ADVERTISEMENT

COVID-19 Monthly Update: Dealing With Potential Long-Term Effects of COVID-19 Infection
INTRODUCTION
At the time this program was prepared, the number of documented cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was continuing a steady march toward 200 million worldwide, with hundreds of thousands new cases being diagnosed in India each day. Within the United States, more than 30 million cases and nearly 600,000 deaths have been documented since the beginning of the pandemic in early 2020. There is definitely good news in that 3 vaccines have been authorized by the FDA and more than 200 million doses administered, including 50% of the adult population and more than 80% of those age 65 or older having received at least 1 dose of vaccine.
While the vast majority of patients recover successfully from coronavirus disease 2019 (COVID-19), there is much to learn regarding the potential long-term sequalae of the disease. With the pandemic now more than 1 year in duration, research is demonstrating significant effects of COVID-19 illness for weeks and months after the acute episode in a number of patients. While there is no consensus definition to describe these findings, terms such as “long COVID,” “post-COVID syndrome,” and “post-acute COVID-19 syndrome” have been used. Among the lay public, the term “long haulers” is commonly used. These effects result in a number of different syndromes affecting many different organs and tissues (Figure 1).
The purpose of this program is to highlight the primary issues identified with COVID-19 that continue after convalescence from the initial infection.
Figure 1. Organ Systems Affected by Post-COVID Syndrome
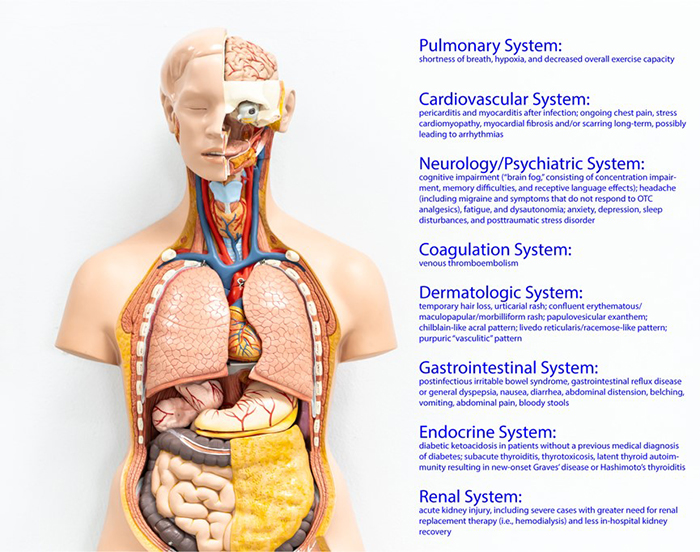
POTENTIAL PATHOPHYSIOLOGY FOR LONG-TERM COVID-19 EFFECTS
COVID-19 disease is mediated by SARS-CoV-2, a single-stranded RNA coronavirus. One of the distinguishing characteristics of SARS-CoV-2 and other coronaviruses are surface spike glycoproteins that bind to the surface of host respiratory epithelial cells to obtain entry into cells. These spike proteins are what the successful mRNA vaccines use to form a spike protein–type analogue that the human immune system recognizes and generates protective antibodies; these decrease SARS-CoV-2 infections and most likely transmission.
Angiotensin-converting enzyme 2 (ACE2) is a primary component of the renin–angiotensin–aldosterone system, which is expressed in nearly every tissue of the human body, including lung, kidney, and intestinal cells, and coronary myocytes. ACE2 converts angiotensin II into angiotensin 1–7, which opposes the deleterious effects of angiotensin II, such as vasoconstriction, sodium and water retention, increased sympathetic tone, and decreased nitric oxide availability.
