
Expired activity
Please go to the PowerPak
homepage and select a course.
Module 11: Diabetes Control in the Hospital Setting: What Pharmacists Need to Know
Introduction
Over the past 15 years, blood glucose (BG) management in the inpatient setting has advanced considerably.1-8 Hyperglycemia is common in the hospital environment, with up to 40% of patients experiencing hyperglycemia and up to one-third of these patients having no previous diagnosis of diabetes.
Many observational trials have suggested worse outcomes associated with both hyperglycemia and hypoglycemia in many hospitalized patient populations. 1,8-22 Hyperglycemia has been linked to poor outcomes in several patient populations that include noncardiac surgery patients and those with aneurysmal subarachnoid hemorrhage, chronic obstructive pulmonary disease, community-acquired pneumonia, critical illness, acute myocardial infarction (MI), or requiring total parenteral nutrition (TPN). Hypoglycemia, both moderate (i.e., BG of less than 70 mg/dL) and severe (i.e., BG of less than 40 mg/dL), are linked to poor outcomes.16,17,23 Efforts to manage BG in the inpatient setting should focus on minimizing hyper- and hypoglycemia.
Causes of Hyperglycemia
There are several proposed causes of hyperglycemia in hospitalized patients, including endogenous stress-induced hyperglycemia, exogenous dextrose-containing fluids, drug-induced hyperglycemia, and inadequate management of preexisting diabetes.17,24-27 Stress-induced hyperglycemia is thought to cause peripheral insulin resistance secondary to hormones and other inflammatory markers released during acute illness. Endogenous norepinephrine, epinephrine (adrenaline), cortisol, and growth hormones are associated with stress-induced hyperglycemia. Many patients received exogenous intravenous (IV) dextrose, either from maintenance fluid or in IV admixtures of medications, in the hospital that, in turn, led to hyperglycemia. Several commonly used medications can cause hyperglycemia, most notably glucocorticoids (prednisone, methylprednisolone, dexamethasone), catecholamines, and tacrolimus (Prograf).
Hyperglycemia is common in patients with preexisting diabetes, regardless of whether they have been previously diagnosed or not. All patients with hyperglycemia in the hospital setting are recommended to have a glycosylated hemoglobin (HbA1C) laboratory test—unless there is a result within the past month—for a marker of BG over the past few months. For patients with diabetes, this test is useful as a marker of outpatient diabetes management and as an assist with discharge planning. For individuals with hospital-related hyperglycemia and without a current diagnosis of diabetes, the A1C would help determine whether the hyperglycemia is unmasking undiagnosed diabetes or if the patient has isolated hyperglycemia of critical illness. A high A1C in a previously undiagnosed person is an opportunity for follow-up and a complete work-up in the ambulatory setting postdischarge.
Inpatient Glucose Goals
Several groups of experts have given recommendations for glucose targets to noncritically ill hospitalized patients, as well as critically ill hospitalized patients (Tables 1 and 2).28-33 Ideally, each health system and institution should create, implement, monitor, and adjust management strategies tailored to the needs of the patients for which they care. (This material is discussed further in the “Team Approach” section below).
| Table 1. Glucose goals in noncritically ill hospitalized patients |
| Year |
Organization |
Patient Population |
BG Treatment Threshold (mg/dL) |
BG Target
(mg/dL) |
BG Hypoglycemia Definition
(mg/dL) |
| 2009 |
AACE and ADA Consensus Statement |
Noncritically ill patients |
180 mg/dL |
Premeal <140 mg/dL |
<70 mg/dL
(Reassess treatment if <100 mg/dL) |
| 2012 |
Endocrine Society Clinical Practice Guideline |
Noncritically ill patients |
180 mg/dL |
Premeal <140 mg/dL |
(Reassess treatment if <100 mg/dL) |
| Table 2. Glucose goals in critically ill hospitalized patients |
| Year |
Organization |
Patient Population |
BG Treatment Threshold (mg/dL) |
BG Target
(mg/dL) |
BG Hypoglycemia Definition
(mg/dL) |
| 200846 |
American Heart Association |
ICU(Intensive Care Unit) patients with ACS (Acute Coronary Syndrome) |
180 |
90 – 140 |
Not stated |
| 200928 |
AACE and ADA |
ICU patients |
180 |
140 – 180 |
<70 |
| 201229 |
American College of Critical Care Medicine (ACCM) |
ICU |
150 |
<150 (Trauma)
<180
(Stroke+) |
<70 |
| 201331 |
Surviving Sepsis Campaign |
ICU patients |
180 |
<180 |
Not stated |
| 201433 |
American College of Physicians |
ICU patients |
Not stated |
140 – 200 |
Not stated |
Most patients managed with insulin in the noncritically ill inpatient setting should have a premeal BG of less than 140 mg/dL and most BG values should be less than 180 mg/dL. If the individual experiences hypoglycemia (i.e., a BG of less than 70 mg/dL), the insulin regimen should be modified. When the BG is relatively low—less than 100 mg/dL—the insulin regimen should be reassessed for appropriateness.7,28 This reassessment may well help prevent hypoglycemic events later in the admission.
In general, critically ill hospitalized patients using insulin therapy should have a goal glucose value between 140 mg/dL and180 mg/dL. In the published literature, there is plenty of controversy concerning the goal glucose in the critically ill setting versus the noncritically ill setting.
