ADVERTISEMENT

COVID-19 Monthly Update: Focus on the Vaccine Endgame
INTRODUCTION
With 175 candidate severe acute respiratory syndrome virus 2 (SARS-CoV-2) vaccines in some form of development and more than a dozen products in clinical trials around the world, public health officials are actively looking at how to take a licensed product forward into a mass immunizations campaign. As they do so, surveys of the American public are increasingly showing that millions of Americans — perhaps even a super majority in some polls — do not intend to take a SARS-CoV-2 vaccine, sometimes because of hesitancy but also due to concern that the vaccine has been rushed through clinical trials.
As the 6-month mark passed in July since the first case of coronavirus syndrome 2019 (COVID-19) in the United States, the number of identified cases was moving steadily toward 4 million, including nearly 150,000 Americans who have died of the disease. The actual number of cases could be from 6 to 24 times this figure, one study showed,1 a figure that if correct, could mean millions of Americans already have some degree of exposure to this virus.
Pharmacists and pharmacies are an important part of the immunization neighborhood in the United States, and their accessibility, parking lots at convenient locations, and long hours will be important in rolling out a mass immunization campaign for the country. In addition, pharmacists are important clinical opinion leaders in communities. Their voices will be important in helping patients sort out fact from social media misinformation and making good decisions about getting a COVID-19 vaccine.
In this installment of Power-Pak’s COVID-19 offerings, the current status of public opinion regarding a vaccine is reviewed, ways of building vaccine confidence presented, and plans for the rollout of a COVID-19 discussed.
HERD IMMUNITY
The goal of immunization programs is to control spread of a disease by inducing sufficient immunity in a population through administration of a safe, efficacious biologic product. To achieve the threshold of protection, or herd immunity, needed to reduce the risk of outbreaks in the population, not every individual has to be vaccinated. Some people may have natural immunity because of prior exposure to the pathogen. If enough other people are vaccinated to reach the protective threshold, herd immunity can be achieved. However, it is important to remember that herd immunity does not guarantee that a person will not get the disease; this threshold interrupts the epidemic or pandemic spread of a pathogen but does not stop all outbreaks.
The threshold for herd immunity depends on the infectivity of the infecting organism. For measles, a high level of herd immunity is needed because an infected person typically spreads the pathogen to an average of 15 others. SARS-CoV-2 is easily spread, but not as easily as measles. The number of contacts likely to acquire SARS-CoV-2 from an infected person appears to be more in the 2.5 range, meaning that an infected person spreads the virus to an average of 2 to 3 other people.
This figure is known in epidemiology as the basic reproductive number, or R0. The level of required herd immunity is calculated as 1 – 1/R0, or for SARS-CoV-2, 1 – 1/2.5, or 0.60. This means that immunity among 60% of a population should protect against rampant spread of the virus, but outbreaks can still occur.
At a June virtual meeting of the National Vaccine Advisory Committee (NVAC), David Dowdy, MD, PhD, of the Johns Hopkins Bloomberg School of Public Health, said that about 8% of the American population had antibodies from natural exposure to SARS-CoV-2, and this figure is rising at 1.5% per month. Without a vaccine and given an R0 of 2.5, it will take a lot of time and COVID-19 cases (and the associated deaths) to get the country to the 60% or 70% needed for herd immunity, Dowdy said. Since it is not currently known how long antibodies remain protective, the duration of natural immunity is likewise unclear. If antibodies do persist, the threshold for herd immunity is raised. Similarly, if a vaccine becomes available but does not induce long-lasting immunity in all recipients, the threshold for herd immunity will also be higher.
Other principles of herd immunity also come into play, Dowdy added. If the susceptibility in the population is nonuniform, the herd immunity threshold is lower. For instance, if many of the most susceptible people get the virus first, the rest of the population is at lower risk of later infection. The herd immunity threshold could be as low as 30% in that case. Another instance involves health care workers, first responders, and other categories of essential workers. Since they are at increased risk of viral exposure, vaccinating them could reduce the risk for everyone else, lowering the threshold for herd immunity.
