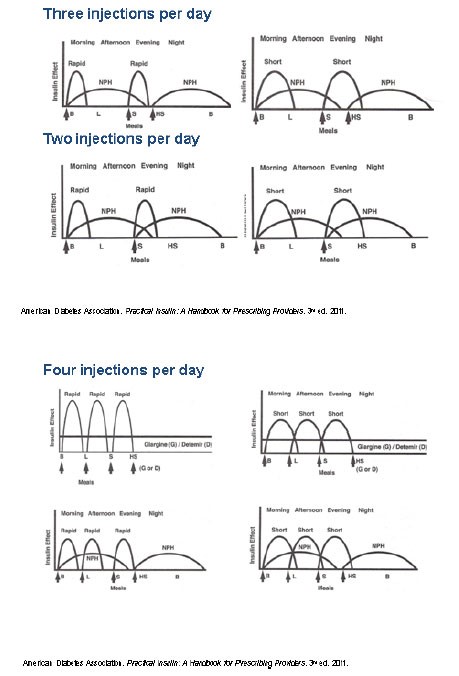 |
American Diabetes Association. Practical Insulin:
A Handbook for Prescribing Providers. 3rd ed. 2011. |
INSULIN ADMINISTRATION
Properly preparing and administering the insulin dose is essential to ensure that patients receive the correct dose and to prevent injection-site reactions. Tables 6 and 7 provide stepwise insulin preparation and administration instructions. Insulin administration can be influenced by several factors listed in Table 8. As an inhaled insulin product, Afrezza is administered by inhaling at a 45-degree angle as shown in Figure 8.
| Table 6. Preparing the Insulin Dose: Vial and Pen Methods40 |
| Guidelines for Preparing an Insulin Dose |
| Using a Vial and Syringe |
- Wash hands with soap and warm water.
- Check the insulin label on the vial to verify the type of insulin to be injected.
- Visually inspect the insulin vial for signs of contamination or degradation (e.g., white clumps, color changes).
- For all cloudy insulins, roll the vial gently back and forth between the hands to re-suspend the insulin.
- Remove the cap and wipe the top of the vial off with an alcohol swab or cotton ball dipped in alcohol.
- Remove the protective coverings over the plunger and needle of the syringe.
- Taking care not to touch the needle, draw up air equal to the insulin dose to be administered into the syringe.
- Inject the air into the insulin vial.a
- With the syringe still inserted, invert the vial and withdraw the insulin dose.b
- Be sure to keep the hub of the needle below the surface of the insulin to prevent creating air bubbles within the syringe.
- If bubbles are present, gently tap the syringe to coax air to the top of the barrel where it can be injected back into the vial.
- Remove the syringe from the vial and self-inject the dose using proper injection technique.
|
| Using a Prefilled Insulin Pen |
- Wash hands with soap and warm water.
- Check the insulin label on the device (e.g., pen) to verify the type of insulin to be injected.
- Remove the protective pen cap and visually inspect the insulin for signs of contamination or degradation.
- For all cloudy insulins, invert and roll the device (e.g., pen) gently back and forth between the hands to re-suspend the insulin.
- Wipe the rubber stopper with an alcohol swab.
- Attach a needle onto the device according to the manufacturer's directions.
- Remove the outer and inner needle caps.
- Follow any manufacturer's recommendations for priming the device (e.g., 2-unit air shot).
- Making sure pen dose selectors are first set to zero, then dial the insulin dose to be injected.
- Use proper injection technique.
- To deliver insulin when injecting, push down on the plunger button and hold for 5 to 10 seconds.
- Remove the needle from the device after injection to avoid allowing air into the insulin reservoir.
|
NPH = neutral protamine Hagedorn
aPatients mixing rapid- or short-acting insulin with NPH into the same syringe for injection should be instructed to inject air first into the NPH vial, then into the rapid- or short-acting vial.
bPatients mixing rapid- or short-acting insulin with NPH into the same syringe for injection should be instructed to withdraw the dose of the rapid- or short-acting insulin before the NPH.
*Patients should refer to the product information for specific dosing, storage, and administration details. |
| Assemi M, Morello CM. Table 47-11. In: Berardi RR, Ferreri S, Hume AL, et al, eds. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care. 16th ed. Washington, DC: American Pharmaceutical Association; 2009:837-850. |
| Table 7. Injecting Insulin44 |
| Insulin Subcutaneous Self-Injection Technique |
- Prepare insulin dose for administration.