Management of Hyperglycemia in Noncritically Ill Hospitalized Patients
It is important to note that patients admitted to the hospital typically have vastly different needs than do ambulatory patients. Therefore, the following 5 general principles are designed to guide glucose management in the inpatient environment: 1) inpatient situations are unstable; 2) there are changes from a home regimen to an inpatient regimen; 3) no single algorithm is suitable for all patients; 4) frequent daily adjustments made to the inpatient regimen are vital; and 5) medications must be reassessed when preparing for discharge and modified to an appropriate ambulatory regimen (often, the previous home regimen). 6,7,28,30,34
The inpatient regimen of choice for most hospitalized noncritically ill patients with hyperglycemia or preexisting diabetes is scheduled subcutaneous (SC) insulin, which is known as the basal/bolus approach.20,35,36 As insulin is considered the medication of choice to manage BG in the hospital setting, noninsulin-based diabetic therapies—oral or noninsulin SC medications—are not considered standard practice and should usually be discontinued on admission. The inpatient insulin regimen generally consists of the following 3 insulin orders: a basal insulin to manage glucose between meals, prandial insulin for meal-related nutritional BG spikes, and a correctional insulin given in addition to the prandial insulin dose in the event the patient might have premeal hyperglycemia (Table 3). Sliding-scale insulin is not recommended for glucose management in the hospitalized patient.
| Table 3. Basal/Bolus/Correctional Insulin |
| |
Basal insulin |
Prandial (nutritional) insulin |
Correctional (Supplemental) Insulin |
| Rational |
Controls glucose between meals |
Prevents meal/nutritional related glucose spikes |
Given in addition to prandial insulin if needed for premeal hyperglycemia |
| Patient eating meal |
long-acting insulin once- or twice daily
Or
NPH (intermediate-acting insulin) twice-daily |
Rapid-acting insulin given with meal |
Rapid-acting insulin with meals (in addition to prandial insulin if hyperglycemic) |
| Patient not eating meal |
long-acting insulin once or twice-daily
or
NPH (intermediate acting insulin) twice-daily |
Not applicable |
Regular insulin every 4-6 h
Or
Rapid-acting insulin every 4 h |
| Enteral tube feeding or continuous dextrose |
long-acting insulin once- or twice-daily
Or
NPH (intermediate acting insulin) twice-daily |
Regular insulin every 4 – 6 h
Or
Rapid-acting insulin every 4 h |
Regular insulin every 4 – 6 h (in addition to prandial insulin if hyperglycemic)
Or
Rapid-acting insulin every 4 h (in addition to prandial insulin if hyperglycemic) |
Insulin has a narrow therapeutic index and is categorized as a “high alert” class of medications, meaning that it has the potentially high risk of causing injury when misused.37 Insulin management strategies are important to ensure that the correct drug, or dosage form of the drug, is given properly to the appropriate patient at the correct time, as well as through the optimal route of administration.
Randomized clinical trials have shown the superiority of the basal/bolus approach in controlling hyperglycemia and for clinical outcomes compared with sliding-scale insulin in noncritically ill hospitalized patients.35,36 Typically, the starting estimated total daily dose (TDD) required of SC insulin is calculated using the patient’s weight. A conservative standard starting dose for an individual who has never been on insulin before is usually 0.2 to 0.5 units/kg/day.6,7,28,30,35,36 It is best to begin at the lower end (0.2 units/kg/day) for patients at risk of hypoglycemia, such as those presenting with renal or liver disease. The TDD is then split, with one-half into the basal insulin and the other half into the nutritional insulin. The following is a quick example: A 220 lb (100 kg) patient who is hyperglycemic and on a general medical service who has never been on insulin prior to admission and with minimal risk factors for hypoglycemia could be started at 0.4 unit/kg/day or 40 units. Precisely 20 units would be given as basal insulin, such as 10 units of NPH SC every 12 hours and 7 units of rapid-action with each meal. If the patient has premeal hyperglycemia, augment quickly acting correctional insulin to the nutritional insulin.
It is important to note that this conservative beginning TDD dose of 0.2 to 0.5 units/kg/day is a starting place, with critical patients being monitored and dose titrations made at least once daily (see Case 1). Dose adjustments, either up or down, depending on the individual need of the person, are likely for all patients. For example, a person with stress-induced hyperglycemia will likely require less insulin over time during admission as a result of the correction of the underlying cause. Those who are insulin-resistant because of acute illness or chronic disease may require a TDD up to 1.5 to 2 units/kg/day. 7,38
| Case 1 |
| Basal/Nutritional insulin in non-critically ill patient |
RP is a 58-year-old woman with a history of type 2 diabetes mellitus. She is admitted to the internal medicine ward (non-critically ill) with shortness of breath.
Home medications for diabetes include glipizide 10 mg/day and metformin 1000 mg twice/d. She weighs 176 lb (80 kg) and currently maintains a good appetite. Glucose on admission is 242 mg/dL.
