ADVERTISEMENT

The Role of Continuous Glucose Monitoring in Pharmacy Practice
Overview
Diabetes mellitus is a chronic condition characterized by hyperglycemia. Treatment often requires insulin or other antihyperglycemic medications that put people at risk of hypoglycemia. Frequent monitoring of glucose is associated with lower hemoglobin A1C levels and improved self-management, particularly in people taking insulin.1 Continuous glucose monitoring (CGM) is a form of technology that continuously checks glucose readings to directly measure the impact of food, physical activity, medications, stress, and other factors on glucose levels. Accuracy, accessibility and affordability of CGM devices have dramatically improved in recent years. Pharmacists play an important role in counseling people with diabetes (PWD) on how to use these devices, selecting the best one to fit their unique needs, and helping them to understand the data. This module will discuss important differences between CGM devices, guideline recommendations for use, and counseling and education strategies to successfully incorporate CGM technology into pharmacy practice.
Introduction
The hallmark of diabetes is high blood glucose levels. Approximately 90%-95% of cases are type 2 diabetes mellitus, which is characterized by tissue insulin resistance often in combination with a relative deficiency in insulin production. The majority of the other 5%-10% of cases are type 1 diabetes mellitus, which is caused from an autoimmune destruction of pancreatic beta cells. A total of 30.3 million people have diabetes in the United States, which corresponds to 9.4% of the population. Additionally, 84.1 million adults have prediabetes.2 Diabetes was the seventh leading cause of death in the United States in 2015. The total direct and indirect costs of diabetes in the United States was $327 billion in 2017.3
Chronic hyperglycemia leads to microvascular and macrovascular complications. Diabetes is associated with increased rates of obesity, hypertension, hyperlipidemia, and kidney disease. In 2014, 14.2 million visits to the emergency department and 7.2 million hospital discharges involved patients with diabetes. Of the emergency department visits, 245,000 and 207,000 were for hypoglycemic and hyperglycemic events, respectively.2
Burden of Hypoglycemia
Foundational treatment for type 1 diabetes is insulin. Many people with type 2 diabetes also need insulin or are prescribed medications that can cause hypoglycemia such as sulfonylureas and meglitinides. Hypoglycemia with these agents often limits the ability to achieve glycemic goals. Hypoglycemia is classified into 3 levels (Table 1).1
| Table 1. Levels of Hypoglycemia |
| Hypoglycemia Level |
Glucose |
| Level 1 |
< 70 mg/dL |
| Level 2 |
< 54 mg/dL |
| Level 3 |
A severe event characterized by altered mental and/or physical status requiring assistance |
Level 1 hypoglycemia is defined as a glucose less than 70 mg/dL. Symptoms vary and may include shakiness, irritability, confusion, tachycardia, and hunger. Even level 1 hypoglycemia is associated with a negative impact on quality of life.4 In older adults, hypoglycemia is associated with increased risk for dementia and cognitive impairment.1 Level 2 hypoglycemia is defined as a glucose less than 54 mg/dL and can lead to more serious symptoms including loss of consciousness, seizure, coma, or death. As hypoglycemia becomes more frequent, counterregulatory responses may be impaired leading to hypoglycemia unawareness. This increases the risk of level 3 hypoglycemia, defined as severe hypoglycemia requiring the assistance of others. Hypoglycemia unawareness is associated with increased risk in mortality as well as cardiovascular and neurological complications including cardiac arrhythmias resulting in sudden death.5 A CGM device can be prescribed to reduce the incidence of hypoglycemia.6
CGM Technology
A CGM device measures glucose in the interstitial fluid every 1-5 minutes and records the data every 5-15 minutes depending on the specific device. A recording every 5 minutes is equivalent to 288 glucose readings per day. Even compared to the very engaged patient checking glucose frequently, this provides much more data. These data help PWD understand how they respond to food, medications, physical activity, stress, and other factors that affect glucose levels.
At least 42 factors have been described to affect glucose levels.7 Figure 1 lists the 42 factors along with the expected effect: increase, decrease, or possibly either.7 Each person is unique and may have different responses to these factors. For example, one person may notice a spike in glucose after drinking black coffee while another person may have no effect. One person may notice that running raises their glucose while another person may find it lowers their glucose. These individual differences are easier to see from CGM compared with self-monitoring of blood glucose (SMBG). The CGM information is very useful for the health care team to recommend medication and lifestyle changes to help people manage their diabetes based on how they uniquely respond to different factors. The goal of these data is to help stabilize glucose levels and reach glucose targets.
| Figure 1. Forty-two Factors that Affect Glucose Levels.7 |
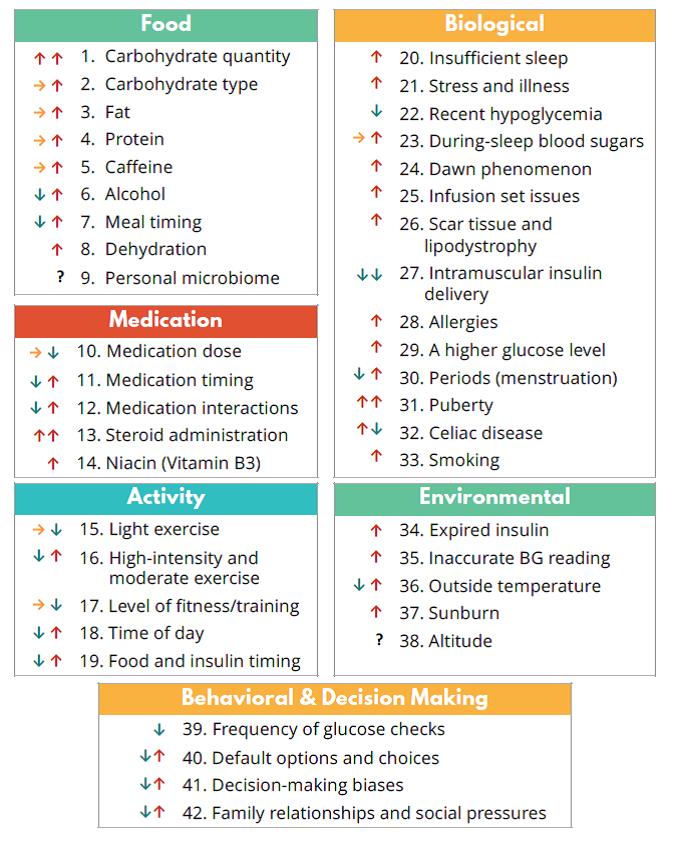 |
Source: Brown A. 42 factors that affect blood glucose. Diatribe. Feb 23, 2018.