SARS-CoV-2 binds to membrane-attached ACE2 on the surface of epithelial cells in the respiratory tract, thereby gaining entry and producing to further pathophysiologic changes. Host proteases — including transmembrane protease serine protease 2 (TMPRSS2), cathepsin L, and furin — are key to process the S protein within SARS-CoV-2 to drive endocytosis within respiratory cells. The SARS-CoV-2–ACE2 complex is endocytosed, leading to a drop in ACE2 expression and overall activity in infected cells. Other potential factors leading to decreased ACE2 expression include viral-mediated ACE2 cell shedding as well as ACE2 downregulation. These activities at the cellular level are believed to increase angiotensin II-mediated inflammation, which may increase acute lung injury and fibrosis associated with severe COVID-19 illness.1
In patients with acute respiratory distress syndrome, a correlation between inflammatory markers and ACE/ACE2 activity was found within bronchoalveolar lavage fluid. An early concern during the COVID-19 pandemic was whether patients receiving ACE-inhibitor or angiotensin receptor blocker therapy would be at increased risk of acquiring SARS-CoV-2 infection due to upregulation of ACE2 receptors.2 Others hypothesized that ACE2-associated modulation due to these agents could suppress SARS-CoV-2 replication. Investigations to determine the ultimate role of these agents within the management of SARS-CoV-2 infection are ongoing.3
Several explanations have been developed for long-term symptoms after COVID-19 infection. One study revealed presence of SARS-CoV-2 nucleic acids from gastrointestinal biopsies 4 months after illness in 7 of 14 cases, demonstrating the possibility of continued live virus.4 Second, due to pathophysiologic changes from the SARS-CoV-2–ACE2 complex, fibrosis could occur in a number of different tissues/organs and produce disease long after the virus is dead. A study of more than 200 patients with long-term COVID-19 symptoms showed multiorgan involvement primarily in heart and lungs around 4 months after infection.5Autoantibody production in some studies is higher post-COVID compared with uninfected people, which may also provide rationale for long-term symptoms or a waxing/waning type symptom presentation.
PATIENTS AT RISK
While specific risk factors are being further studied, long COVID clearly can develop with any clinical presentation of COVID-19, including in those patients who have a mild-to-moderate presentation or are asymptomatic upon isolation of SARS-CoV-2. These findings may lead to underreporting of long-term symptoms or correlation to SARS-CoV-2, especially in asymptomatic individuals who do not seek testing. Increased severity of illness, such as requirement for intensive care unit admission with subsequent mechanical ventilation, has been significantly associated with persistent symptoms after infection as well as decreased overall quality of life.6
ORGAN SYSTEMS AFFECTED BY LONG COVID
As described earlier, SARS-CoV-2 can infect several tissues and produce organ dysfunction through the ACE2 receptor. Because ACE2 receptors are ubiquitous throughout the body, nearly every major organ system can be affected in some manner during variable timeframes after COVID-19 infection. These can range from very mild inconveniences, such as anosmia (loss of sense of smell), to severe findings such as myocardial fibrosis or thromboembolism.
Summarized below are the most common findings within various organ systems and in patients experiencing thrombosis after COVID-19 infection.
Pulmonary System
Changes in the pulmonary system are commonly found in patients with long COVID, a plausible finding given the pathophysiology of SARS-CoV-2 and the predominance of presentation of primary pulmonary infections. A number of changes on various imaging studies have been demonstrated, including ground-glass opacities as well as fibrosis that may explain some of the following symptoms.
Persistent signs and symptoms include shortness of breath, hypoxia, and decreased overall exercise capacity. Shortness of breath is the most common finding, occurring in up to two-thirds of patients 2–3 months post-infection at follow-up.6 These findings are similar to patients who survive acute respiratory distress syndrome from a number of various types of infections.
In a Chinese study of post-COVID-19 patients, nearly 25% of patients experienced decreased 6-minute walking distances compared with noninfected patients at 6 months. Approximately 1 in 15 patients in a post-acute COVID-19 study in the United States required new supplemental oxygen due to hypoxia or sleeping breathing support, such as a continuous positive airway pressure machine, at 60-day follow-up from acute episode. A decrease in diffusion capacity is one of the most common impairments resulting in these findings, especially in patients with severe acute COVID-19 illness.