Herd immunity is a continuum, Dowdy said. While it is usually discussed in terms of a specific figure based on R0, any amount of protection from vaccine- or disease-induced immunity will help flatten the infection curves. “Even a vaccine that doesn’t achieve a prespecified threshold will save lives,” Dowdy said. “The goal of vaccination should really be to optimize the number of people we are able to render immune, not to meet a specific threshold.”
VACCINE CONFIDENCE AND HESITANCY
When the inactivated poliovirus vaccine was introduced in the mid-1950s, uptake was rapid. People lined up to get their children immunized against this disease that had caused so much morbidity and mortality for centuries. And when the oral version of the vaccine was introduced in the early 1960s, the lines formed again to get children this additional protection against this virus.
Given the hysteria over COVID-19 and societal and economic upheaval it has caused, the demand for an effective, safe vaccine should be immense and immediate. Increasingly, though, a number of factors appear to be creating doubt in the minds of many people about the wisdom of being first in this line.
Surveys are quantifying the depth of this doubt. Jennifer Benz of the Associated Press–National Opinion Research Center (AP-NORC) at the University of Chicago told NVAC that a May 2020 survey showed few people (20%) believe a COVID-19 vaccine will be available this year; most respondents (61%) believed it would reach the market in 2021, and 17% were not expecting a licensed product until 2022. The survey also showed differences in vaccine acceptance among different groups (Figure 1). While some groups at higher risk reported greater intent to be immunized (adults aged 60 years or older), Blacks and Hispanics reported more hesitancy than the overall population.
Figure 1. Plans to Receive COVID-19 Vaccine by Americans, May 2020
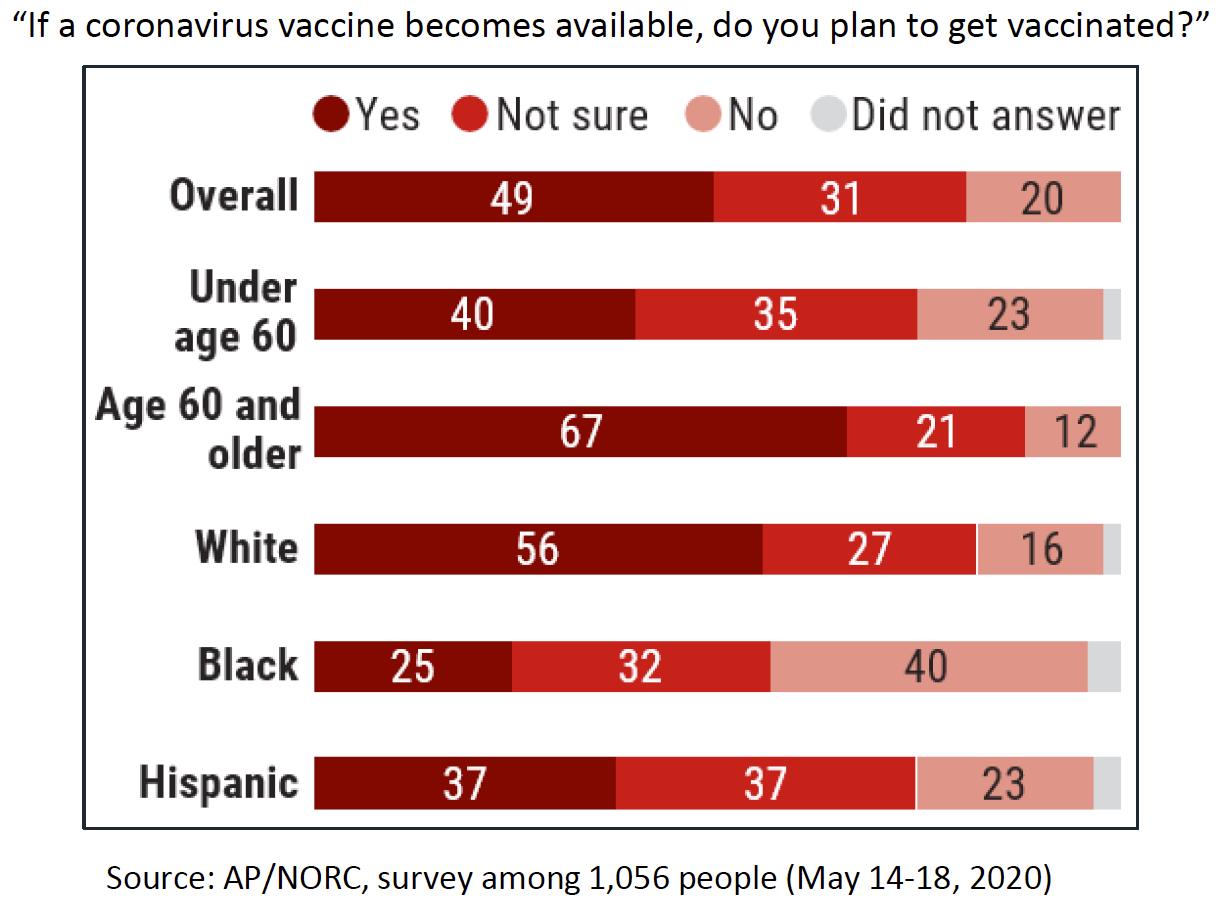
Similar survey findings were reported to NVAC by Chris Jackson of Ipsos Public Affairs based on an April/May 2020 survey conducted with Reuters. Women and Blacks in the United States were more skeptical about whether they will receive a COVID-19 vaccine, with only 28% of women and 25% of blacks very interested. In this survey, older adults were not confident about getting COVID-19 vaccine.
The speed with which COVID-19 vaccines are being developed was named by nearly half (48%) of the Ipsos survey respondents when asked why they were not interested in getting a COVID-19 vaccine. Other reasons were the risks associated with taking new vaccines (42%) and lack of trust in the those developing them (35%).