Available at: https://diatribe.or/42factors. Reprinted with permission. |
Barriers to Traditional Glucose Testing
Traditional SMBG has several limitations. Monitoring is often infrequent or intermittent, and it may be challenging to stop an activity to lance the finger and draw a drop of blood for the test. Overnight glucose levels are seldom measured often resulting in the presence of unrecognized nocturnal hypoglycemia. SMBG additionally offers no real-time alerts for high or low glucose readings often causing episodes of hypoglycemia and hyperglycemia to be missed. For example, many people only check glucose readings first thing in the morning and miss the excursions that happen during the day. Figure 2 shows the visual difference between SMBG and CGM.
The FDA requires that 95% of all measured blood glucose meter values be within 15% of the true lab measurement and 99% of values must be within 20%.8 Glucose meters can introduce additional user error such as squeezing the site too much, collecting too little or too much blood, or using a site that is not fully clean with residual food or alcohol causing falsely high or low blood glucose readings that are outside of this 15%-20% range.
The gold standard measure of long-term glycemic control, hemoglobin A1C, also has limitations. It is a surrogate marker that is based on average glycemia over a 2- to 3-month period. It provides no information about hypoglycemia and glycemic variability. Factors that affect red blood cell turnover, such as anemia, can also make this measure inaccurate.9
| Figure 2. Self-Monitoring of Blood Glucose versus Continuous Glucose Monitoring |
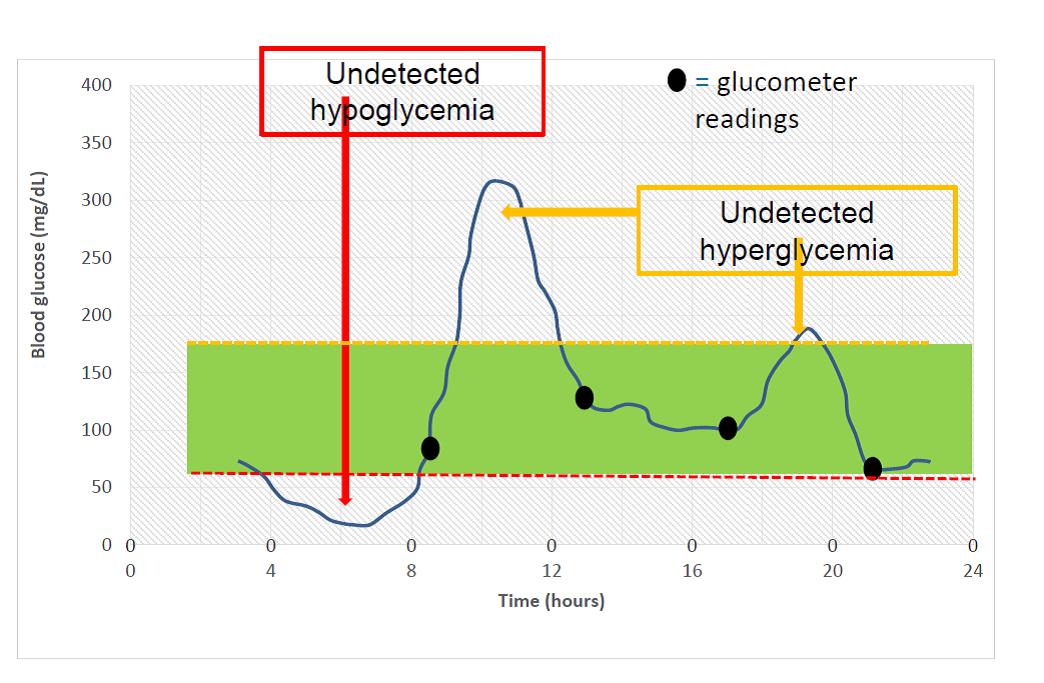 |
In this example, a person is checking glucose 4 times daily. All SMBG appear to be within target range 70-180 g/dL. Based on these values, one would assess the patient is meeting goals especially if A1C was also at target. However, based on the CGM data, there are episodes of hypoglycemia and hyperglycemia that were not detected. This person has a lot of glucose variability that was missed from SMBG alone.
CGM Terminology
| Figure 3. CGM Sensor and Transmitter.10 |
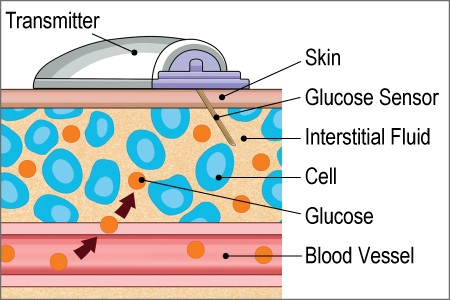 |
CGM consists of three components: a sensor, transmitter, and receiver. The sensor and transmitter are typically inserted on the back or the arm or abdomen. The sensor connects the device to the interstitial fluid through a small filament that stays under the skin. Some devices require a separate step where the transmitter is attached after the sensor is inserted (Figure 3).10 The transmitter communicates with the receiver. Several devices are compatible with cell phones, allowing the cell phone to serve as the receiver. Some devices are compatible with insulin pumps and show the data on the pump.