- Clean the skin using an alcohol swab.
- Pinch the area to be injected.
- Insert the needle at a 90-degree angle to the skin in the center of the pinched area (a 45-degree angle for insertion may be used in small children and very thin adults).
- Release the pinch.
- Press down on the syringe or device plunger to inject insulin.
- Hold the syringe or device in the area for 5 to 10 seconds to ensure full delivery of insulin. This step is particularly important for insulin pen devices.
- Remove the syringe or device.
- Throw needle away in Sharps container.
|
| Assemi M, Morello CM. Table 47-12. In: Berardi RR, Ferreri S, Hume AL, eds.Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care, 16th ed. Washington, DC: American Pharmaceutical Association; 2009:837-850. |
| Table 8. Influences on Insulin Injection Routes and Absorption Rates |
| Insulin Influence |
Contributing Factor |
| Administration Route |
|
| Injection Site |
- Abdomen (preferred site) > deltoid > thighs > hips
- Rotate sites
- Avoid 2-inch radius around navel
|
| Increased Absorption Rate |
- Warmth and increased blood flow (e.g., massage, strenuous exercise, fever, sauna or hot tub)
|
| Decreased Absorption Rate |
- Ice, cold packs, cold extremities
|
| Erratic Absorption |
- Large insulin doses or improper mixing
- Lipohypertrophy
|
| IM = intramuscular; IV = intravenous; SC = subcutaneous; Lipohypertrophy = a thickened area of tissue that can develop in the subcutaneous fat layer where repeated injections of insulin are given |
Figure 8. Inhaled Insulin Administration25 |
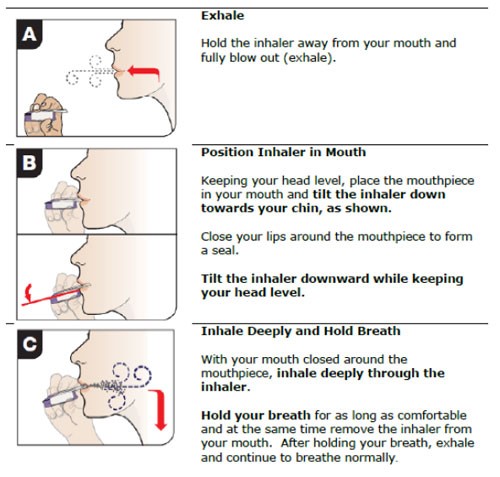 |
WARNINGS AND PRECAUTIONS FOR THE USE OF INSULIN
With the purity of today's insulin options, injection-site reactions rarely occur. Since insulin is an anabolic hormone, fatty accumulation may occur if the patient regularly uses the same injection site. As such, it is important to remind patients to rotate their injection site and use a new needle/pen needle for each injection to avoid lipohypertrophy. The abdominal area is the preferred injection site, avoiding 1 to 2 inches around the navel, although some patients prefer injecting in the thighs or deltoids.
While weight gain may also be a concern with insulin use, coupled with a healthy diet and regular daily physical activity, insulin use does not have to result in added pounds.45,46 Moreover, regular physical activity has been shown to improve BG control, lipids, blood pressure, cardiovascular events, mortality risk, and quality of life.47
Hypoglycemia is among the most concerning adverse events with insulin use. Personalizing glucose target goals, self-monitoring blood glucose, and managing glucose patterns can help prevent hypoglycemia. Mild-to-moderate hypoglycemia is more common and easily treated compared with severe episodes requiring medical assistance. Research indicates an increased risk of cognitive impairment,48 arrhythmias,49 and cardiovascular and all-cause mortality48-53 is associated with severe hypoglycemic episodes. Educating patients on prevention and treatment remains key for dealing with hypoglycemia. For these reasons, the ADA recommends a premeal glucose target of 80 to 130 mg/dL, instead of the previous 70 to 130 mg/dL.7 Several factors can contribute to hypoglycemia, including insufficient caloric intake (e.g., skipped or delayed meals, vomiting), inaccurate insulin dosage (e.g., depot effect, too high, frequent adjustments, incorrect insulin given), use of other hypoglycemic medications (i.e., insulin secretagogues), vigorous exercise, and excessive alcohol intake.
Since detecting hypoglycemia early is important, educating patients about potential symptoms is crucial. Early symptoms of hypoglycemia may include trembling, shaking, sweating, heart palpitations, or tachycardia. Other symptoms (e.g., slow mentation, difficulty concentrating, slurred speech, uncoordinated, dizziness) may occur as hypoglycemia worsens.