In one study from Spain, only approximately 50% of patients who received tracheostomy were successfully weaned from the ventilator 1 month later.8
Patients at highest risk for developing long-term pulmonary sequelae seem to be those with the greatest pulmonary severity during the acute COVID-19 illness, manifested by requirement of mechanical ventilation or even high-flow nasal cannula. Data are lacking at this point regarding monitoring and management, with some suggestions of serial pulmonary function tests, 6-minute walk tests, and home pulse oximetry being useful in patients with persistent symptoms and computed tomography of the chest at 6 and 12 months. A more long-term, structured approach will likely evolve over time, one involving coordination of care of pulmonary findings as well as effects on other organ systems.
Cardiovascular System
One of the earliest findings during the COVID-19 pandemic was in athletes experiencing cases of pericarditis and myocarditis after infection; this complication has been associated with numerous other viruses. Fortunately, with continued research, these cases tend to be rare, but a number of other significant cardiac manifestations are being researched. The underlying finding from magnetic resonance imaging suggests persistent myocardial inflammation, which may be responsible for some of the chronic findings.
Two of the more common findings include ongoing chest pain, reported in a studies to be anywhere from 5% to 20% at 60–180 days after infection.6 A 4- to 5-fold increased risk of stress cardiomyopathy has been demonstrated in some patients, but morbidity and mortality to this point have been similar to non-COVID-19 patients.9
Myocardial fibrosis and/or scarring long-term could potentially lead to arrhythmias, similar to patients with cardiomyopathy. Patients with cardiac complications during acute COVID-19 illness or those with persistent cardiac symptoms should be considered for follow-up echocardiogram and/or electrocardiogram 1–3 months after infection on a case-by-case basis. Competitive athletes with documented cardiac manifestations of COVID-19 should abstain from competitive sports including normal aerobic exercise for 3–6 months until resolution of myocardial inflammation via imaging or normalization of troponin concentrations.6 Patients receiving any renin-angiotensin-aldosterone inhibitor for a chronic disease state, such as an ACE-inhibitor for heart failure, should continue to receive these agents as previously administered before COVID-19 illness.
Neurologic/Psychiatric System
A number of significant findings have been reported by patients after COVID-19 that persist for variable amounts of time. One of the more common complaints by patients has been cognitive impairment known as “brain fog.” This can manifest in a variety of ways, including concentration impairment, memory difficulties, and receptive language effects. Other complaints include headache, fatigue, and dysautonomia (autonomic dysfunction) that wax and wane or can be daily in some circumstances. Headaches, including migraines, are often refractory to traditional over-the-counter analgesics. Some late-onset headaches have been correlated to high cytokine levels.
As with other coronavirus infections, a number of patients experiencing COVID-19 have anxiety, depression, sleep disturbances, and posttraumatic stress disorder (PTSD). A number of pathologic factors are responsible for these symptoms, including immune system dysfunction and small vessel thrombosis.6
A recently published study in Lancet Psychology has shed light on some of the long-term effects of COVID-19 in adults. Using an electronic health records network of more than 81 million patients, the incidence of 14 neuropsychiatric outcomes was compared between patients diagnosed with COVID-19 versus 2 other cohorts: those with influenza diagnosis and those with any respiratory tract illness including influenza. Of the 236,379 patients diagnosed with COVID-19, the incidence at 6 months of a neuropsychiatric diagnosis was 33.6% with nearly 40% of these being new diagnoses. Compared to the other cohorts, patients post-COVID-19 had statistically significant increases in nearly every neuropsychiatric diagnosis, including intracranial hemorrhage, ischemic stroke, mood and anxiety disorders, psychotic disorders, insomnia, and even substance use disorders.10
An Italian cohort study of approximately 400 COVID-19 survivors found that more than half screened positive in at least 1 domain for a psychiatric diagnosis, including PTSD, depression, and obsessive-compulsive behavior.11
Standard screening tools should be used to detect new diagnoses of the neuropsychiatric illnesses noted above. Long-term management of neurologic manifestations should be completed as usual, with neurologic evaluation and further imaging appropriate in patients who do not respond to traditional therapies.