There is a lag time for CGM glucose measurements when compared to glucose measured in the capillary through a traditional glucose meter. This lag time is more apparent at times when glucose is rapidly rising or falling and, with the lag time typically ranging from 5 to 15 minutes. Mean Absolute Relative Deficiency (MARD) is a term used to describe CGM accuracy compared to laboratory blood glucose values. The lower the MARD, the more accurate. Device accuracy has greatly improved in recent years. Three devices (Eversense, Freestyle Libre, Dexcom G6) have nonadjunctive indications, meaning they are FDA approved to dose insulin without performing a confirmatory finger stick.
Types of CGM
Differences between professional CGM and personal CGM are outlined in Table 2.10 Professional CGM is owned by the clinic and worn by the patient for 3-14 days depending on device. There are blinded and unblinded versions. Blinded CGM is when the person is not able to see the data in real-time. After wear, the data are downloaded to review retrospectively. Some providers prefer a blinded version to get a better sense of frequency of hypo and hyperglycemia without any interference from the patient’s response to the CGM. When using a blinded device, it’s especially important that the patient keeps a food and medication log to pair up glucose changes with daily activities.
Personal CGM is owned by the patient and changed by the patient every 7-14 days depending on device. Personal devices include real-time feedback where a person can see their glucose readings and receive alerts. There is also a flash device, where the sensor is scanned to see glucose readings. With this version, the patient will not be alerted for high and low glucose readings unless it is scanned at the time of the event.
| Table 2. Differences in Professional and Personal CGM.11 |
| Professional |
Personal |
| Owned by the clinic |
Owned by the patient |
| Blinded and unblinded (real-time feedback) options |
Real-time feedback or scan for feedback (flash device) |
| Short-term use (3-14 days) |
Long-term use |
| Alarms for hypo/hyperglycemia in select devices |
Alarms for hypo/hyperglycemia in select devices |
| Insurance coverage for most people with type 1 and type 2 diabetes |
Insurance coverage more limited to type 1 diabetes or those on MDI insulin |
| Not compatible with smartphones or insulin pumps |
Compatible with smartphones and insulin pumps with select devices |
| MDI, multiple daily injections |
CGM Outcomes Data
Increased glucose monitoring leads to lower A1C in people with type 1 diabetes. This has been demonstrated by data from 20,555 participants with type 1 diabetes in the type 1 diabetes exchange clinic registry. The more often patients monitored their glucose, the lower their A1C levels were, which was observed in all age groups (Figure 4). Although these data are not specific to the use of CGM, they demonstrate that more frequent glucose monitoring is associated with lower A1C levels.12
| Figure 4: Data from the Type 1 Diabetes Exchange: A1C and Frequency of Glucose Testing.12 |
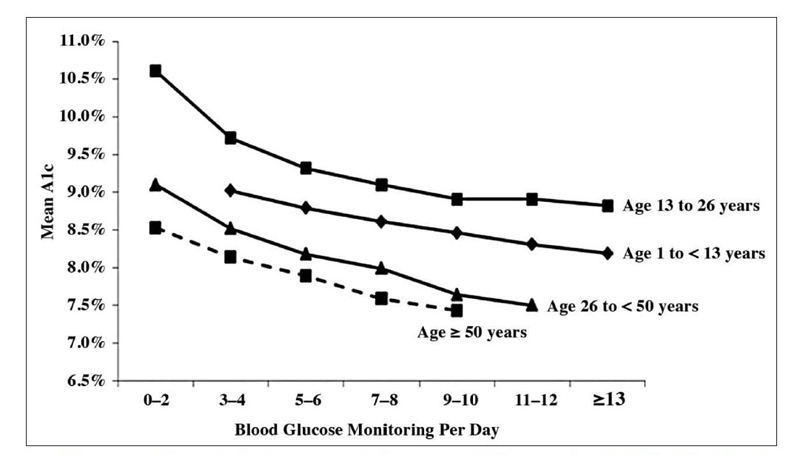 |
Association between blood glucose monitoring frequency and A1C in patients with T1DM (70).
A1C, glycolated hemoglobin. T1DM, type 1 diabetes mellitus
Source: Miller KM, et al. Diabetes Care. 2013;36(7):2009-2014. Reprinted through Creative Commons license. |
Large randomized trials demonstrate efficacy of CGM in people with type 1 diabetes13-15 and insulin-treated type 2 diabetes.16-17 Specific benefits of CGM in people with type 1 and type 2 diabetes include a reduction in A1C, reduction in glycemic variability, decreased time in hypoglycemia, improvement in diabetes related quality of life, and increased hypoglycemia confidence. The low alerts that some CGM devices can provide may increase confidence in preventing and treating hypoglycemia.18
The Juvenile Diabetes Research Foundation CGM Trial was conducted over 6 months in 322 children, adolescents, and adults with type 1 diabetes and a baseline A1C 7%-10%. An A1C decrease of 0.53% was observed in adults over 25 years old (P < 0.001) and in children and adolescents who wore the device at least 6 days per week.13 A similar study conducted in a more clinical environment showed similar A1C reductions in those with baseline A1C > 7%. Reductions in hypoglycemia were seen in all age groups. Greater benefit was observed in participants that wore the device more frequently.19
The DIAMOND Study was a large, randomized, controlled trial series. Phase 1 was conducted in people with type 1 diabetes taking multiple daily injections of insulin. It included 158 adults with A1C levels of 7.5% to 9.9% assigned 2:1 to CGM or usual care. The primary outcome after 24 weeks was change in A1C from a mean baseline of 8.6% in both groups. In the usual care group, there was a modest improvement by week 24, to 8.2%, compared to the CGM group, which decreased to 7.7%. Overall, there was a 0.6% greater improvement in A1C in the CGM group, which was statistically significant (P < 0.001).14 Phase 2 of the Diamond Study was conducted in 158 adults with type 2 diabetes taking multiple daily injections; mean baseline A1C was 8.5%, which improved significantly in both the CGM and usual care arms. The CGM group had a greater reduction in A1C at 24 weeks, going from 8.5% to 7.7%, while the usual care group’s final A1C was 8.0%. In total, this was a 0.3% difference between groups (P = 0.02). There was a greater difference of 0.7% between groups when comparing baseline A1C ≥ 9.0% (P = 0.04).15
The IMPACT study included 241 adults with mean A1C < 7.0% and type 1 diabetes; one-third were using insulin pump therapy. The primary outcome was time spent in hypoglycemia. Use of flash CGM was associated with a 38% reduction in time spent in hypoglycemia.20 There was an increase in glucose time in range combined with a reduction in glycemic variability without worsening A1C. The REPLACE study was a prospective, randomized, controlled trial in people with type 2 diabetes using multiple daily injections and included 224 participants. The primary outcome was change in A1C at 6 months. Overall, there was no difference in A1C change between groups, but there was a 0.44% A1C reduction in adults under 65 (N = 142). Secondary endpoints showed improvement with CGM including reduced glycemic variability and reduction of hypoglycemia with glucose < 70 mg/dL by 43%, reduction in events with a glucose < 55 mg/dL by 53%, and reduction in nocturnal hypoglycemia by 54%. Additionally, questionnaires showed an increased in treatment satisfaction.16
A meta-analysis, including 7 CGM studies in people with type 2 diabetes, showed an overall average 0.42% A1C reduction.17 Overall, clinical trials demonstrate a wide range of benefits for people with type 1 and type 2 diabetes.