TREATING MILD-TO-MODERATE HYPOGLYCEMIA
Mild-to-moderate hypoglycemia can usually be reversed rapidly, often within 5 to 10 minutes. Using the "Rule of 15" described in Table 9, BG concentrations rise quickly without overtreating, which could lead to elevated glucose concentrations and an undesired BG roller coaster. As a preventative measure, people using insulin should always carry a fast-acting glucose source. For BG of less than 70 to 54 mg/dL, use 15 to 20 grams of carbohydrates. But if the glucose is below 54 mg/dL and the patient can swallow, 20 to 30 grams of carbohydrates should be consumed. Table 10 lists 15-gram amounts of effective fast-acting carbohydrates such as glucose tablets, milk, fruit juice, regular (non-diet) soft drink, sugar, raisins, hard candies, and glucose gels, with the tablets being the preferred source for treatment.
Foods high in fat such as chocolate, doughnuts, potato chips, and pizza are poor choices for treating hypoglycemia. This is mainly because fatty foods slow carbohydrate absorption, delaying glucose correction and adding unnecessary calories.42 However, if these foods are the only sources available, patients should use them, realizing that symptoms may take longer to resolve. Once hypoglycemia is treated and if mealtime is not within 1 hour, a light snack (e.g., crackers, a piece of fruit, a small sandwich) should be consumed to prevent further hypoglycemia.
| Table 9.40 Treating Mild-to-Moderate Hypoglycemia with the "Rule of 15"+ |
| Step |
Directions |
| 1 |
Using a glucose monitor, test to determine blood glucose (BG) is below 70 mg/dL. |
| 2 |
Eat 15 grams of simple, concentrated carbohydrates. |
| 3 |
Wait 15 minutes. |
| 4 |
Check BG again. |
| 5 |
If blood glucose is still below 70 mg/dL, consume an additional 15 grams of carbohydrates. |
| 6 |
Follow up with a light snack (or with a meal if it is mealtime). |
| + If BG is below 50 mg/dL and the patient can swallow, 20 – 30 grams of carbohydrates should be consumed.36 |
| Table 10. Fast-Acting Sources of Carbohydrates |
| Source |
Quantity |
Glucose tablets
Glucose gel
Soft candies (Skittles, Starbursts, jelly beans)
Sugar
Raisins
Non-diet soft drink
Fruit juice (apple/orange)
Milk (non-/low-fat) |
3 – 4 tablets
1 tube of 15 grams' carbohydrates
5 – 6 pieces
3 cubes or 1 tbsp
2 tbsp
4 oz (1/2 cup)
4 oz (1/2 cup)
8 oz (1 cup) |
| oz = ounces; tbsp = tablespoon(s)
|
TREATING SEVERE HYPOGLYCEMIA
Untreated mild-to-moderate hypoglycemia can lead to severe hypoglycemia and unconsciousness. Severe hypoglycemia may result in unconsciousness, coma, seizures, and the inability to swallow and should be treated with glucagon.54 All patients with T1D must have a glucagon emergency kit. For an unconscious patient, keep in mind that the person experiencing severe hypoglycemia cannot swallow. Force-feeding food or liquid to an unconscious person is unsafe and can lead to choking.
Treatment of severe hypoglycemia requires a glucagon emergency kit, which is available only by prescription. The kit contains a 1-mg ampule of glucagon, a syringe filled with diluent, and administration directions. Glucagon, a natural counter-regulatory hormone to insulin, works quickly to increase BG concentrations. The glucagon mixing and administration instructions may seem confusing during an emergency. To prevent confusion in a stressful situation, it is vital to educate people around the patient (family, coworkers, close friends, teachers, caregivers) on how to prepare and administer glucagon before an actual emergency arises. Encourage them to practice the process of glucagon administration ahead of time so they will be prepared. Annual re-education is a practical recommendation.