Plan
- Discontinue home oral hypoglycemic agents
- Send HbA1C
- Add basal/bolus insulin regimen
- Calculate estimated total daily dose
- 80 kg x 0.5 U/kg/d = 40 U per day
- 50% of dose for basal requirements
- 20 U of long-acting insulin daily
- 50% of dose for prandial requirements
- 7 U of rapid-acting insulin with meals
- Add correctional insulin
- Rapid-acting insulin to be given with prandial insulin
- Monitor glucose values
- Hypoglycemia prevention and treatment protocol ordered
- Adjust the insulin regimen at least daily based on glucose values
- Follow-up on A1C
- Patient education should begin as early in the admission as possible
- Discharge preparation as early in the admission as possible.
|
This general approach to determining the TDD and splitting half for basal and half for nutritional insulin is a positive start for many patients; however, this is not appropriate for all people and variable nutritional statuses. There are many patients for whom the standard approach may not exactly fit, making disease management challenging. For example, those with a nothing-by-mouth status will not require the prandial insulin. Patients on continuous enteral tube feeds may benefit from a more nutrition-centric insulin regimen (e.g., TDD split, 60% nutritional and 40% basal). Complications appear for patients who are on cycled tube feeds (8 PM to 8 AM) but eat meals during the day. In these cases, it may be prudent to add 2 doses of regular insulin to the basal insulin to cover the cycled tube feeds and rapidly acting insulin to cover the meals during the day.7
Management of Hyperglycemia in Critically Ill Hospitalized Patients2,21,39-46
The practice standard for treatment and prevention of hyperglycemia for the critically ill patient population is IV insulin infusion via a validated protocol that allows for predefined adjustments in the insulin infusion rate based on insulin dose and glycemic fluctuations (see Case 2).28 Although controversy exists regarding the goal glucose, the current recommendations indicate a moderate glucose goal (i.e., 140 to 180 mg/dL) for most patients on IV insulin therapy in the intensive care unit, not to target the tight glucose goal (i.e., 80 to 110 mg/dL). IV insulin protocols should be assessed for the glucometrics (i.e., the systematic analysis of blood glucose data) to ensure that the numbers are safe and effective at reaching the goal with minimal hypoglycemia events.47
| Case 2 |
| IV Insulin management in critically ill patient |
DC is a 62-year-old man with no known history of diabetes who is admitted to the surgical intensive care unit after complicated intra-abdominal surgery. His current glucose is 195 mg/dL, and on recheck DC's glucose is 201 mg/dL.
Plan
- Initiate IV insulin protocol with a goal glucose of 140 mg/dL – 180 mg/dL
- Send HbA1C to the lab
- Check glucose and adjust the insulin rate frequently (according to protocol)
- Hypoglycemia prevention and treatment protocol ordered
- When he is stable and getting ready for discharge, consider transitioning the insulin to SC insulin regimen.
|
Randomized trials of intensive versus conventional glucose goal management in critically ill populations have reported mixed results. Mortality was substantially decreased in the intensive BG control arm in 1 randomized trial, increased in another trial, and with no difference in 3 others.2,39,40,42,48 One result seems evident from these 5 randomized trials; that is, patients with tight glycemic goals using IV insulin therapy have a higher rate of severe (i.e., less than 40 mg/dL) hypoglycemia. A meta-analysis of these trials, along with several other observational trials, suggests no mortality benefit from tight glycemic control with an increased risk of hypoglycemia.45 Ultimately, the majority of critically ill patients should target moderate glucose goals.
There are a number of areas of research on goal glucose in the critically ill patient subpopulations. Some clinicians suggest that surgical patients may benefit from tight glycemic control versus populations of medical patients. Those without the diagnosis of diabetes may benefit from tight glycemic control versus moderate control.18,19 Patients with an established diabetes diagnosis may actually be associated with worse outcomes when targeting tight glucose targets. 18,19
Several treatment recommendations provide guidance on the role of IV insulin in critically ill patients.28,29,34 As mentioned, an IV insulin infusion is the preferred therapy for treating hyperglycemia in critically ill patients because it is potent, rapidly acting, and easily titrated. Institutions are advised to use a single insulin concentration in these infusions to avoid any confusion about doses or potential errors relevant to having more than 1concentration; suggested standard concentrations are 1 unit/mL or 0.5 unit/mL.29,49-51
Many protocols have been published, the majority of which use manual calculations. A handful of these protocols use computerized algorithms to adjust insulin rates based on the resulting BG.52 There are components of an IV insulin protocol to examine, such as target glucose levels, time to achieve target glucose levels, incidence of hypoglycemia, rationale for adjusting the rates of insulin infusion, ease of utilization for end-users, and methods of BG measurements.