CGM Devices
| Table 3: Professional CGM Options |
| Type |
Abbott Freestyle
LibrePro21 |
Dexcom G422 |
Medtronic IPro223 |
| Equipment needed |
Sensor, reader |
Sensor, transmitter, receiver |
Sensor, transmitter, receiver |
| Blinded vs unblinded |
Blinded |
Both |
Blinded |
| Maximum wear time |
14 days |
7 days |
6 days |
| Calibration |
12-h warm up, no calibration |
2-h warm up, 2 per day |
2-h warm up, 3-4 per day |
| Downloading and data reports |
LibreView-does not have any BG or event data within the report, so must be manually compared to the food/activity log |
Clarity Studio -shows user-entered events |
Carelink-BG from meter, app or manually, must be entered at the time of download to appear on the report |
| Reimbursement (CPT) codes |
95250 for removal with a minimum of 3-day wear; 95251 for interpretation by licensed health care provider |
95250 for removal with a minimum of 3-day wear; 95251 for interpretation by licensed health care provider |
95250 for removal with a minimum of 3-day wear; 95251 for interpretation by licensed health care provider |
| Care between uses |
Disposable sensors, one clinic reader for multiple users |
Must be cleaned and disinfected |
Must be cleaned and disinfected |
| Insertion |
1-step process with auto inserter, the transmitter is incorporated into the sensor |
2-step process; insertion of the sensor and attachment of the transmitter |
Multiple step process with insertion of the sensor, taping the sensor, attaching the transmitter and taping over the transmitter |
| MARD (accuracy-the lower the better) |
12.3% |
9% |
11.05% |
| Alarms for high and low alerts |
No |
Yes |
No |
| FDA-approved insertion sites |
Arm |
Abdomen |
Abdomen |
| Interfering substances |
Salicylic acid and high dose Vitamin C |
Acetaminophen |
Acetaminophen |
| Table 4: Personal CGM Options |
| Type |
Abbott Freestyle Libre Flash24 |
Dexcom G625 |
Medtronic Guardian Connect26 |
Senseonics Eversense27 |
| Equipment needed |
Sensors, reader or compatible mobile device |
Sensors, transmitter, receiver (must be within 20 ft of transmitter) or compatible mobile device |
Sensors, mobile device app Sugar.IQ (no receiver, compatible with iPhone or iPod), rechargeable transmitter |
Implanted sensor, rechargeable transmitter, mobile device |
| Maximum wear time |
14 days |
10 days |
7 days |
3 months |
| Warm-up and calibration |
1-h warm up, no calibration |
2-h warm up, no calibration |
Up to 2-h warm up, 2 calibrations per day |
2-h warm up, 2 calibrations per day |
| Downloading and data reports |
LibreView |
Dexcom Clarity |
Carelink and mySugr |
Eversense Data Management Software (DMS) program |
| Reimbursement (CPT) codes |
95249 for removal with a minimum of 3-day wear; 95251 for interpretation by licensed health care provider |
95249 for removal with a minimum of 3-day wear; 95251 for interpretation by licensed health care provider |
95249 for removal with a minimum of 3-day wear; 95251 for interpretation by license health care provider |
Insertion code used by MD or advanced practice provider;95251 for interpretation by licensed health care provider |
| FDA-approved for insulin dosing |
Yes |
Yes |
No |
Yes |
| Data sharing |
Yes |
Yes |
Yes |
Yes |
| Insertion |
1-step with auto inserter |
1-step with auto-inserter, attach transmitter |
1-step with auto-inserter; attach and tape transmitter |
Physician inserted and removed every 3 months; transmitter placed on skin with adhesive |
| MARD (accuracy-the lower the better) |
9.45% |
9% |
Arm: 8.7% (3 calibrations/d), 8.1% (2 calibrations/d)Abdomen: 9.6% (3 calibrations/d), 10.5% (2 calibrations/d) |
8.5% |
| Alarms for high and low alerts |
No unless during a scan |
Yes; absolute and predictive alerts |
Yes, absolute and predictive alerts |
Yes, absolute and predictive alerts |
| FDA-approved insertion sites |
Arm |
AbdomenUnder 18: abdomen and upper buttocks |
Abdomen, arm |
Arm |
| FDA-approved ages |
Over 18 years |
Over 2 years |
Over 14 years |
Over 18 years |
| Interfering substances |
Salicylic acid and high dose Vitamin C |
No |
Acetaminophen |
No |
| Figure 5: CGM Data Display Interfaces.18 |
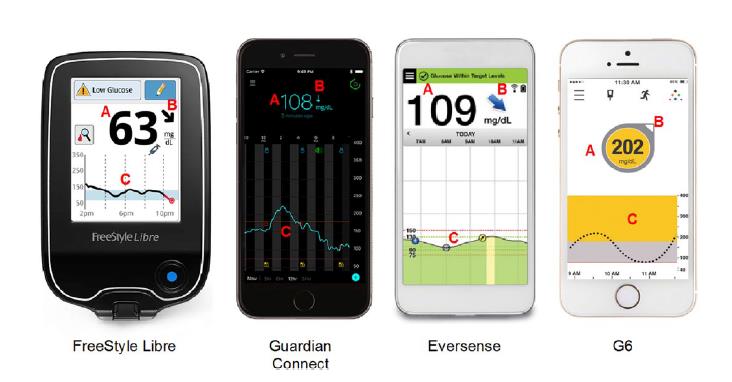 |
| A. Current glucose reading. B. Trend arrow. C. Retrospective analysis of glucose. |
CGM Device Selection
There are 3 choices for professional CGM and 4 choices with personal CGM, which are summarized in Tables 3 and 4, respectively. There are many similarities between the currently available CGM devices. For example, they are all waterproof in the sense that one can shower and bathe without issues, and the sensors can also be submerged in water for varied amounts of time. They must be removed for MRI or CT scans. All personal devices have data-sharing capabilities, meaning a certain number of followers can access the data when using the mobile application through a smart phone. The first 2 to 12 hours of use with all devices can result in more variance between CGM values and true glucose values. One reason for this is the body’s innate response to the introduction of the sensor, and the wound healing that accompanies this. This process is accounted for with the manufacturers’ recommendations and warm-up periods.