Figure 9 contains a summary of the steps for treating severe hypoglycemia with glucagon. This hormone peptide may cause vomiting that lasts up to 24 hours. Unconscious individuals should, therefore, be turned on their side before glucagon is administered to prevent choking. The usual dose is 1 mg for adults and children 5 to 8 years old who weighing more than 44 pounds (20 kg), and 0.5 for children younger than 5 years of age who weigh less than 44 pounds (20 kg). Glucagon usually works within 5 to 10 minutes, but the effects are short-lived. If no response is seen after 5 to 10 minutes, a second injection may be given. If that is ineffective, the caregiver should call 911 immediately. Once the person is conscious and can swallow, the caregiver should give him or her a carbohydrate-containing liquid (e.g., juice, milk, a non-diet soft drink) followed by a carbohydrate snack (small sandwich and/or crackers with peanut butter). For the next 24 hours, regular SMBG is necessary as well as adequate food intake to replenish hepatic glycogen stores. The primary care provider should be informed of the episode.
It is important to remind patients to regularly check expiration dates on glucagon kits. It is a good practice to obtain a new glucagon kit annually. Although some patients go for years without having to use their glucagon emergency kits, it is very important that every insulin-treated patient has a kit available and keeps it easily accessible. The kits should be stored in several places, such as in the bedroom or in a purse, a desk, a briefcase, or a backpack, and those individuals around the patient should know where the kits are located.
Patients with repeated hypoglycemic episodes can develop hypoglycemia unawareness. This condition is characterized by a progressive loss of symptoms to hypoglycemia, such as sweating, tremor, anxiety, and palpitations.55 This loss of symptoms may result in BG levels falling to potentially dangerous levels since the patient does not experience the typical signs. Hypoglycemic unawareness can in some cases be reversible by strict avoidance of hypoglycemia for even a few days.56 This may require blood glucose targets to be higher (i.e., 90-150 mg/dL) temporarily depending on the individual patient to help avoid severe hypoglycemia.
Some medications such as beta-blockers can decrease the response to hypoglycemia by blocking most sympathetic warning symptoms, except for sweating. According to the ADA Standards of Care, the benefits of beta-blocker use in patients who have had a myocardial infarction outweigh the potential consequences of hypoglycemia.7 If nocturnal hypoglycemia occurs, individuals should keep a fast-acting glucose source such as a 4-ounce bottle/box or container of apple juice at the bedside for easy treatment. If nocturnal hypoglycemia recurs, the insulin regimen should be re-evaluated.
Figure 9. Treatment of Severe Hypoglycemia With A Glucagon Kit |
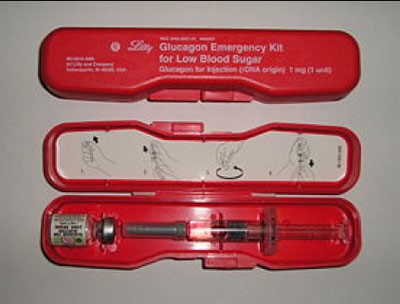
Use a glucagon emergency kit only if the person is unconscious or unable to swallow. |
Follow instructions on the brightly colored box carefully:
- Reconstitute 1 mg of glucagon in 1 mL of sterile water.
- Mix.
- Turn patient on side.
- Inject into arm, abdomen, or rear end.
Administer the following amounts:
- 1 mL for adults and children older than 5 to 8 years of age (weighing more than 44 lb [20 kg])
- 0.5 mL for children weighing less than 44 lb (20 kg) irrespective of age
Reconstituted glucagon must be used right away - discard any unused drug. |
| When the person is able to swallow, give a carbohydrate liquid such as juice, soda, or milk followed by a carbohydrate-and-protein snack such as crackers with peanut butter or a meat sandwich. |
Response generally takes 5 to 20 minutes.
IF THE PERSON DOES NOT RESPOND TO GLUCAGON, SEEK MEDICAL ATTENTION IMMEDIATELY BY CALLING 911. |
PERCEIVED BARRIERS TO INSULIN USE
Despite the advances and improved safety of newer insulin formulations, push back still persists with insulin use in many patients with T2D. A systematic review evaluating 25 studies (15 qualitative and 10 quantitative) indicated that barriers to insulin use tend to fall into 3 main categories: patient-, health care provider (HCP)-, and system-related.57 The 3 most common barriers for patients were fear of painful injections, adverse effects of insulin, and the perception that insulin hinted at the end stages of diabetes. Patient barriers can be broken down by the provider if each is addressed and the patient is educated. Table 11 lists related barriers with practical talking points and solutions that providers can use to address patient-related barriers as well as common clinician- and health-system-related barriers.