There are a host of advantages to computer-directed algorithms compared with paper-based protocol. The computer algorithms typically allow for adjusting the goal as opposed to paper-based protocols that have static goals. Depending on patient requirements or patient populations, there are pluses to changing the goal. The computer-directed algorithms typically adjust insulin rates using a multiplication factor that takes into account the rates of insulin and change in BG over time as opposed to fixed adjustments in many paper-based protocols. Fixed protocols often do not always account for the rate of change in BG levels over time or give dose recommendations based on the current BG. For example, a patient whose BG level has dropped by 70 mg/dL in an hour but is still in a safe range (e.g., dropped from 190 mg/ dL to 120 mg/dL) might be counseled to keep the same rate of insulin by a fixed protocol. But if that rate of change were to continue, at the next check, the patient’s BG level could drop at the same rate, potentially putting the individual in danger.53
In critically ill patients, bedside BG monitoring should be performed regularly—every 30 minutes to every 2 hours—based on the protocol suggestion and clinical status.28,29 Ideally, the patient’s BG level would be monitored continually (similarly to blood pressure). But this technology is not yet ready for clinical practice in the critically ill patient population, although there is some excellent data suggesting that this technology can be helpful in the ambulatory setting.54,55
Before IV insulin is initiated and while on therapy, the individual’s potassium levels should be assessed because insulin is known to shift potassium intracellularly, thereby lowering the potassium and putting the patient at risk of arrhythmias.56 It is important for clinicians to monitor and replete potassium and then consider delaying the initiation of insulin infusion until the potassium level is greater than 3.0 to 3.5 mEq/L. 57
When patients are ready to be transferred out of the critical care setting and off the IV insulin drip, many of them will require a transition to SC insulin (see Case 3). Similar to the management of SC insulin in the noncritically ill hospitalized population, this transition will typically require a basal, nutritional, and correctional insulin regimen. The TDD is typically derived by taking 80% of the average hourly rate of IV insulin over a period of time when the patient has been stable, multiplied by 24 hours.6 For instance, if a patient is on 3 units/hour of IV insulin, the TDD = 3(24) (0.8), for a TDD of 58 units. The 58 units would then be split by 50% basal and 50% nutritional in the typical patient. Approximately 80% of the TDD is used because the concentration in an IV bag of 100 units/100 mL is around 0.8 units/mL, likely traceable to absorption of insulin to the IV bag.44[] Because IV insulin has a rapid offset of action, basal insulin should be administered 2 hours before discontinuing IV insulin.6
| Case 3 |
| Transition from IV to SC |
DC from Case #2 is ready to be transitioned to an SC insulin regimen based of a stable IV insulin rate and clinically close to being ready to transfer to the floor. He is currently eating his meal, and his current rate of IV insulin is on 4 U/h (stable for past several hours).
Plan
- Transition from IV to SC utilizing basal/bolus insulin regimen
- Calculate his estimated total daily dose
- 4 U (24) (0.8) = 76 U
- 50% of dose for basal requirement
- 38 U of long-acting insulin daily
- 50% of dose for nutritional requirements
- 12 U of rapid-acting insulin with meals
- Add correctional insulin
- Rapid-acting insulin to be given with nutritional insulin
- Monitor glucose values
- Hypoglycemia prevention and treatment protocol ordered
- Adjust the insulin regimen at least daily based on glucose values
- Follow-up on HbA1C
- Patient education should begin as early in the admission as possible
- Discharge preparation as early in the admission as possible.
|
Point-of-Care Monitoring
The most common methods for glucose monitoring in the hospitalized patients are bedside or point-of-care testing (POCT) devices and central laboratory. POCT instrumentation usually uses capillary blood obtained by a finger stick; however, central venous blood from venous lines, and arterial blood from arterial lines can also be used (typically in the critically ill patient population). Of note, variability exists between each site; therefore, when possible, individuals should be assessed at the same site to minimize confusion, as well as potentially inappropriate pharmacotherapeutic adjustments.
The major benefit of POCT monitoring is the convenience of bedside testing and the relatively fast results (within minutes). POCT devices have variable accuracy, though, and the use of such instrumentation requires training and continual competency assessment of nursing and nursing support staff to ensure proper technique. The International Organization for Standardization (ISO) criteria requirement for accuracy is that displayed values be within 20% of the actual BG level for all readings greater than 75 mg/dL.58,59
The convenience of POCT for glucose monitoring in the hospitalized patient population has made it a standard of care in many, if not all, institutions, including in the critically ill population. However, there is a considerable amount of controversy about the future standards that may be put into place for POCT devices for hospitalized patients and, partially, for those who are critically ill. This debate has not yet been resolved and will continue to unfold and shape clinical practice for the next several years.
Blood samples, collected by venipuncture, venous line, or arterial line and sent to a central laboratory, are usually processed with a whole-blood analyzer or an arterial blood-gas analyzer. Such methods are thought to produce the most accurate results; however, the BG-value results can take considerably longer if one is waiting for the centralized laboratory’s report. Blood analyzers are considerably more expensive than POCT devices; therefore, they are typically not used in clinical areas (e.g., bedside).
Management of Hypoglycemia
The American Diabetes Association (ADA) defines hypoglycemia as a BG level of less than 70 mg/dL, and both the American Association of Clinical Endocrinologists (AACE) and the ADA agree that insulin therapy should be adjusted if this occurs and that insulin regimens are re-evaluated at levels of 100 mg/dL.7,28 Due to the negative consequences of hypoglycemia as well as increased morbidity and mortality, all efforts should be made to limit the incidence of hypoglycemia. This disease should be anticipated and a plan put in place to systematically allow nurses to treat without delay, rather than waiting for new orders from a clinician.16,17,23
Hospitalized patients are at a unique risk of hypoglycemia because the nutritional needs of these patients can change frequently, including the patient alternating on and off nothing-by-mouth status, inconsistent nutritional intake while eating, and some altered mental status. A protocolized plan is warranted to monitor for, prevent, and treat hypoglycemia. This plan should involve a clear definition of and plan to manage patients at risk of hypoglycemia in the hospital environment.
One preventive strategy is to use a glucose value as a signal to reassess insulin therapy before hypoglycemia occurs. For example, you can use fasting BG levels of less than 100 mg/dL for patients on insulin therapy. This type of proactive approach for prevention of hypoglycemia should be a part of the guidelines of each institution’s protocols and guidelines.