There are also many differences between CGM devices and some of the key differences are outlined below (Figure 5).
Professional CGM
If there is a preference for a real time professional CGM device, then Dexcom G4 is the best option. For blinded CGM, the LibrePro and IPro2 are acceptable choices. The LibrePro is widely used due to the 14- day wear, lack of a need for calibration, and disposable sensors that eliminates the need for cleaning that is required between uses of the other two devices. There are differences between interfering substances and accuracy, which may guide a clinic to choosing one device over another.
Personal CGM
Alerts
A key difference between devices are the ability to receive high, low, and predictive alerts, which can occur with the Eversense, Guardian, and G6, but not with the Freestyle Libre unless actively scanned. The Freestyle Libre is considered a flash CGM and works differently from real-time CGM in that it must be scanned to see the current glucose value. It provides similar information in terms of current reading and direction, but there are no high and low alerts. The sensor can only hold 8 hours of data, so it must be scanned every 8 hours to have the full CGM tracing.
Calibration
The Guardian Connect and Eversense require calibrations with a glucose meter. The accuracy of the CGM readings can be undermined by not washing the hands before testing, using a meter that is not accurate, or calibrating at a time when glucose is rapidly rising or falling. The best times to calibrate are before meals when glucose is steady. The G6 and Freestyle Libre are factory calibrated and do not require routine finger stick calibrations. The G6 has the ability to perform optional calibrations. With all devices, patients should check finger stick glucose readings whenever symptoms do not match the displayed readings. Of note, the reader of the Freestyle Libre includes a glucose meter that uses freestyle Neo test strips. The other devices require the user to carry a glucose meter separately.
Transmitter
The Eversense is the only implantable sensor. It is currently approved for 3 months of wear and the sensor sends raw data by radio frequency to a removable, rechargeable transmitter on the skin, which provides on-body vibration alerts even if the receiver is not carried. The transmitter is taped onto the skin and is easy to remove and reattach compared to other CGM devices. It also allows for less supplies to be carried during travel as the patient would only need to carry extra tape strips and not the bulky sensors.28 The Guardian and G6 require the person to save the transmitter and reuse for a period of time (3 months for G6, 1 year for the Guardian). The Libre is a disposable transmitter.
Interfering Substances
The MARD values vary slightly between devices and may vary on location of insertion. Substances like acetaminophen, salicylic acid, and vitamin C can interfere with the glucose readings, often causing falsely high readings. The FDA-approved site location varies between arm and abdomen, and accuracy may be affected if using an alternative site. Additionally, if a person is dehydrated or has excess scar tissue, that can interfere with the sensor’s ability to work. This is one of the advantages of the implantable CGM, which may allow for improved accuracy.
Insulin Pump Compatibility
The G6 is compatible with the T:Slim insulin pump and offers a feature called Basal IQ, which suspends insulin when glucose is predicted to fall below 80 mg/dL. There is a similar Guardian transmitter called the Guardian 3 that is compatible with the Medtronic 670G hybrid closed loop insulin pump. Medtronic offers a pathway for people using the Guardian Connect who want to upgrade to the full insulin pump system.