HCP barriers include lack of knowledge and concerns about insulin's adverse events as well as fear that the patient will either not be willing to use it or have poor adherence.57,60-63 With the advances in insulin therapy described above, adding basal insulin to an oral regimen can considerably control BG levels while minimizing risks of weight gain and hypoglycemia. HCP may need diabetes education and training that includes the pathophysiology of diabetes and the benefits and ease of insulin use in addition to patient-directed pattern management.
| Table 11. Insulin Use: Perceived Barriers and Solutions57-63 |
| Patient-Related Barriers |
Talking Points with Patients |
Solutions/Actions |
| Fear of pain and injection |
Abdominal fat tissue has very few nerves. More nerves exist in the fingertips. Injections should not be painful, especially compared to testing blood glucose on fingertips. |
Demonstrate in clinic. |
| Adverse effect: Hypoglycemia |
May be prevented with longer-acting basal inulin products along with proper dosing and monitoring. |
Educate patient on prevention and treatment of hypoglycemia. |
| Adverse effect: Weight gain |
May be prevented by regular, daily physical activity that the patient likes to perform (e.g., biking, walking, swimming, dancing). |
With patient, identify a daily activity and a schedule the patient can easily maintain. |
| Perception insulin used for end stage diabetes |
This may have been the case 30 to 40 years ago when only sulfonylureas and insulin were available for use. Now, the ADA recommends that basal insulin be considered earlier in treatment. |
Share and discuss the most recent ADA guidelines with the patient. Find these guidelines annually at www.diabetes.org. |
| Inconvenience |
Most people with T2D who add insulin to their treatment regimen only require 1 injection daily. Insulin pens can be used for added convenience. |
Educate patient on basal insulin and insulin pens. |
| Administration difficulty |
Insulin comes in vials, pens and pumps. Pens are the easiest to use, especially for patients who frequently travel, but the vial/syringe method is also quite easy. |
Demonstrate easy steps of insulin administration. |
| System Barriers |
| Time |
Inquire if the patient will attend an educational class or a one-on-one get-together with a diabetes educator. Insulin administration can be taught outside of the clinical setting. |
Refer patient to diabetes educational services. |
BREAKING DOWN THE BARRIERS: EMPOWERING PATIENTS TO CONTROL THEIR DIABETES
A more personalized care approach is recommended to help patients achieve glycemic control.6 Patients need information regarding the progressive nature of diabetes, factors that can control diabetes, current glycemic control status, current or potential complications, how to prevent complications, and the importance of glycemic control. A key component to diabetes control is empowering patients.64,65 Empowerment encompasses listening, acknowledging, believing, and guiding. (Figure 10) HCPs can listen to patients' fears, concerns, and interests to help them identify a regimen that will best suit them. Acknowledging patients' concerns and discussing their issues should help them know that they have been heard, and it is helpful in breaking down barriers.
Figure 10. Empowerment Concept |
 |
Patients may need help identifying motivating factor(s) for taking control of their diabetes. What is the carrot? Is it improving their well-being by experiencing less nighttime urination, getting better sleep, having more energy, wanting to see improvement quickly, performing better at their job, living longer to experience a significant life event, or understanding the reason behind taking medications? Whatever the key element is, identifying and addressing the issue can help patients to control their diabetes. In a cross-sectional survey asking 524 patients with diabetes and caregivers about motivational factors and adherence methods used, medication education was identified as a key factor.66 The 3 motivating factors most commonly identified as improving medication adherence were: 1. Knowing that diabetes medications work effectively to lower BG, 2. Managing those medications' adverse effects, and 3. Understanding the benefits of the agents used. To empower patients to overcome barriers to compliance, HCPs can help to educate patients about diabetes-drug indications, mechanisms of action, and therapeutic effects. Pharmacy technicians can help identify patients who would benefit from this education.
Understanding treatment options and addressing barriers are essential to helping patients adhere to insulin therapy. Empowering patients to understand that ultimately they are in charge of their diabetes is key. HCPs can provide patients with the tools, education, medication, and knowledge to assist them, but it is up to the patients to utilize this information to help control their diabetes. We can believe in them and help guide them toward achieving their goals. Empowerment is a vision that helps patients modify their behavior and make better decisions about their health care.64,65 Using patient empowerment and motivation as key elements in a diabetes-intensive medical management tune-up clinic model has demonstrated significantly improved glycemic controlled compared with regular care supervised by a primary care provider, even in complex patients (-2.4% versus – 0.8% A1C reduction in 6 months, P < .05).45
Once barriers are addressed with insulin therapy and patients are educated, seeing a positive impact from initiating insulin therapy is almost immediate. Finding the right dose of basal insulin plus daily SMBG is a good way for patients to see a difference in their diabetes control. Improvement in hyperglycemic symptoms of poor energy, frequent urination, and excessive thirst soon follows. Glycemic control has been shown to prevent or reduce long-term complications. Achieving diabetes control appears to have a legacy effect, with the benefits of previous glycemic control lasting over a decade in preventing complications.66-70
IMPORTANT POINTS FOR PATIENTS USING INSULIN
Pharmacy technicians are an integral part of the pharmacy team and can identify potential counseling opportunities during interactions with patients. For patients who use insulin, ask the following open-ended questions to assess their comfort levels with insulin therapy and discern if a pharmacist consultation is needed:
- How does your insulin work?