The standing hypoglycemia management protocol/guideline should encompass the explicit route, dose, and form of glucose administration, depending on the patient’s level of cognition. Insulin infusions should be discontinued (or reduced, if the patient has type 1 diabetes mellitus [T1DM]), and glucose should be administered. Repetition of BG monitoring and administration of glucose are required until the patient is stabilized. Assessing causality and adjusting treatment are then necessary. Look for the cause of hypoglycemia and determine whether other treatment modifications are needed.
Hyperglycemic Emergencies
The most serious acute complications of hyperglycemia are diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state (HHS).57 DKA is characterized by hyperglycemia, metabolic acidosis, and an increase in ketone production, whereas HHS is distinguished by dehydration, hyperglycemia, mental status change, and hyperosmolality (usually in the absence of significant acidosis). Both DKA and HHS are caused by a complex metabolic process that results from the combination of absolute or relative insulin deficiency.
Most patients presenting with DKA have T1DM or another cause of inadequately circulating insulin. However, DKA can happen in patients with type 2 diabetes mellitus (T2DM) when exacerbated by a stressful event, such as infection and MI(myocardial infarction).57 Several medications are known precipitants for hyperglycemic emergencies, including glucocorticoids, atypical antipsychotics, and thiazide diuretics.
The most recent consensus statement from the ADA about the management of DKA and HHS was published in 2009.57 The ADA put forth treatment strategies that covered classification, monitoring, correction of fluid and electrolyte status, and insulin therapy for patients with DKA and HHS. Most patients with the 2 conditions will require a significant amount of fluid resuscitation and electrolyte repletion in addition to closely monitored glucose and ketones. Initial management with IV insulin infusion is typically indicated, with or without an initial bolus, although in mild-to-moderate DKA, rapid-acting SC insulin can be given every 1 to 2 hours without an IV infusion. Importantly, the goal of insulin therapy in DKA is beyond glucose lowering; insulin is required to prevent ketone formation. It is important to follow DKA-specific endpoints of pH, serum bicarbonate levels, and anion gaps as well as BG. In HHS, the key endpoints to monitor are BG, blood osmolality, and mental status.
Barriers to Glucose Control in Hospitalized Patients
Despite published guidelines and best-practice examples in the literature, the attainment of goal glucose remains difficult in clinical practice.8 Health care providers face diverse potential barriers to providing optimal glycemic control to the different patient populations throughout their institution. The barriers to achieving glucose control in hospitalized patients listed in Figure 1 include fear of hypoglycemia, lack of institutional consensus, lack of education and inadequacy protocols. Identifying and developing strategies to overcome these barriers is necessary for ensuring glycemic control and improving patient outcomes.60,61
| Figure 1. Barriers to Glucose Management in Hospitalized Patients |
| Barrier to Address when Managing Glucose in Hospitalized Patients |
Lack of consensus regarding goals with clinicians
Lack of institution-specific published "how to"
Lack of a standardized approach to testing and treatment
Inadequate insulin-drip protocol
Compliance with established treatment guidelines and protocols
Fear of hypoglycemia
Culture of acceptance of hyperglycemia
Point-of-Care-Testing device accuracy
Provider and clinician education
Communication among health care providers
Health care resources |
To overcome the barriers to glucose management in the hospital environment, a multidisciplinary team approach should be employed. Each health system or institution will face unique challenges, making a cookie-cutter solution to the barriers difficult. The multidisciplinary team allows for a systematic evaluation of each barrier and plans to overcome it.
Team Approach
Managing diabetes and hyperglycemia in hospitalized patients requires multidisciplinary resources and coordination of care.34,61-63 A high-risk medication, such as insulin, as the primary management for inpatients with hyperglycemia warrants a protocolized approach to improving patient outcomes while maximizing patient safety.37 A standardized, multidisciplinary team approach should be employed for implementation of best-practice recommendation guidelines to achieve glucose management metrics and improve patient care outcomes.
The following are 2 general types of glucose teams described in the literature: First, the Steering Committee or Task Force Team, with a focus on global oversight of BG management for an institution or health care system; and, second, the best practice Clinical Management Team that highlights the bedside clinical management of patients’ glucose on the referral list.
The multidisciplinary Steering Committee or Task Force Team is responsible for the management of formulary agents, the development of policies/guidelines, and order sets and standards of practice for an institution, as well as providing a means of education (both patient and staff), implementation, and continuous quality improvement. The Steering Committee also advocates for resources to ensure patients have access to critical glycemic management medications and materials, including glucose monitors and test strips for self-testing, before being discharged.
This Steering Committee or Task Force is crucial to ensure policies, order sets, protocols, and guidelines are appropriate for the specific needs of the institution and are developed, implemented, and continually monitored and improved in a stepwise fashion (Figure 2). Members of the Steering Committee or Task Force should include representation from all disciplines as a means of providing insight into all potential treatment barriers and areas for improvement. Representation should generally include hospitalists, intensivists from multiple units, nurses from all care areas, pharmacists, endocrinologists, diabetes educators, nutritionists, information-systems personnel administrators, clinical laboratory technicians, and others responsible for patient care, depending on the structure of the institutions.