Coagulation System
The risk of venous thromboembolism (VTE) is variable but important in patients with confirmed SARS-CoV-2, with higher incidence among those patients hospitalized for COVID-19. Of particular interest, up to 80% of these events occur in the post-hospital discharge period, typically in the 30–45 days after infection, possibly because of an extended inflammatory state. The challenge at this time is figuring out what to do to mitigate this risk; few data are available to guide use of medical VTE prophylaxis with low-molecular-weight heparin or direct oral anticoagulants at the time of discharge.
Aspirin, which has been investigated in low-risk surgical patients postdischarge, is being evaluated after COVID-19 illness. The current National Institutes of Health guidelines currently state that patients with low bleeding risk and high VTE risk may be considered for prophylaxis.12 A published tool is available for assessing VTE risk known as the Modified International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) score. Higher-risk patients have an IMPROVE score of ≥4 or ≥2 with a D-dimer level of >2 times the upper limit of normal. Components of the IMPROVE score include previous VTE, known thrombophilia, current lower-limb paralysis, current cancer, immobilized more than 7 days, critical-care unit stays, and age of more than 60 years.
Larger ongoing studies will help determine overall risk and incidence of VTE post-COVID-19 to determine optimal populations of patients to provide medical prophylaxis. Patients should be counseled and encouraged to resume or increase activities of daily living and other physical activity as able to decrease the risk of VTE.
Dermatologic System
Dermatologic findings can occur in a high number of patients after COVID-19. A recent international study of approximately 700 patients demonstrated dermatologic findings in nearly 70% of patients approximately 1 week after initial respiratory symptoms.
The most common finding in several studies has been hair loss, noted in about 1 in 5 patients. This may be a result of telogen effluvium, a finding resulting in temporary hair loss due to excessive shedding of resting or telogen hair after viral infection or stress response. The nature of a high number of COVID-19 dermatological manifestations has led 1 group to propose a classification based on 6 main clinical patterns: urticarial rash; confluent erythematous/maculopapular/morbilliform rash; papulovesicular exanthem; chilblain-like acral pattern; livedo reticularis/racemose-like pattern; purpuric “vasculitic” pattern.14
Fortunately, 1 study has shown a high resolution rate of rash at the 6-month timeframe post-COVID-19.7 Pharmacists should be keen on these findings, as patients with dermatologic complaints commonly present for consultation.
Gastrointestinal System
Gastrointestinal flora may be altered during a number of bacterial and viral respiratory tract infections, resulting in longer term effects such as postinfectious irritable bowel syndrome or gastrointestinal reflux disease or general dyspepsia. Enteric symptoms are prevalent within COVID-19 illness, manifested through a variable number of clinical presentations and can be present alone without respiratory symptoms or before respiratory symptoms.13 The mechanisms of SARS-CoV-2 specifically are still being elucidated; decreases in normal commensal flora as well as amplification of opportunistic infective organisms have been postulated.15
While some data suggest a lower incidence of gastrointestinal symptoms after COVID-19, a recent analysis published in Lancet Gastroenterology and Hepatology suggests these symptoms may be more common than initially thought 90 days after discharge. This report included 117 patients discharged from Chinese hospitals after COVID-19, many of whom had severe illness with the median length of stay of 19 days in 12 hospitals. Gastrointestinal symptoms were documented for 13% of patients on admission and 42% during their hospital stay.16
In that study, gastrointestinal long-term symptoms were only counted if they were not present in the month before the COVID-19 illness. Nearly half of all patients reported gastrointestinal symptoms at a 90-day telephone interview, with loss of appetite (24% of patients) the most common finding. Nausea (18%), gastrointestinal reflux (18%), and diarrhea (15%) were also reported, as were abdominal distension (14%), belching (10%), vomiting (9%), abdominal pain (7%), and bloody stools (2%).16
None of these patients had long-term gastrointestinal symptoms unless they had experienced them before admission for COVID-19 illness or during their hospitalization. It is important to note that a number of these patients likely had their stress ulcer prophylaxis discontinued after hospitalization, which may have induced some “rebound” gastrointestinal symptoms. In addition, it is important to note that this study was completed solely in China, so data will be important to collect and assess to further answer long-term gastrointestinal questions in the United States population.16
Endocrine System
It has been long postulated that type I diabetes may be caused by a number of various viral infection etiologies.17 Potential long-term endocrine effects of COVID-19 illness include direct viral damage to beta cells and/or significant inflammation, but to date, no evidence suggests long-term beta-cell damage due to SARS-CoV-2.