- What difficulties do you have with your injections?
- How are you tracking your blood sugars?
- How often do you test your blood sugars? What do the results mean to you?
- How have you been feeling since you started your insulin?
The main message patients should understand is that insulin use does not have to be feared, and it can very quickly help control diabetes.
| Counseling Points Pharmacists May Use for Patients Who Use Insulin Therapy |
| Category |
Potential Counseling Tips |
| General |
- Insulin therapy is not difficult
- Insulin therapy produces a rapid, measurable response and can be used safely to help patients achieve their glycemic goals.
- Testing BG at home is a must.
- When starting insulin, patients should test at least once each morning before they eat.
- When patients have reached their goals, they can switch to twice-weekly testing.
- Remember to rotate the injection site. The abdominal area is preferred but avoid 1 to 2 inches around the navel.
- Detecting hypoglycemia early is important. Early symptoms of hypoglycemia may include trembling, shaking, sweating, heart palpitations, or tachycardia. Other symptoms such as slow mentation, difficulty concentrating, slurred speech, uncoordinated, dizziness may occur as hypoglycemia worsens.
|
| Basal Insulin |
- The main function of basal insulin is to suppress liver glucose production between meals and overnight.
|
Long-acting and
Intermediate Insulin |
- Long-acting and intermediate insulins are used to mimic normal insulin secretion.
|
| Bolus Insulin |
- The primary function of bolus insulin replacement is to control hyperglycemia following food consumption.
- Insulin lispro (Humalog; Admelog), aspart (Novolog; Fiasp) and glulisine (Apidra) have a fast onset. Thus, they should be injected right before a meal or within 15 minutes of eating a meal.
- Humulin R or Novolin R have a longer onset and should be administered 30 minutes before a meal.
- Inhaled insulin (Afrezza) is only used as mealtime insulin with fixed doses. Before starting inhaled insulin, lung function tests are required. The most common adverse events are hypoglycemia, cough, and throat pain or irritation.
|
| Combining Insulins |
- When combining insulins, the bolus insulin should be drawn up in the syringe first, followed by NPH.
|
REFERENCES
- Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA: US Department of Health and Human Services; 2017.
- Cefalu WT, Rosenstock J, LeRoith D, Riddle MC. Insulin's role in diabetes management: after 90 years, still considered the essential "black dress." Diabetes Care.2015;38(12):2200-2203.
- United Kingdom Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). 1998;352(9131):837-853.
- Saisho Y. Beta-cell dysfunction: its critical role in prevention and management of type 2 diabetes. World J Diabetes. 2015;6(1):109-124.
- Centers for Disease Control and Prevention. Age-Adjusted Percentage of Adults with Diabetes Using Diabetes Medication, by Type of Medication, United States, 1997–2011,Atlanta, GA: US Department of Health and Human Services; 2012.
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care.2018;41(12):2669-2701.
- American Diabetes Association. Standards of Medical Care in Diabetes – 2019. Diabetes Care.2019;42(Suppl1):S1-S193.
- Powers AP, D'Alessio D. Endocrine pancreas and pharmacotherapy of diabetes mellitus and hypoglycemia. In: Brunton L, Chabner B, Knollman B..Goodman & Gilmans' The Pharmacological Basis of Therapeutics, 12th ed. New York, NY: McGraw-Hill Education; 2011. accessmedicine.mhmedical.com/content.aspx?/bookid=374§ionid=41266252.
- Moghissi E, King AB. Individualizing insulin therapy in the management of type 2 diabetes. Am J Med.2014;127(10 Suppl):S3-S10.
- McCall AL. Therapy and hypoglycemia. In: Insulin Therapy. Leahy J, Cefalu W, eds. New York, NY: Marcel Dekker, Inc; 2002:193.
- Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26(3):881-885.
- Bethel MA, Feinglos MN. Basal insulin therapy in type 2 diabetes. J Am BoardFam Pract. 2005;18(3):199-204.
- Edelman SV, Morello CM. Strategies for insulin therapy in type 2 diabetes. South Med J. 2005;98(3):363-371.
- Morello CM. Pharmacokinetics and pharmacodynamics of insulin analogs in special populations with type 2 diabetes mellitus. Int J Gen Med.2011;4(1):827-835.
- Humalog [package insert]. Indianapolis, IN: Eli Lilly and Company;2018.
- Novolog [package insert]. Plainsboro, NJ: Novo Nordisk;2019.
- Apidra [package insert]. Bridgewater, NJ: Sanofi-aventis;2015.
- Humulin R [package insert]. Indianapolis, IN: Eli Lilly and Company;2018.
- Novolin R [package insert]. Princeton, NJ: Novo Nordisk;2012.
- Humulin N [package insert]. Indianapolis, IN: Eli Lilly and Company;2018.
- Novolin N [package insert]. Princeton, NJ: Novo Nordisk;2018.
- Fiasp [package insert]. Plainsboro, NJ; Novo Nordisk;2018.
- Bowering K, Case C, Harvey J, et al. Faster aspart versus insulin aspart as part of a basal-bolus regimen in inadequately controlled type 2 diabetes: The onset 2 trial. Diabetes Care. 2017;40(7):951-957.
- FDA approves Admelog, the first short-acting “follow on” diabetes product to treat diabetes. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm588466.htm Accessed April 2, 2019.
- Afrezza (inhaled insulin powder) [package insert]. Danbury, CT:MannKind Corp;2017.
- Levemir [package insert].Bagsvaerd, Denmark; 2019.
- Lantus [package insert]. Bridgewater, NJ: Sanofi-aventis; 2015.
- Trujeo (insulin glargine U300) [package insert]. Bridgewater, NJ: Sanofi-Aventis; 2015.
- Tresiba [package insert]. Bagsvaerd, Denmark: Novo Nordisk;2016.
- Wallace JP, Wallace JL, McFarland MS. Comparing dosing of basal insulin analogs determir and glargine: is it really unit-per-unit and dose-per-dose? Ann Pharmacother. 2014;48(3):361-368.
- FDA approves Basaglar, the first "follow on" insulin glargine product to treat diabetes. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm477734.htm Accessed April 2, 2019.
- Humulin R U-500 [package insert]. Indianapolis, IN: Eli Lilly and Company;2018.
- FDA approves a dedicated syringe to be used with Humulin R U-500. http://www.fda.gov/Drugs/DrugSafety/ucm510318.htm. Accessed April 2, 2019.
- Humulin 70/30 [package insert]. Indianapolis, IN; Eli Lilly and Company;2018.
- Novolin 70/30 [package insert]. Plainsboro, NJ: Novo Nordisk;2018.
- Humalog Mix 50/50 [package insert]. Indianapolis, IN: Eli Lilly and Company;2018.
- Humalog Mix 75/25 [package insert]. Indianapolis, IN: Eli Lilly and Company;2018.
- Novolog Mix 70/30 [package insert]. Bagsvaerd, Denmark: Novo Nordisk;2018.
- Ryzodeg 70/30 [package insert]. Plainsboro, NJ: Novo Nordisk;2016.
- Kroon LA, Williams C. Diabetes mellitus. In: Alldredge BK, Corelli RL, Ernst ME, et al, eds. In: Koda-Kimble and Young's Applied Therapeutics: The Clinical Use of Drugs.10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013:1223-1300.
- American Diabetes Association. Practical Insulin: A Handbook for Prescribing Providers. 3rd ed. 2011.
- Trimble LA, Meneilly GS. Optimizing insulin absorption and insulin injection technique in older adults. Diabetes Care. 2014;37(6):e127-e128.
- Sussman JB, Kerr EA, Saini SD, et al. Rates of deintensification of blood pressure and glycemic medication treatment based on levels of control and life expectancy in older patients with diabetes mellitus. JAMA Intern Med. 2015;175(12):1942-1949.
- Assemi M, Morello CM. Diabetes mellitus. In: Berardi RR, Ferreri S, Hume AL, et al, eds.Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care.16th ed. Washington, DC: American Pharmaceutical Association; 2009:837-850.