Figure 2
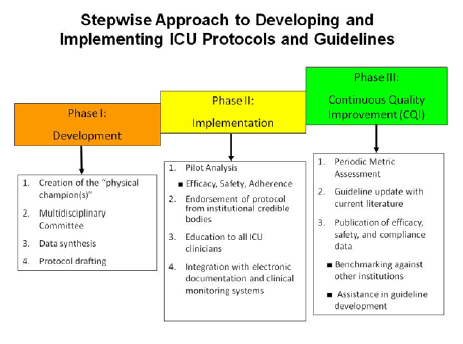 |
Strong team members are required to model and encourage positive changes in clinical practice.34 A designated champion or champions from each discipline may be necessary to positively reinforce the initiatives of the team on a departmental basis. These physicians, nurses, pharmacists, and nutritionists are the core driving force behind implementation and improvement of glucose control to achieve performance metrics and optimize patient outcomes. Champions can facilitate communication between the steering committee or task force and the health-system committees.
In addition to the Steering Committee or Task Force, several health care systems may have a Clinical Management Team performing consultative services for individual patients. Although the order sets, protocols, and guidelines developed by the Steering Committee or Task Force are intended for use by everyone in the organization, the complexity of managing BG in the acute care setting may necessitate the expert guidance offered by the clinical management group.
Such teams typically focus on the management of diabetes and hyperglycemia in those patients on their consult. However, these clinicians can also take part in staff education, discharge planning and counseling, and facilitating change in culture as institutional leaders of the clinical topic. The makeup of the groups in the literature varies slightly, but can include endocrinologists, endocrine fellows, hospitalists, nurse practitioners, physician assistants, and clinical pharmacists. This team is often considered the glucose swat team, which works clinically to improve the care of diabetic patients and those with hospital-related hyperglycemia.
Whether a part of the steering committee, glucose clinical team, or just working clinically in the inpatient setting, pharmacists are uniquely qualified in the management of diabetes and glucose in the inpatient setting. Clinical pharmacists often are key members of the care team in many patient populations, including, but not limited to, oncology, critical care, cardiology, internal medicine, perioperative, nutritional support, and emergency medicine. Pharmacists involved in patient care have the opportunity to identify at-risk patients, monitor therapy, suggest changes in pharmacotherapy management, and help with transitions of care.
Information technology can help in the management of inpatient glucose. Often, hospital pharmacies have rule-based surveillance tools in place to help identify potential adverse drug reactions and areas for intervention. One example of this in clinical practice is having a rule-based alert for any glucose value greater than 200 mg/dL. When the rule fires, the responsible clinical pharmacists would evaluate the case and make recommendations appropriate for that patient. This may seem simple, but these types of rules help busy clinicians make best-practice recommendations in a timely manner.
Transition/Discharge
Clinicians should take time during any hospitalization to promote best practices for the ambulatory management of diabetes, particularly for patients who may have a new diagnosis of diabetes or patients not well-controlled in the ambulatory setting prior to admission. Pharmacists may be ideal for this transitional teaching of high-risk patients with diabetes. When an individual is transitioning from being an inpatient to the ambulatory setting, there is an opportunity to enhance patient understanding and adherence. Importantly, the discharge teaching should start as early in admission as possible and not wait until the patient is actually being discharged. Ideally, the outpatient regimen should be finalized at least 24 hours prior to actual discharge in order to identify and target any immediate issues that may occur.64,65
Each patient’s discharge plan should be tailored to his or her unique needs. Depending on the many factors (e.g., recent A1C, ambulatory goal glucose, individualized tailored plan), many people can be discharged on a regimen similar to the home regimen prescribed by their ambulatory care provider. Reassessment of the home regimen is warranted, particularly when the patient has a new contraindication to an ambulatory medication, shows evidence of poor ambulatory management (e.g., very high A1C), or presents with hypoglycemia on the admission regimen.
In those who have not previously been on insulin prior to admission and require insulin at the time of discharge, the discharge insulin regimen should usually be as simple as possible (e.g., a single injection of bedtime basal insulin). As with most general principles, there are several exceptions to the rule. One example would be the newly diagnosed patient with T1DM, who should be discharged with a home regimen of 3 to 4 injections per day, including basal and nutritional coverage. Individuals generally should not be discharged to home on sliding-scale insulin. Patients who require insulin injections after discharge should receive education and instruction on how to monitor their glucose with a POCT testing machine, how to inject the insulin, what the signs and symptoms are of hypo- and hyperglycemia, and how to manage the adverse of either of these events.
If a medication, insulin or otherwise, has been adjusted or added, patients should have prompt follow- up with their primary care clinician. People newly diagnosed with diabetes should be discharged on a simple regimen and provided with close follow-up by a dedicated provider who will carefully oversee their progress.28,65 For patients with no history of diabetes, but who are found to have hyperglycemia during hospital admission, studies show that approximately 60% of this population will meet diagnostic criteria for diabetes at follow-up testing; therefore, these patients should have follow-up testing for diabetes (e.g., fasting plasma glucose) within 1 to 2 months following hospital discharge.28,65 After discharge, continual reviews of the patient are paramount, given the likely changes in endogenous stress hormones, diet, physical activity, and medications; all of which may contribute to the necessity for pharmacotherapeutic alterations.