Diabetic ketoacidosis has been observed in patients without a previous medical diagnosis of diabetes as long as months after resolution of initial COVID-19 infection.6 Since many people with type 2 diabetes are undiagnosed, COVID-19 illness could simply occur in such patients. COVID-19–related diabetes should be treated similarly to non-COVID-19–related diabetes, assuming the causality is ultimately proven.6An international registry has been established in an effort to ascertain risks of COVID-19 with long-term diabetes risk (CoviDiab registry).
Thyroid manifestations have been sporadically reported in patients who have had COVID-19, with subacute thyroiditis and thyrotoxicosis presenting several weeks after initial resolution of respiratory symptoms. In addition, latent thyroid autoimmunity may be increased by COVID-19 resulting in new-onset Graves’ disease or Hashimoto’s thyroiditis.18,19
Renal System
Renal injury is common in hospitalized patients with COVID-19; approximately 24% to 57% of hospitalized patients develop acute kidney injury (AKI). This incidence increases further in patients admitted to intensive care units, with approximately 61% to 78% of critically ill patients experiencing AKI. These patients have an increased risk of experiencing severe AKI, greater need for renal replacement therapy (i.e., hemodialysis), and less in-hospital kidney recovery.20
Autopsies and kidney biopsies have found significant acute tubular necrosis upon examination. Detection of SARS-CoV-2 is seen in some patients as well when evaluated. Chemokine and interferon synergy may result in focal segmental glomerulosclerosis in addition to acute tubular necrosis. Data on long-term outcomes related to COVID-19 illness have been limited. While dialysis-dependent renal function is not common at discharge, longitudinal evaluation of overall renal function remains important for determining overall risk.
Nugent et al. evaluated more than 1,600 patients with AKI in 5 hospitals in Connecticut and Rhode Island who were monitored after hospitalization for COVID-19. In this evaluation, patients with COVID-19 and AKI who did not require hemodialysis within 3 days of discharge and had at least 1 outpatient serum creatinine value after discharge were included. Compared with AKI patients without COVID-19, those with COVID-19 were more likely to have decreases in overall estimated glomerular filtration rates, even after adjusting for baseline comorbidities. These findings persisted when controlling for peak serum creatinine and in-hospital dialysis requirement.20 Further study is needed to determine overall long-term trajectory of renal function in patients discharged from the hospital after COVID-19 illness.
Stockmann et al. also investigated long-term renal recovery in survivors of COVID-19 illness with AKI who required renal replacement therapy. All patients were treated in intensive care units within a large tertiary care center in Germany over a 4-month period. Data demonstrated that a median period of 5 months after initiation of renal replacement therapy, the median duration of renal replacement therapy was approximately 1 month after discharge. Approximately 92% of patients had achieved varying intervals of renal recovery, with 62.2% achieving full recovery. These data need to be duplicated in larger, longer studies, but the results give hope that even in patients requiring renal replacement therapy for COVID-19–associated illness, many can achieve complete remission of kidney injury.21
Pharmacists managing patients’ medication therapy should review all patients after COVID-19 to ensure appropriate doses and frequencies of medications that require renal adjustment. In addition, if renal recovery occurs, changing to normal kidney function doses for renally cleared medications would be appropriate to ensure effectiveness.