- Morello CM, Christopher ML, Ortega L, et al. Clinical outcomes associated with a collaborative pharmacist-endocrinologist diabetes intense medical management "tune up" clinic in complex patients. Ann Pharmacother. 2016;50(1):8-16.
- Evert AB, Boucher JL, Cypress M, et al; American Diabetes Association. Nutrition therapy recommendations for the management of adults with diabetes [position statement]. Diabetes Care.2013;36(11);3821-3842.
- Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33(12):e147-e167.
- Feinkohl I, Aung PP, Keller M, et al; Edinburgh Type 2 Diabetes Study (ET2DS) Investigators. Severe hypoglycemia and cognitive decline in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study.Diabetes Care. 2014;37(2):507-515.
- Chow E, Bernjak A, Williams S, et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes. 2014;63(5):1738-1747.
- Elwen FR, Huskinson A, Clapham L, et al. An observational study of patient characteristics and mortality following hypoglycemia in the community. BMJOpen Diabetes Res Care. 2015;3(1):e000094.
- Zoungas S, Patel A, Chalmers J, et al; ADVANCE Collaborative Group. Severe hypoglycemia and risks of vascular events and death. N Engl J Med.2010;363(15):1410-1418.
- McCoy RG, Van Houten HK, Ziegenfuss JY, et al. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care.2012;35(9):1897-1901.
- Khunti K, Davies M, Majeed A, et al. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care.2015;38(2):316-322.
- Pearson T. Glucagon as a treatment of severe hypoglycemia: safe and efficacious but underutilized. Diabetes Educ. 2008;34(1):128-134.
- Oltmanns KM, Deininger E, Wellhoener P, et al. Influence of captopril on symptomatic and hormonal responses to hypoglycaemia in humans. Br J Clin Pharmacol.2003;55(4):347-353.
- Raskin P, Klaff L, Bergenstal R, et al. A 16-week comparison of the novel insulin analog insulin glargine (HOE 901) and NPH human insulin used with insulin lispro in patients with type 1 diabetes. Diabetes Care.2000;23(11):1666-1671.
- Ng CJ, Lai PS, Lee YK, et al. Barriers and facilitators to starting insulin in patients with type 2 diabetes: a systematic review. Int J Clin Pract.2015;69(10):1050-1070.
- Edelman S, Pettus J. Challenges associated with insulin therapy in type 2 diabetes mellitus. Am J Med. 2014;127(10 Suppl):S11-S16.
- Wallia A, Molitch ME. Insulin therapy for type 2 diabetes mellitus. 2014;311(22):2315-2325.
- de Pablos-Velasco P, Parhofer KG, Bradley C, el al. Current level of glycaemic control and its associated factors in patients with type 2 diabetes across Europe: data from the PANORAMA study. Clin Endocrinol (Oxf).2014;80(1):47-56.
- Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29(5):682-689.
- Ratanawongsa N, Crosson JC, Schillinger D, et al. Getting under the skin of clinical inertia in insulin initiation: the Translating Research Into Action for Diabetes (TRIAD) Insulin Starts Project. Diabetes Educ.2012;38(1):94-100.
- Funnell MM. Overcoming barriers to the initiation of insulin. Diabetes.2007;25(1):36-38.
- Baghbanian A, Tol A. The introduction of self-management in type 2 diabetes care: a narrative review. J Educ Health Promot.2012;1:35.
- Tol A, Alhani F, Shojaeazadeh D, Sharifirad G, Moazam N. An empowering approach to promote the quality of life and self-management among type 2 diabetic patients. J Educ Health Promot.2015;4:13.
- Morello CM, Chynoweth M, Kim H, et al. Strategies to improve medication adherence reported by diabetes patients and caregivers: results of a Taking Control of Your Diabetes Survey. Ann Pharmacother. 2011;45(2):145-153.
- Nathan DM; for the DCCT/EDIC Research Group. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 years: overview. Diabetes Care. 2014;37(1):9-16.
- ACCORD Study Group; ACCORD Eye Study Group, Chew EY, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med.2010;363(3):233-244.
- Gore MO, McGuire DK. The 10-year post-trial follow-up of the United Kingdom Prospective Diabetes Study (UKPDS): cardiovascular observations in context. Diab Vasc Dis Res.2009;6(1):53-55.
- Zoungas S, Chalmers J, Neal B, et al; ADVANCE-ON Collaborative Group. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med.2014;371(15):1392-1406.
Back to Top