Summary
Management of diabetes and hyperglycemia for hospitalized patients can be challenging and each clinician working with these patients should be able to recognize the proper management to improve outcomes and minimize adverse events. The principles of decreasing hyperglycemia and hypoglycemia and maintaining normoglycemia should be a priority for all clinicians treating the hospitalized patient with diabetes or hospital-related hyperglycemia. Pharmacists have many important roles in this management, including being members of a glucose Steering Committee or a clinical team, and involvement with discharge/transitional care, teaching and coaching, monitoring, pharmacotherapy modifications, and staff education.
| Guide for Hospital Pharmacists |
| What Pharmacists Must Know Article Category |
Diabetes Management |
| General |
- Most patients managed with insulin in the noncritically ill inpatient setting should have a premeal blood glucose (BG) of less than 140 mg/dL and most BG values should be less than 180 mg/dL.6
- In general, critically ill hospitalized patients using insulin therapy should have a goal glucose value between 140 mg/dL and180 mg/dL.
- Patients admitted to the hospital typically have different needs than ambulatory patients.
- The following 5 general principles are designed to guide inpatient glucose management:
- 1) Inpatient situations are unstable;
- 2) There are changes from a home regimen to an inpatient regimen;
- 3) No single algorithm is suitable for all patients;
- 4) Frequent daily adjustments made to the inpatient regimen are vital; and
- 5) Medications must be reassessed when preparing for discharge and modified to an appropriate ambulatory regimen.8
- Insulin is considered the medication of choice to manage blood glucose in the hospital setting; noninsulin-based diabetic therapies should usually be discontinued on admission.
- The inpatient insulin regimen generally consists of the following 3 insulin orders:
- A basal insulin to manage glucose between meals;
- Prandial insulin for meal-related nutritional BG spikes; and
- A correctional insulin given in addition to the prandial insulin dose in the event the patient might have premeal hyperglycemia
- Sliding-scale insulin is not recommended for glucose management in the hospitalized patient.6
- An IV insulin infusion is the preferred therapy for treating hyperglycemia in critically ill patients because it is potent, rapidly acting, and easily titrated.
- In critically ill patients, bedside BG monitoring should be performed regularly—every 30 minutes to every 2 hours—based on the protocol suggestion and clinical status.28,29
- When an individual is transitioning from being an inpatient to the ambulatory setting, there is an opportunity to enhance patient understanding and adherence. The outpatient regimen should be finalized at least 24 hours prior to actual discharge in order to identify and target any immediate issues that may occur.
- If a medication, insulin or otherwise, has been adjusted or added, patients should have prompt follow-up with their primary care clinician. People newly diagnosed with diabetes should be discharged on a simple regimen and provided with close follow-up by a dedicated provider who will carefully oversee their progress.
|
REFERENCES
- Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78(12):1471-1478.
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359-1367.
- Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3): 978-982.
- Siegelaar SE, Hermanides J, Oudemans-van Straaten HM, et al. Mean glucose during ICU admission is related to mortality by a U-shaped curve in surgical and medical patients: a retrospective cohort study. Crit Care. 2010;14(6):R224.
- Estrada CA, Young JA, Nifong LW, Chitwood WR Jr. Outcomes and perioperative hyperglycemia in patients with or without diabetes mellitus undergoing coronary artery bypass grafting. Ann Thorac Surg. 2003;57(5):1392-1399.
- Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591.
- Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16-38.
- Swanson CM, Potter DJ, Kongable GL, Cook CB. Update on inpatient glycemic control in hospitals in the United States. Endocr Pract. 2011;17(6):853-861.
- Pasquel FJ, Spiegelman R, McCauley M, et al. Hyperglycemia during total parenteral nutrition: an important marker of poor outcome and mortality in hospitalized patients. Diabetes Care. 2010;33(4):739-741.
- Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33(8):1783-1788.
- Schlenk F, Vajkoczy P, SarrafzadehA. Inpatient hyperglycemia following aneurysmal subarachnoid hemorrhage: relation to cerebral metabolism and outcome. Neurocrit Care. 2009;11(1):56-63.
- Bochicchio GV, Joshi M, Bochicchio KM, et al. Early hyperglycemic control is important in critically injured trauma patients. J Trauma. 2007;63(6):1353-1358; discussion 1358-1359.
- Baker EH, Janaway CH, Philips BJ, et al. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax. 2006;61(4):284-289.
- McAlister FA, Majumdar SR, Blitz S, et al. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28(4):810-815.
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31(3):596-615.
- Bagshaw SM, Egi M, George C, et al. Early blood glucose control and mortality in critically ill patients in Australia. Crit Care Med. 2009;37(2):463-470.
- Kosiborod M, Inzucchi SE, Krumholz HM, et al. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation. 2008; 117(8):1018-1027.
- Krinsley JS, Egi M, Kiss A, et al. Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: an international multicenter cohort study. Crit Care. 2013;17(2):R37.
- Lanspa MJ, Hirshberg EL, Phillips GD, et al. Moderate glucose control is associated with increased mortality compared with tight glucose control in critically ill patients without diabetes. Chest. 2013;143(5):1226-1234.
- Goyal A, Mahaffey KW, Garg J, et al. Prognostic significance of the change in glucose level in the first 24 h after acute myocardial infarction: results from the CARDINAL study. Eur Heart J. 2006;27(11):1289-1297.
- Penning S, Pretty C, Preiser JC, et al. Glucose control positively influences patient outcome: a retrospective study. J Crit Care. 2015;30(3):455-459.
- Krinsley JS, Preiser JC. Time in blood glucose range 70 to 140 mg/dl >80% is strongly associated with increased survival in non-diabetic critically ill adults. Crit Care. 2015;19:179.
- NICE-SUGAR Study Investigators, Finfer S, Liu B, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367(12):1108-1118.