CGM Guidelines
The American Association of Clinical Endocrinologists recommend professional CGM if A1C is not at goal after 3 months or if patients are taking agents that can cause hypoglycemia. The guideline recommends personal CGM devices for people taking multiple daily injections or in those with a history of hypoglycemia unawareness.29 The American Diabetes Association recommends real-time CGM to be considered in children and adolescents with type 1 diabetes as a tool to help improve glucose control and reduce risk of hypoglycemia. The guideline stresses that when prescribing CGM, robust diabetes education, training, and support are required for optimal implementation and ongoing use. The guidelines also recommend that people who have been successfully using CGM should have continued access across third-party payers. Insurance coverage varies greatly and so life transitions such as going off parent’s insurance, changing jobs, losing a job, or retiring may affect access to devices and supplies in the current health care model.30
Several organizations came together to write a consensus report for clinically meaningful outcomes beyond A1C for type 1 diabetes. The purpose of this report was to acknowledge that the newer technology of CGM makes it possible to assess efficacy of therapy beyond the A1C and glucose meter readings, and therefore, it’s important to standardize definitions of glycemic metrics for the purpose of clinical management and regulatory and reimbursement decisions.31 The international consensus on use of CGM recommends standardizing key metrics on all reports, including use of an ambulatory glucose profile (Figure 6).32
| Table 5. CGM Metrics.33-34 |
| CGM Metric |
Measure |
| Number of days CGM is worn |
14 days recommended |
| Percentage CGM is active |
70% recommended |
| Standardized visualization of data |
Ambulatory glucose profile (AGP) |
| Mean glucose |
Calculated |
| Hypoglycemia, Level 1 |
< 70mg/dL |
| Very low/clinically significant hypoglycemia, Level 2 |
< 54mg/dL |
| Hyperglycemia, Level 1 |
> 180mg/dL |
| Very high/clinical significant hyperglycemia, Level 2 |
> 250mgdL |
| Time in range |
70-180 mg/dL |
| Glycemic variability (coefficient of variation) |
Standard deviation/mean, |
| Glucose management indicator (GMI) |
CGM version of estimated A1C |
| Table 6. CGM Treatment Targets Care.33 |
| Value |
Type 1 and Type 2 Diabetes |
Older/High-Risk Type 1 and Type 2 Diabetes |
| > 250 mg/dL |
< 5% |
< 10% |
| > 180 mg/dL |
< 25% |
< 50% |
| 70-180 mg/dL |
> 70% |
> 50% |
| < 70 mg/dL |
< 4% |
< 1% |
| < 54 mg/dL |
< 1% |
0 |
| Coefficient of Variation |
< 36% |
< 36% |
| Figure 6. Example of Ambulatory Glucose Profile. |
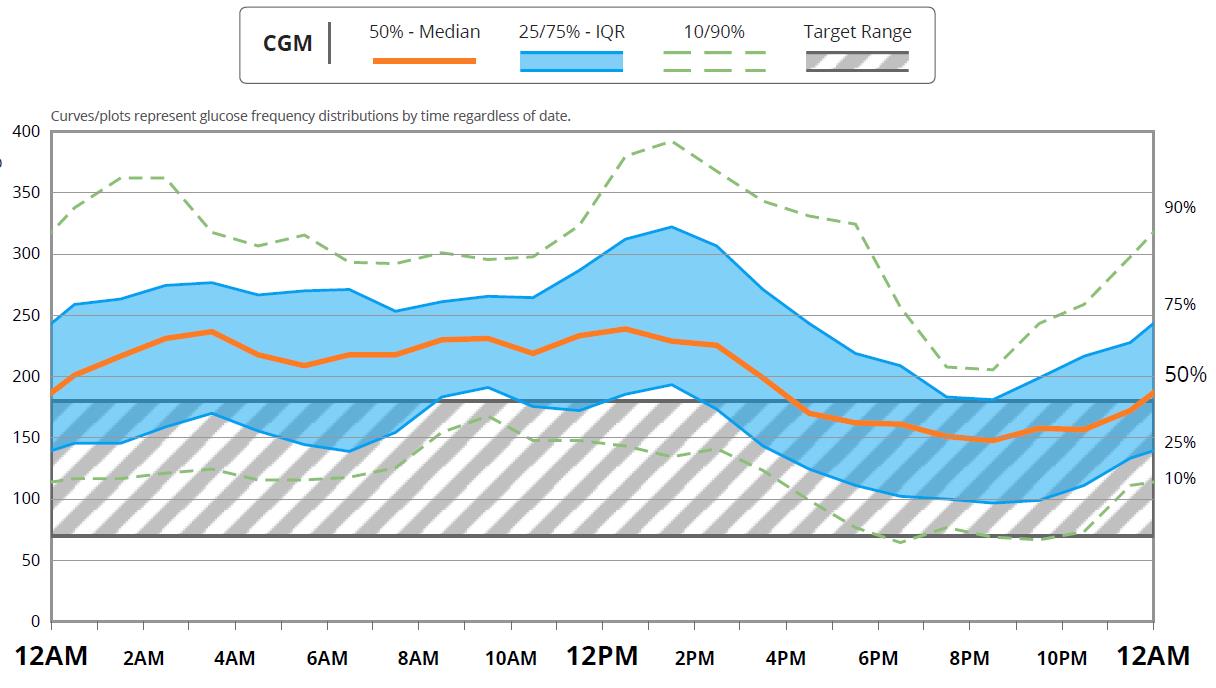 |
| Source: Dexcom.com. Dexcom CLARITY Diabetes Management Software. https://www.dexcom.com/clarity |
Understanding Reports
The reports will look different depending on the device and the system used for the download. However, there are certain key components that should be available on every report. One is the ambulatory glucose profile (AGP), which is a standardized way of visualizing the data. It typically provides a median line and shows glucose from the 10th to 90th percentile. The other CGM Metrics are outlined in Table 5.33-34 An International Consensus agreed on certain goals for time in range and other parameters of the reports, which are listed in Table 6.33 Of note, time in range can often be adjusted on reports, but the standard is to use 70-180 mg/dL since under 70 is classified as hypoglycemia and over 180 mg/dL is classified as hyperglycemia. Hyperglycemia statistics are further broken down into very high or clinically significant hyperglycemia, which is glucose over 250 mg/dL. Glycemic variability or the coefficient of variation is the standard deviation divided by the mean. It gives a measure for how much variation there is between the high and low glucose reading. If there is a lot of variation, it is harder to make adjustments or find patterns since every day may be different. Guidelines recommend that this be less than 36%.33
The glucose management indicator is an estimate of what the A1C would be based on the average glucose data. This term is preferred over estimated A1C since there may be differences between the actual A1C, which is impacted by red blood cell life span.34
To properly analyze a CGM report, it’s important that the sensor is consistently used. If it’s not used consistently, then you may only be getting a snapshot of the data. Higher is better, recommended data sufficiency is at least 70% sensor use over 14 days for personal CGM.33 This is different from professional CGM, which is generally worn for 6 to 14 days.