PEDIATRIC LONG-TERM COVID?
Data regarding the incidence of potential long-term COVID in pediatric patients is minimal and challenging for a number of reasons. First, children tend to have milder presentations and even more asymptomatic cases than adults. This makes causation difficult as many of these patients are never tested for SARS-CoV-2.
Children in rare circumstances can present with multisystem inflammatory syndrome (MIS-C), a condition in which systemic inflammation occurs in a number of organs. Many of the symptoms are vague: fever, abdominal pain, neck pain, rash, bloodshot eyes, and significant fatigue. Fortunately, the vast majority of children recover completely, but long-term effects are still unknown.
A recent study out of Australia has evaluated 171 pediatric patients (≤18 years of age) at a follow-up clinic. Children could be enrolled in the study if they had tested positive for SARS-CoV-2. A standardized form was used to collect data on symptoms, transmission, medical history, and post-acute COVID-19 symptoms. Data were obtained from the medical record, and acute disease severity was determined by World Health Organization criteria.22
Overall, 36% of patients were asymptomatic, 58% had mild disease, and 5% had moderate disease. Most hospitalizations consisted of brief observation or volume rehydration. Follow-up data at 90–180 days were available for approximately 90% of children with acute COVID-19 presentations (36% asymptomatic, 64% symptomatic). Of note, post-COVID-19 cough lasted from 3 weeks to 8 weeks, and post-COVID-19 fatigue endured for 6–8 weeks from start of symptomology. All 151 patients available for follow-up had returned to baseline health with post-acute COVID-19 symptoms resolving.22
Longer-term evaluations of pediatric patients are needed, especially with COVID-19 vaccination and full-time in-person schooling now beginning. In addition, comprehensive outcome studies on the long-term effects of COVID-19 on mental health are significantly needed, including assessments of isolation and lockdown effects on overall health and well-being.
NATIONAL INSTITUTES OF HEALTH INITIATIVE TO STUDY LONG COVID
With the success of the mRNA vaccines by Pfizer/BioNTech and Moderna respectively, a shift of federal research and resource dollars is being directed toward a number of unanswered questions regarding long COVID. In December 2020, Congress has provided $1.15 billion in funding to the National Institutes of Health (NIH) for research into the long-term effects of SARS-CoV-2 on health, known collectively as the Post-Acute Sequelae of SARS-CoV-2 infection (PASC). A number of underlying questions will be investigated with this initiative, including the following:
- What does the spectrum of recovery from SARS-CoV-2 infection look like across the population?
- How many people continue to have symptoms of COVID-19, or even develop new symptoms, after acute SARS-CoV-2 infection?
- What is the underlying biological cause of these prolonged symptoms?
- What makes some people vulnerable to these sequelae but not others?
- Does SARS-CoV-2 infection trigger changes in the body that increase the risk of other conditions, such as chronic heart or brain disorders?
Current research along with new projects will be organized within a SARS-CoV-2 Recovery Cohort. The goal of these studies is to study a diverse population of patients, measuring symptoms over a long period of time. A multidisciplinary team of scientists and investigators will work together to coordinate across these various studies. Two primary complementary studies will be supported involving biological specimens to evaluate organ injury as well as large data evaluations to get a sense of the breadth and depth of the long-term symptoms as well as predictive factors for recovery.