The survey showed that people will have more confidence in COVID-19 vaccines when results of large scientific studies are available (62%), the vaccine is approved by the U.S. Food and Drug Administration (57%), U.S. health authorities reassure Americans that the vaccine will prevent a repeat of the pandemic (57%), taking the vaccine means that life can return to the way it was before the pandemic (57%), and their personal physician recommends the vaccine (54%), Dowdy said.
Understanding the reasons people might refuse a COVID-19 vaccine provides a window into the challenges pharmacists and technicians face as vaccine advocates. By knowing why people feel as they do, more effective counter messaging can be developed. Because people are more likely to accept vaccinations that are recommended by health professionals they trust — and who have protected themselves by getting vaccinated — an important first step is to vaccinate the vaccinators. Fortuitously, that also makes sense from an epidemiologic standpoint.
PRIORITIZING COVID-19 VACCINE DISTRIBUTION AND ADMINISTRATION
People can get vaccinated only when vaccines are available. Even with the unprecedented federal effort to fund the manufacturing of hundreds of millions of doses of 5 COVID-19 vaccines before they even enter phase 3 trials, it will take time for these products to enter distribution channels, reach vaccinators, and be administered.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) held virtual meetings in June and July to begin planning for its recommendations regarding a rollout of one or more licensed COVID-19 vaccines and prioritizing access to the initial supply of products. More ACIP meetings on this topic are scheduled for August and September, and the group convenes again October for its regular meeting. The activities of this group complement a recent report from the Johns Hopkins Bloomberg School of Public Health and the Texas State University Department of Anthropology2 and dovetails with discussions at the National Academies of Sciences, Engineering, and Medicine concerning the equitable allocation of COVID-19 vaccine supplies.
While vaccine hesitancy may reduce the demand for vaccine, a number of concerns with the rollout are already evident. Even if only half of Americans want the vaccine on day 1, that’s 170 million people. Some vaccines require 1 dose but others 2 doses. They are administered differently. Products have different cold storage requirements. All of these factors will create confusion and could lead to errors during a massive rollout. Even the 1 versus 2 doses will be difficult to figure out if more than one vaccine reaches the market; people won’t know which one they already received, and without a linked network of state immunization information systems, providers will be hard pressed to help them or to follow up if they do not return for the second dose.