- Smith WD, Winterstein AG, Johns T, et al. Causes of hyperglycemia and hypoglycemia in adult inpatients. Am J Health Syst Pharm. 2005;62(7):714-719.
- Turina M, Fry DE, Polk HC Jr. Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med. 2005;33(7):1624-1633.
- Mizock BA. Alterations in carbohydrate metabolism during stress: a review of the literature. Am J Med. 1995;98(1):75-84.
- McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17(1):107-124.
- Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353-369.
- Jacobi J, Bircher N, Krinsley J, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012;40(12):3251-3276.
- American Diabetes Association. (13) Diabetes care in the hospital, nursing home, and skilled nursing facility. Diabetes Care. 2015;38 Suppl:S80-S85.
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580-637.
- McMahon MM, Nystrom E, Braunschweig C, et al. A.S.P.E.N. clinical guidelines: nutrition support of adult patients with hyperglycemia. JPEN J Parenter Enteral Nutr. 2013;37(1):23-36.
- Qaseem A, Chou R, Humphrey LL, et al. Inpatient glycemic control: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Am J Med Qual. 2014;29(2):95-98.
- Maynard G, Wesorick DH, O'Malley C, et al. Subcutaneous insulin order sets and protocols: effective design and implementation strategies. J Hosp Med. 2008;3(5 Suppl):29-41.
- Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181-2186.
- Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261.
- Joint Commission IDs five high-alert meds. ED Manag. 2000;12(2):21-22.
- Lane WS, Cochran EK, Jackson JA, et al. High-dose insulin therapy: is it time for U-500 insulin? Endocr Pract. 2009;15(1):71-79.
- Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449-461.
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125-139.
- Preiser JC, Devos P. Steps for the implementation and validation of tight glucose control. Intensive Care Med. 2007;33(4):570-571.
- NICE-SUGAR Study Investigators, Finfer S, Chittock DR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283-1297.
- Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014;370(17):1626-1635.
- Rocchio MA, Belisle CD, Greenwood BC, et al. Evaluation of the maximum beyond-use-date stability of regular human insulin extemporaneously prepared in 0.9% sodium chloride in a polyvinyl chloride bag. Diabetes Metab Syndr Obes. 2013;6:389-392.
- Griesdale DE, de Souza RJ, van Dam RM, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180(8):821-827.
- Deedwania P, Kosiborod M, Barrett E, et al. Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2008;117(12):1610-1619.
- Goldberg PA, Bozzo JE, Thomas PG, et al. "Glucometrics"--assessing the quality of inpatient glucose management. Diabetes Technol Ther. 2006;8(5):560-569.
- Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35(10):1738-1748.
- Hellman R. A systems approach to reducing errors in insulin therapy in the inpatient setting. Endocr Pract. 2004;10 Suppl 2:100-108.
- Hellman R. Patient safety and inpatient glycemic control: translating concepts into action. Endocr Pract. 2006;12 Suppl 3:49-55.
- Cohen MR. Pharmacists' role in ensuring safe and effective hospital use of insulin. Am J Health Syst Pharm. 2010;67(16 Suppl 8):S17-S21.
- Krikorian, A., Ismail-Beigi F, Moghissi ES. Comparisons of different insulin infusion protocols: a review of recent literature. Curr Opin Clin Nutr Metab Care. 2010;13(2):198-204.
- Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract. 2004;10 Suppl 2:21-33.
- Ryan EA, Germsheid J. Use of continuous glucose monitoring system in the management of severe hypoglycemia. Diabetes Technol Ther. 2009;11(10):635-639.
- Joseph JI, Hipszer B, Mraovic B, et al. Clinical need for continuous glucose monitoring in the hospital. J Diabetes Sci Technol. 2009;3(6):1309-1318.
- Ford W. Self WH, Slovis C, McNaughton CD. Diabetes in the emergency department and hospital: acute care of diabetes patients. Curr Emerg Hosp Med Rep. 2013;1(1):1-9.
- Kitabchi AE, Umpierrez G, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343.
- Guimont MC, Desjobert H, Fonfrède M, et al. Multicentric evaluation of eight glucose and four ketone blood meters. Clin Biochem. 2015;48(18):1310-1316.
- Inoue S, Egi M, Kotani J, Morita K. Accuracy of blood-glucose measurements using glucose meters and arterial blood gas analyzers in critically ill adult patients: systematic review. Crit Care. 2013;17(2):R48.
- Anger KE, Szumita PM. Barriers to glucose control in the intensive care unit. Pharmacotherapy. 2006;26(2):214-228.
- Reynolds LR, Cook AM, Lewis DA, et al. An institutional process to improve inpatient glycemic control. Qual Manag Health Care. 2007;16(3):239-249.
- Szumita PM. The hospital pharmacist: an integral part of the hyperglycaemic management team. J Clin Pharm Ther. 2009;34(6):613-621.
- Garber AJ, Moghissi ES, Bransome ED Jr, et al. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10 Suppl 2: 4-9.
- Hsu CW, Lin CS, Chen SJ, et al. Risk of type 2 diabetes mellitus in patients with acute critical illness: a population-based cohort study. Intensive Care Med. 2016;42(1):38-45.
- Lien LF, Angelyn Bethel M, Feinglos MN. In-hospital management of type 2 diabetes mellitus. Med Clin North Am. 2004;88(4):1085-1105, xii.
.
Back to Top