OTHER COVID-19 NEWS
A complete review of progress with COVID-19 vaccines is the topic of the next Power-Pak monthly update, but pharmacists are no doubt aware of recent developments concerning prevention and treatment of SARS-CoV-2 infections. Certainly, the biggest recent surprise was CDC’s announcement that many fully COVID-19–vaccinated people no longer need to wear masks or physically distance from other individuals, whether vaccinated or not. Details on CDC’s new advice are in an interim guidance on the agency’s website. International travel continues to involve testing before returning to the United States, and immunocompromised people should consult their health care providers before dropping their guard on avoiding the virus. Otherwise, though, fully vaccinated people can:
- Resume activities without wearing masks or physically distancing, except where required by federal, state, local, tribal, or territorial laws, rules and regulations, including local business and workplace guidance
- Resume domestic travel and refrain from testing before or after travel or self-quarantine after travel
- Refrain from testing before leaving the United States for international travel (unless required by the destination) and refrain from self-quarantine after arriving back in the United States
- Refrain from testing following a known exposure, if asymptomatic, with some exceptions for specific settings
- Refrain from quarantine following a known exposure if asymptomatic
- Refrain from routine screening testing if feasible
With FDA’s expansion of the emergency use authorization for the Pfizer-BioNTech COVID-19 vaccine to include adolescents 12 through 15 years of age (2 doses, 3 weeks apart), the number of doses administered daily should be going back up. Daily doses administered had dropped from 3 million to closer to 2 million since mid-April as most adults who intend on getting vaccinated had received their shots. While issuing its endorsement of use of this vaccine, the Advisory Committee on Immunization Practices (ACIP)23 noted a decrease in vaccines administered to adolescents during the pandemic and encouraged vaccinators to talk with those presenting for COVID-19 doses to catch up on their other immunizations. While there is concern about the effects on reactogenicity when other immunizations are administered at the same time as COVID-19 products, the ACIP and the American Academy of Pediatrics are recommending coadministration of routine childhood and adolescent immunizations with COVID-19 vaccines (or vaccination in the days before or after) for children and adolescents who are behind on or due for immunizations.25
Use of the Janssen/Johnson & Johnson COVID-19 vaccine was restarted after a prolonged pause with a new warning about thrombosis with thrombocytopenia syndrome (TTS) in recipients, and ACIP emphasized the need for education about this adverse events.24 Clinicians should investigate serious thrombotic events in recent vaccine recipients with headache or other suspicious symptoms, evaluate vaccinated patients with a thrombotic event and thrombocytopenia using a platelet factor-4 ELISA assay as in heparin-induced thrombocytopenia (HIT), and not use heparin unless the HIT test is negative. Problems were also continuing with supply of this product following quality control problems at a Baltimore plant.
On the treatment front, FDA has revoked the emergency use authorization for bamlanivimab monotherapy for the treatment of mild-to-moderate COVID-19 in adults and certain pediatric patients. Alternative monoclonal antibody therapies remain available under emergency use authorizations, including REGEN-COV (casirivimab and imdevimab, administered together), and bamlanivimab and etesevimab, administered together, for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients 12 years of age and older weighing at least 40 kg with positive direct SARS-CoV-2 viral testing, and who are at high risk for progressing to severe COVID-19 and/or hospitalization.
ROLES OF PHARMACISTS
It is important to be aware of the various signs and symptoms detailed in this article as patients will often have many questions regarding potential long-term symptoms, especially as more frequent lay press articles are published on “long COVID.” Avoiding acute and long-term effects of COVID-19 can be an important message as pharmacists encourage people to get their COVID-19 vaccination and provide education to vaccine-hesitant individuals.
In patients with long-term symptoms, some of these can be treated with over-the-counter medications, such as ibuprofen or acetaminophen for headaches. Pharmacists should emphasize with patients that if relief is not obtained with short-term use of these therapies, an appointment with a healthcare provider should be scheduled to obtain a more thorough evaluation of any organ systems that may be affected. The science and understanding of these sequelae will continue to improve with each passing month so that pharmacists can help patients better understand the manifestations of COVID-19 as well as potential options for management.