The ACIP workgroup is preparing to recommend tiered priority groups that can be used for the rollout of COVID-19 vaccine. Tiers will be based on the burden of disease and severity in risk groups, impacts on society and critical infrastructure, the characteristics of vaccines, and the number and timing of doses available, the CDC’s Sarah Mbaeyi, MD, MPH, told ACIP. Informing this planning are the experiences with the novel influenza A virus (H1N1), which emerged in April 2009 and led to a pandemic.
By the time an H1N1 vaccine became available during a second wave in October of that year, cases were being diagnosed at the rate of more than 2 million per week. Because of limited vaccine supply, immunization was limited initially to health care workers and those at highest risk. However, by early November 2009, cases began declining, and “once vaccine was widely available, the pandemic had started to wane in the United States and public demand for vaccination was low,” Mbaeyi said. “In late January [2010], vaccine coverage was 20% among the U.S. population.” Millions of available doses were never used.
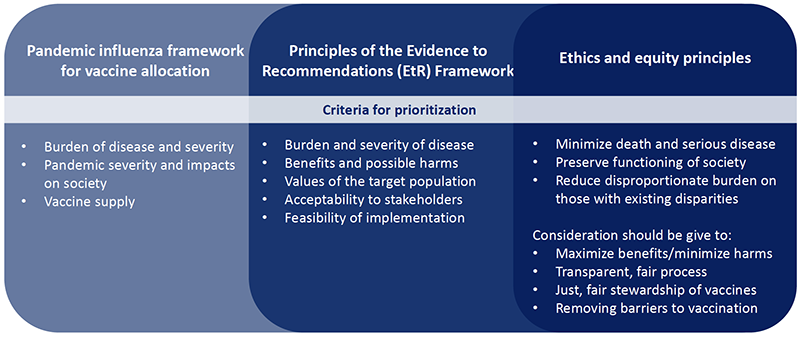
Using the pandemic influenza framework for vaccine allocation, the workgroup is preparing recommendations for ACIP using the evidence and ethical principles shown in Figure 2. “As part of this, the workgroup affirmed that consideration should be given to maximize benefits and minimize harms, utilize a transparent and fair process, assure the just and fair stewardship of vaccines, and remove barriers to vaccination,” Mbaeyi said. “The discussions guided by these frameworks led to a proposed general approach for prioritization, primarily to help with operational planning for vaccine implementation. The workgroup recognizes that this is an iterative process with priority groups to be refined as more information become available.”
CDC data through July 27, 2020, show that 113,730 health care workers (HCWs) were among 688,270 Americans with COVID-19 for whom data were available. A total of 576 deaths occurred among these HCWs, a mortality rate of 0.5%.
Nurses are the largest single group of HCWs affected by COVID-19, according to analysis of information gleaned from the CDC’s COVID-NET.3 From March 1 through July 11, 2020, 512 HCWs were among 9,195 hospitalized patients who had employment data, according to a July ACIP presentation by Sara Oliver, MD, MSPH, of the CDC. Disproportionate numbers of Black and Latinx HCWs were in the group, and 87% had underlying conditions, usually obesity but also hypertension and diabetes. Nurses were the largest single group of HCWs with COVID-19 (24%), followed by nursing assistants (14%); 9 HCWs worked in the pharmacy.
Staff at U.S. long-term care facilities have also been affected greatly by the extensive viral spread in that setting, Oliver said, with 69,438 identifiable cases in 36 U.S. states and territories. A total of 342 confirmed or probably COVID-19 deaths of HCWs working in this setting have been reported by 17 states and territories. Social and racial determinants of health are also important to keep in mind for HCWs, many of whom are in high-risk minority groups and live in lower-income neighborhoods. Oliver told ACIP that 82% of workers in long-term care are women, 26% are non-Hispanic Blacks, and 39% of workers are age 50 years or older.
Other essential workers are included in the first tier, including first responders, homeland and national security workers, and staff at correctional facilities and workers in food-processing plants or agriculture. Like HCWs in hospitals and nursing homes, staff in correctional and detention facilities carry the virus into and out of prisons and jails, and those have been super-spreader locations in several states. Workers in food-processing plants and agriculture have also been hard hit by COVID-19, partly because of conditions on the job but also due to race/ethnicity (predominance of Latinx workers) and crowded living conditions with other workers or intergenerational families.