The Role of the Pharmacist: Device Selection
Many CGM devices are now available through pharmacy benefits. Pharmacists have a vital role in guiding device selection, teaching patients how to use the device, and helping patients understand their data. Pharmacists can also use the data to make treatment recommendations or changes depending on their state-specific scope of practice. There may also be unique billing opportunities for CPT codes 95249 (personal CGM training/download), 95250 (professional CGM insertion/download), and CPT 95251 (CGM interpretation). Medicare guidelines state CPT 95251 should be performed by an advanced practice nurse, physician assistant, or physician, but there can be opportunities for collaboration with local clinics.35 Pharmacists can identify potential candidates that would benefit from the systems and review this with their provider. Providing information, skills, and support will result in empowered individuals that can embrace this innovative technology.11,36
Table 7 summarizes some of the key differences between devices that can help guide device selection in addition to insurance coverage.
| Table 7. Differences Between CGM Devices. |
| Criteria |
Preferred Device(s) |
| No calibration |
Libre, G6 |
| Ability to easily remove transmitter |
Eversense |
| Low cost |
Libre |
| Alerts for hypo/hyperglycemia |
G6, Eversense, Guardian |
| Share data with family/friends |
Eversense, Guardian,
G6, Libre-only if using cell phone application |
| No smartphone required |
Libre, G6 |
| Track responses from certain foods (Sugar IQ) |
Guardian |
| Compatible with insulin pump |
G6 (T:Slim), Guardian 3 (Medtronic) |
| Unlikely medication interference |
G6, Eversense |
| 1 step sensor insertion |
Libre, G6 |
| Disposable transmitter |
Libre |
| Non-adjunctive indication |
Libre, G6, Eversense with mobile app |
| Glucose meter embedded in the reader |
Libre |
| Longest wear time |
Libre (14 days), Eversense (3 months) |
| Use in kids |
Dexcom (over 2), Guardian (over 14) |
Counseling and Education
Pharmacists can also help download devices on the corresponding software and help patients understand what their data means. With cell phone apps, the various systems can update in real time through blue tooth allowing for remote monitoring. There are guidelines that address insulin dose adjustments based on CGM trend arrows, thus allowing patients to add or subtract from insulin doses to reduce risk of hypo and hyperglycemia.37-39 These are some of the important counseling and education points that pharmacists and diabetes educators can be involved with:
- Insertion of the sensor
- Attachment of the transmitter to the sensor when required
- Any required taping/securing of the sensor/transmitter
- Connection of the transmitter to the receiver
- Difference in sensor glucose and blood glucose
- Calibration (if required) including timing, frequency, and importance of accurate meter/finger stick technique
- Setting and managing high and low alerts
- Problem solving for site adhesiveness
- Understanding CGM data and trends
- Help PWD understand real time data and the meaning of the arrows.11
Case 1
Tabitha is 42-year-old woman who takes metformin, insulin glargine, and insulin lispro for type 2 diabetes. She complains of cold sweats overnight but is not sure if this is due to hypoglycemia. She checks her blood glucose 2-4 times daily before meals. She is concerned about her lows, which she is often unaware of. Her most recent A1C = 7.2%. She is willing to wear a professional CGM for 1 week.
Plan:
- Tabitha will wear the LibrePro for 14 days. This is a blinded CGM, so Tabitha will not see her glucose readings while she wears it.
- The pharmacist places the sensor on the back of Tabitha’s upper arm.
- After 14 days, the pharmacist removes the sensor and downloads the CGM report to review with Tabitha. Lows are observed overnight. A spike in glucose is also seen after breakfast. Tabitha usually eats cereal with orange juice.
- The pharmacist recommends incorporating protein into breakfast. Instead of cereal only, Tabitha will add a hard-boiled egg. She agrees to try water instead of orange juice. The pharmacist also recommends decreasing the insulin glargine dose since the blood glucose is falling precipitously overnight.
- The professional CGM is billed CPT 95250 (device insertion/download) and is routed to the physician office to bill CPT 95251 for interpretation. The pharmacist performed the preliminary interpretation and notified the doctor’s office about the recommendation to reduce insulin glargine due to hypoglycemia.
Case 2
Alex is a 36-year-old man with type 1 diabetes of 15 years duration. He takes insulin degludec and insulin aspart to treat his diabetes. He enjoyed wearing a professional CGM and asks about obtaining a personal CGM device. He prefers a device that can alert him about high and low glucose readings, and would like the ability to share the data with his wife. He uses an IPhone.
Plan:
- The G6, Guardian Connect, and Eversense could all meet Alex’s needs. The pharmacist shares features of each device.
- Alex decides on the G6 because he likes that it does not require calibrations.
- The pharmacist shows Alex how to insert the sensor and attach the transmitter.
- The pharmacist helps Alex download the Dexcom G6 and Clarity applications on his phone.
- The pharmacist helps Alex set alerts. They agree on a low alert of 70 mg/dL and a high alert of 250 mg/dL.
- The pharmacist counsels about the 2-hour warm-up period every time the sensor is changed. The sensor should be changed every 10 days. The transmitter should be replaced every 3 months.
- The pharmacist discusses times when Alex should calibrate or confirm his glucose with a finger stick.
- One week later, the pharmacist downloads Alex’s data through Clarity and reviews the report with him.
- The pharmacist can bill CPT 95249 for personal device training. This CPT code can be billed once in the life of a personal device.
- The pharmacist notes spikes in glucose after meals and recommends more meal time insulin. The pharmacist performs a preliminary interpretation and routes to the physician office to bill CPT 95251.
Case 2 Continued:
Alex reports that his sensor fell off after only 2 days. How can the pharmacist help Alex to prevent this from happening again?
Plan:
- The pharmacist shows Alex different taping and adhesive options. Skin Tac and mastisol can be placed underneath the device in a donut shape to avoid inserting the sensor directly through it.
- Outer tapes and patches can be placed over it as well. A few options include Tagaderm, IV 3000, and Simpatch.
- If the device falls off before the full wear time, patients can call the company to receive a replacement sensor.