REFERENCES
- South AM, Brady TM, Flynn JT. ACE2 (angiotensin-converting enzyme 2), COVID-19, and ACE inhibitor and ang II (angiotensin II) receptor blocker use during the pandemic. Hypertension. 2020;76:16-22.
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19). A review. JAMA. 2020;324:782-793.
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/
- Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639-644.
- Dennis A, Wamil M, Alberts J, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391.
- Nalbandian A, Sehgal K, Gupta A. et al. Post-acute COVID-19 syndrome. Nature Med. 2021;27:601-615.
- Huang C, Huang L, Wang Y. et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. 2021;397:220-232.
- Martin-Villares C, Perez Molina-Ramirez C, Bartolome-Benito M., et al. Outcome of 1890 tracheostomies for critical COVID-19 patients: a national cohort study in Spain. Eur Arch Oto Rhino Laryngol.https://doi.org/10.1007/s00405-020-06220-3.
- Jabri A, Kaira A, Kumar A, et al. Incidence of stress cardiomyopathy during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3:e2014780.
- Taquet M, Geddes JR, Husain M, et al. 6-month neurological and psychiatric outcomes in 236,379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psych. 2021(Apr 6); doi: 1016/S2215-0366(21)00084-5
- Mazza MG, De Lorenzo R, Conte C, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594-600.
- National Institutes of Health. COVID-19 treatment guidelines: antithrombotic therapy in patients with COVID-19. Last updated February 11, 2021. https://www.covid19treatmentguidelines.nih.gov/antithrombotic-therapy/
- Mao R, Qiu Y, He JS, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667-678.
- Donati Zeppa S, Agostini D, Piccoli G et al. Gut microbiota status in COVID-19: an unrecognized player? Front Cell Infect Microbiol. 2020;10:576551.
- Genovese G, Moltrasio C, Berti E, Marzano AV. Skin manifestations associated with COVID-19: current knowledge and future perspectives. 2021;237:1–12.
- Weng J, Li Y, Li J, et al. Gastrointestinal sequelae 90 days after discharge for COVID-19. Lancet Gastroenterol Hepatol. 2021;6:344-346.
- Coppieters KT, Boettler T, von Herrath M. Virus infections in type 1 diabetes. Cold Spring Harb Perspect Med. 2012(Jan);2(1):a007682.
- Tee LY, Hajanto S, Rosario BH. COVID-19 complicated by Hashimoto’s thyroiditis. Singapore Med J. 2020 Jul 16. doi: 11622/smedj.2020106
- Mateu-Salat M, Urgell E, Chico A. SARS-CoV-2 as a trigger for autoimmune disease: report of two cases of Graves’ disease after COVID-19. J Endocrinol Invest. 2020;43:1527-1528.
- Nugent J, Aklilu A, Yamamoto Y, et al. Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Netw Open. 2021;4:e2111095.
- Stockmann H, Hardenberg JHB, Aigner A. High rates of long-term renal recovery in survivors of coronavirus disease 2019-associated acute kidney injury requiring kidney replacement therapy. Kidney Int. 2021;99:1021-1022.
- Say D, Crawford N, McNab S, Wurzel D, Steer A, Tosif S. Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health. 2021 Apr 20; doi: 1016/S2352-4642(21)00124-3
- Wallace M, Woodworth KR, Gargano JW, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 Vaccine in adolescents aged 12–15 years — United States, May 2021. Morb Mortal Wkly Rep. 2021(May 14);70:15585/mmwr.mm7020e.1
- MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients — United States, April 2021. Morb Mortal Wkly Rep. 2021;70:651-656. doi: 15585/mmwr.mm7017e4
- Committee on Infectious Diseases. COVID-19 vaccines in children and adolescents. 2021 May 11; doi: 10.1542/peds.2021-052336
Back to Top