ACIP’s initial ideas about priority tiers for COVID-19 are shown in Figure 3. In addition to protecting a vulnerable group that is needed during a pandemic, starting the campaign by targeting some 9 million HCWs and 16 million other essential workers has a number of logistic and practical advantages. These include implementation advantages in distribution, planning, microplanning at state/local level, and efficient postvaccination routine monitoring.
Allaying some concerns about ACIP voting to recommend vaccination of HCWs first — nearly all the voting members are in that group — results of focus groups reported at the July ACIP meeting indicate that the public strongly supports their vaccination in the first wave along with essential workers.
As planning continues, the priority groupings in Figure 3 will be refined. “Further tiering may be necessary based on vaccine supply and program planning needs,” Mbaeyi told ACIP members, including addressing the needs of racial and ethnic groups at high risk for severe COVID-19.
One unresolved concern is how to prioritize groups for whom few or no data will be available from clinical trials. Older adults were excluded from phase 1 and 2 trials, but they are being included in some phase 3 trials. Pregnant women and children are excluded from all trials. Mbaeyi specifically said in discussing current challenges that the ACIP COVID-19 workgroup should not preclude high-risk populations from inclusion in priority groups while data are pending.
Figure 2. Criteria for Prioritization for Vaccine Priority Tiers During Pandemics
Sources: Centers for Disease Control and Prevention. Influenza (flu). Interim Updated Planning Guidance on Allocating and Targeting Pandemic Influenza Vaccine During an Influenza Pandemic. June 2, 2020. Available at: https://www.cdc.gov/flu/pandemic-resources/national-strategy/planning-guidance/index.html. Accessed July 20, 2020.
Advisory Committee on Immunization Practices. GRADE (Grading of Recommendations, Assessment, Development and Evaluation). July 19, 2019. Available at: https://www.cdc.gov/vaccines/acip/recs/index.html. Accessed July 20, 2020.
Figure 3. Initial Projections for COVID-19 Vaccine Priority Tiers