Summary
Glycemic variability and episodes of hypoglycemia can go unnoticed with traditional SMBG. Even A1C has limitations. CGM has demonstrated improved glycemic management as well as reduction of hypoglycemia and glucose variability, in both people with type 1 and type 2 diabetes. In recent years, there have been great improvements in accessibility, affordability, and accuracy with CGM devices, which is expected to continue in the future. The pharmacist has an important role in counseling PWD on how to use these devices; selecting the best one to fit their unique needs and helping the PWD understand what the data mean. CGM combined with diabetes education and training can help PWD achieve their glucose targets and improve health outcomes. Pharmacists are one of the most accessible health care team members. With many CGM devices now available in pharmacies, this is a great opportunity for pharmacists.
References
- 1. American Diabetes Association. 6. Glycemic targets: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S61–S70.
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2017.
- Improving care and promoting health in populations: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S7–S12.
- Polonsky WH, Fisher L, Hessler D. The impact of non-severe hypoglycemia on quality of life in patients with type 2 diabetes. J Diabetes Complications. 2018;32(4):373-378.
- Martin-Timon I, del Canzo-Gomez FJ. Mechanisms of hypoglycemia unawareness and implications in diabetes patients. World J Diabetes. 2015;6(7):912-916.
- Sequist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384-1395. https://doi.org/10.2337/dc12-2480.
- Diatribe 42 Factors. Available at: https://diatribe.org/42factors. Accessed 6/15/19.
- Self-monitoring blood glucose test systems for over-the-counter use. Document issued 10/11/ 16. US Food and Drug Administration. Available at: https://www.fda.gov/media/87721/download. Accessed 6/17/19.
- Medtronics. Calibrating Your Sensor. Why Sensor Readings are Different from BG Readings. https://www.medtronicdiabetes.com/customer-support/sensors-and-transmitters-support/calibration-sensor
- American Association of Diabetes Educators (AADE). The diabetes educator role in continuous glucose monitoring. AADE Practice Paper. Updated July 19, 2018. Available at: https://www.diabeteseducator.org/practice/educator-tools/diabetes-management-tools/self-monitoring-of-blood-glucose
- Miller KM, Beck RW, Bergestal RM, et al. Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care. 2013;36(7):2009-14. doi: 10.2337/dc12-1770.
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476.
- Beck RW, Riddlesworth TD, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: The DIAMOND randomized clinical trial. JAMA. 2017;317(4):371-378.
- Beck RW Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167(6):365-437. doi: 10.7326/M16-2855.
- Aleppo G, Ruedy KJ, Riddlesworth, et al. REPLACE-BG: a randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care. 2017;40(4):538-545. doi: 10.2337/dc16-2482.
- AIda S, Kaneko R, Murata K. Utility of real-time and retrospective continuous glucose monitoring in patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Diabetes Res. 2019;2019:4684815. doi: 10.1155/2019/4684815.
- Kruger DF, Edelman SV, Hinnen DA, Parkin CG. Reference guide for integrating CGM into clinical practice. Diabetes Educ. 2019;45(1 Suppl):3S-20S.
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Effectiveness of continuous glucose monitoring in a clinical care environment. Diabetes Care. 2010; 33:17–22.
- 20. Bolinder, J, Antuna, R, Geelhoed-Duijvestijn, P, Kroger, J, Weitgasser, R. Novel glucose‐sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet 2016; 388: 2254– 2263.
- Freestyle Libre Pro System. Available at: https://provider.myfreestyle.com/freestyle-libre-pro-clinical-evidence.html. Accessed on 6/17/19.
- Professional Dexcom CGM. Available at: https://provider.dexcom.com/products/professional-cgm. Accessed 6/17/19.
- Medtronic IPro2. Available at: https://professional.medtronicdiabetes.com/ipro2-professional-cgm. Accessed 6/17/19.
- Freestyle Libre Flash System. Available at: https://provider.myfreestyle.com/freestyle-libre-product.html. Accessed 6/17/19.
- Dexcom Personal CGM. Available at: https://provider.dexcom.com/products/personal-cgm. Accessed 6/17/19.
- Medtronic Guardian Connect. Available at: https://professional.medtronicdiabetes.com/guardian-connect-cgm-system. Accessed 6/17/19.
- Adolfsson P, Parkin CG, Thomas A, Krinelke LG. Selecting the appropriate continuous glucose monitoring system—a practical approach. Eur Endocrinol. 2018;14(1):24-29.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm– 2019 executive summary. Endocrine Practice. 2019;25(1).
- American Diabetes Association. 7. Diabetes technology: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S71–S80.
- Agiostratidou F, Anhalt H, Ball D, et al. Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange Diabetes Care. 2017;40:1622–1630. Available at: https://doi.org/10.2337/dc17-1624.
- Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631-1640.
- Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time-in-range [published online June 8, 2019]. Diabetes Care. 2019; doi.org/10.2337/dci19-0028.
- Bergenstal RM, Beck RW, Close KL, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41(11):2275-2280.
- American Association of Clinical Endocrinologists. CPT Codes 95249, 95250, 95251. Available at: https://www.aace.com/practice-management/cpt-codes-95249-95250-and-95251. Accessed 6/18/19.
- Peters AL, Ahmann AJ, Batelino T, et al. Diabetes technology-continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3922-3937.
- Pettus J, Edelman SV. Recommendations for using real-time continuous glucose monitoring (rtCGM) data for insulin adjustments in type 1 diabetes. J Diabetes Sci Technol. 2017;11(1):138-147.
- Aleppo G, Laffel LM, Ahmann AJ, et al. A practical approach to using trend arrows on the Dexcom G5 CGM System for the management of adults with diabetes. J Endocrinol Soc. 2017;1(12):1445-1460. http://bit.ly/2CXE57P.
- Kudva YC, Ahmann AJ, Bergenstal RM, et al. Approach to using trend arrows in the FreeStyle Libre Flash Glucose Monitoring System in adults. J Endocrinol Soc. 2018;2(12).
Back Top