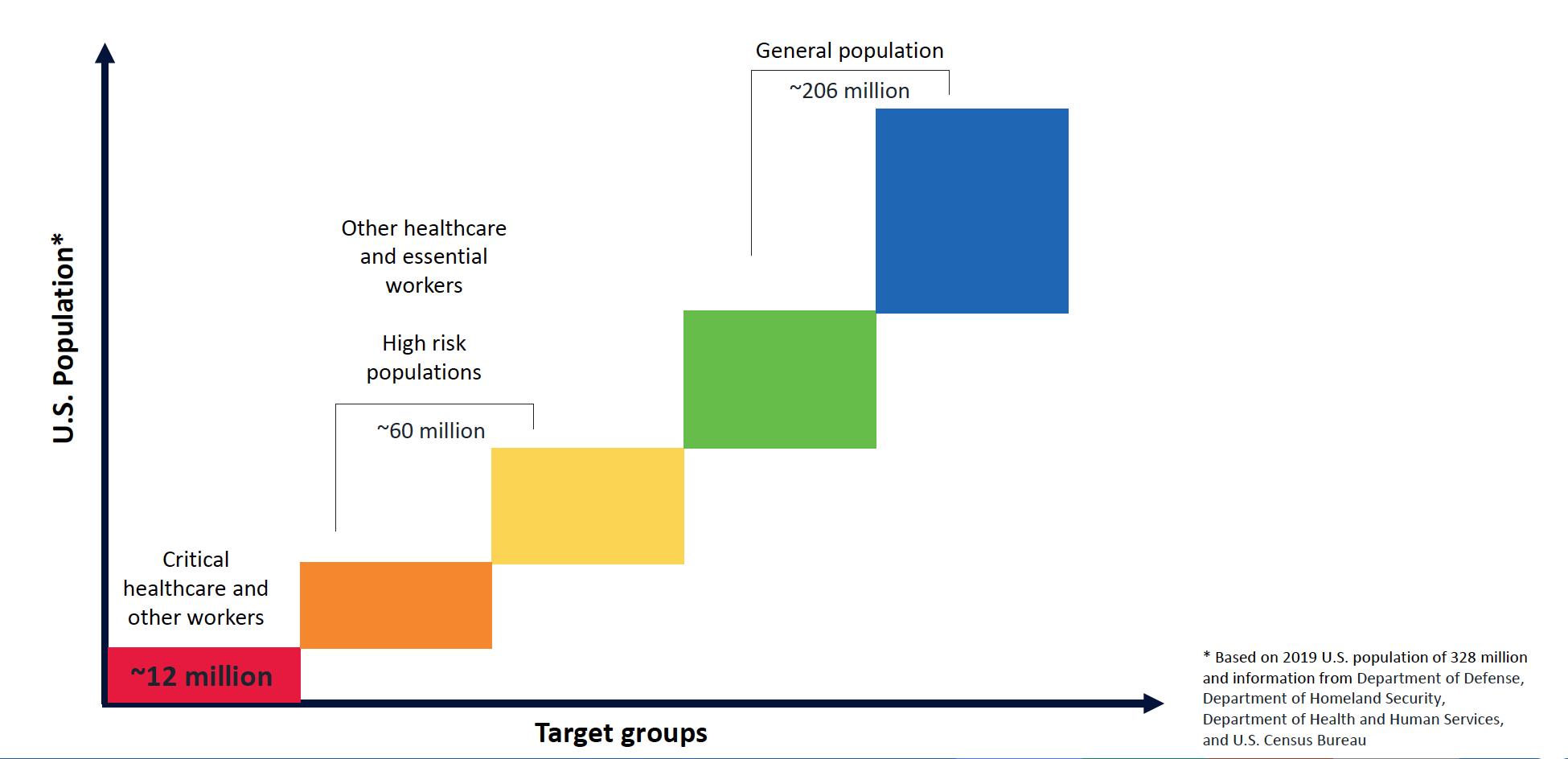
Source: Centers for Disease Control and Prevention Advisory Committee on Immunization Practices.
Perspective authors in a recently published New England Journal of Medicine began a discussion of whether states might mandate immunization for some or all target populations. They proposed 6 trigger criteria for state COVID-19 vaccination mandates4:
- The disease is not controlled in the state.
- ACIP has recommended vaccination for the groups being considered for mandates.
- Adequate vaccine quantities are available.
- Evidence for vaccine safety and efficacy has been communicated transparently.
- The state has created infrastructure for vaccine access without financial or logistic barriers, compensation for adverse effects, and real-time surveillance for adverse effects.
- Mandates are imposed only after a time-limited trial of voluntary vaccination has been unsuccessful.
“As with social distancing orders, we can expect that the advent of SARS-CoV-2 vaccines will spark intense clashes of feeling about what people owe to one another in the fight against the pandemic,” the authors concluded. “In contrast to earlier phases of the pandemic, though, we currently have some time on our side. Careful deliberation now about state vaccination policy can help ensure that we have a strategy when the breakthrough comes.”
REFERENCES
- Havers FP, Reed C, Lim T. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med. 2020 July 21. doi: 10.1001/jamainternmed.2020.4130. [Epub ahead of print]
- Schoch-Spana M, Brunson E, Long R, Ravi S, Ruth A, Trotochaud M on behalf of the Working Group on Readying Populations for COVID-19 Vaccine. The Public’s Role in COVID-19 Vaccination: Planning Recommendations Informed by Design Thinking and the Social, Behavioral, and Communication Sciences. Baltimore, MD: Johns Hopkins Center for Health Security; July 2020.
- Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 states, March 1–30, 2020. Morb Mortal Wkly Rep. 2020;69(15):458–464.
- Mello MM, Silverman RD, Omer SB. Ensuring uptake of vaccines against SARS-CoV-2. N Engl J Med. 2020 June 26. doi: 10.1056/NEJMp2020926. [Epub ahead of print]
Back to Top