
Expired activity
Please go to the PowerPak
homepage and select a course.
Current Strategies for Postprandial Glucose Control
Introduction
According to the Centers for Disease Control and Prevention, 29.1 million individuals in the United States (U.S.) have diabetes.1 Of these, 21 million people have been diagnosed with the disease and 8.1 million remain undiagnosed. Additionally, an estimated 86 million people in the U.S. have pre-diabetes, which is defined as having a blood glucose level that is higher than normal but not high enough for a diagnosis of diabetes. Type 2 diabetes (T2D) accounts for 90% to 95% of all cases of diabetes and is a result of multiple metabolic defects, including insulin resistance and progressive beta cell dysfunction. The risk of T2D is associated with obesity, physical inactivity, family history, and race/ethnicity. T2D is also associated with older age, and more than 40% of all adults with T2D are over the age of 65. Type 1 diabetes (T1D), which accounts for approximately 5% of all cases of diabetes, is an autoimmune disease that leads to complete destruction of pancreatic beta cells and an absolute deficiency of insulin.1 Diabetes is associated with significant morbidity and mortality and is the seventh leading cause of death in the U.S. Diabetes causes both microvascular and macrovascular complications and continues to be a substantial cause of heart disease, stroke, retinopathy, kidney failure, and amputation, making it an important public health concern.1 The total direct medical costs of diabetes in the U.S. in 2012 totaled $176 billion and indirect costs (e.g., disability, work loss, and premature death) totaled $69 billion.1 Clinical evidence shows that appropriate glycemic control can reduce the risk of long-term diabetes-related complications in both T1D and T2D.2-7 Despite this evidence, achieving glycemic control remains challenging and many patients do not reach their glycemic goals. Data from the National Health and Nutrition Examination Survey show that, from 2007 to 2010, only 52.5% of people with diabetes achieved a target hemoglobin A1C (A1C) of less than 7%.8 One major barrier to achieving glycemic control is clinical inertia. A retrospective cohort study of 81,573 patients with T2D revealed that many patients have uncontrolled diabetes for several years before treatment is intensified. In patients with a goal A1C less than 7%, the median time to intensification was 2.9 years in patients taking 1 oral antidiabetic drug (OAD) and more than 7.2 years in patients taking 2 OADs.9 Thus, diabetes remains difficult to control despite a growing number of available treatment options, and there is a continued need for effective and durable treatment approaches that target the pathophysiologic defects of the disease.
Pathophysiology of diabetes
Diabetes is characterized by increased levels of blood glucose as a result of a deficiency in insulin secretion, an abnormality in insulin action, or both. T1D results from destruction of the pancreatic beta cells. This destruction is mediated by the body’s immune system and results in absolute elimination of insulin secretion. Insulin therapy that mimics normal pancreatic insulin secretion is required for survival. T2D is more complex and involves multiple metabolic defects (Figure 1).10-12 The initial, core pathophysiologic defect in T2D is insulin resistance in the muscle, liver, and adipocytes. Insulin resistance in the muscle results in impaired glucose uptake into cells after meals leading to postprandial hyperglycemia. Insulin resistance in the liver results in overproduction of glucose during the fasting state and impaired suppression of hepatic glucose output after meals.11,12 Early in the disease process, beta cells in the pancreas try to compensate for insulin resistance by increasing insulin secretion. Over time, however, this increased workload leads to beta cell failure, depleted insulin secretion, and subsequent hyperglycemia.11,12 Another pathophysiologic defect that contributes to glucose intolerance is decreased incretin hormone secretion. That is, patients with T2D have reduced levels of incretin hormones. The incretin hormones, glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP), are released in the gut in response to carbohydrate ingestion and enhance insulin response to meals.11 It is estimated that GLP-1 and GIP are responsible for 50% to 70% of postprandial insulin release.13 GLP-1 also decreases inappropriately secreted glucagon after meals and slows gastric emptying. Other pathophysiologic defects of T2D include increased glucagon secretion from pancreatic alpha cells leading to increased basal and postprandial hepatic glucose output, increased rate of glucose absorption in the gut, and increased renal glucose reabsorption.10
| Figure 1: Pathophysiology of type 2 diabetes10 |
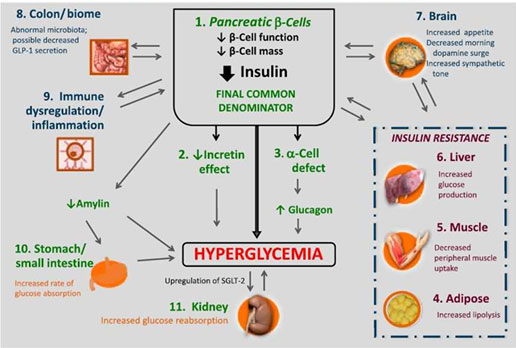 |
T2D is a progressive disease; abnormal beta cell function leading to reduced insulin secretion is the final common denominator of all hyperglycemic characteristics of T2D.10 Reduced insulin secretion begins with an early loss of first-phase insulin secretion, which is the acute insulin surge that occurs immediately after eating, and causes increased postprandial glucose (PPG) levels, followed by a progressive decline in insulin sensitivity and progressive deterioration of beta cell function leading to further reductions in insulin secretion (Figure 2).12,14 Therefore, the typical clinical course of T2D is an initial gradual loss of glycemic control after meals, followed by the development of fasting hyperglycemia in the morning, and, finally, sustained hyperglycemia throughout the day and night.15,16
| Figure 2: Post-meal insulin responses in patients with and without type 2 diabetes14 |
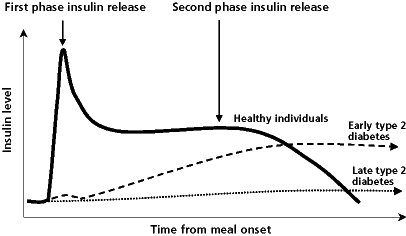 |
Role of postprandial hyperglycemia in T2D
To maintain normal glucose homeostasis, a healthy functioning pancreas secretes approximately 30 to 50 units of insulin each day, half of which is “basal” and half of which is “prandial.” Basal insulin is secreted to manage glucose that is produced by the liver between meals and overnight and to maintain consistent fasting plasma glucose (FPG) levels. Prandial insulin is secreted to manage the oral glucose load after eating.17 First-phase prandial insulin secretion occurs immediately (i.e., onset of 1 minute and duration of 5 to 10 minutes) in response to receptor stimulation by glucose. Postprandial hyperglycemia occurs when the body is not able to produce adequate amounts of insulin to metabolize the glucose load that occurs after a meal.18 PPG levels are typically measured 1 to 2 hours after a meal and are affected by a variety of factors including carbohydrate absorption, insulin and glucagon secretion abnormalities, glucose metabolism, and the timing, quantity, and composition of the meal.19
In individuals with normal glucose tolerance, PPG concentrations generally rise no higher than 140 mg/dL after a meal and return to normal levels within 2 to 3 hours. In individuals with T2D, PPG levels commonly increase to greater than 140 mg/dL, even in individuals who have A1C levels below target.16 Postprandial hyperglycemia is common in patients with T2D and is frequently the first glucose abnormality detected in the disease process.
In patients with diabetes, the A1C level reflects average glucose control over a 2- to 3-month period, with contributions of both FPG and PPG levels. The relative contribution of each component varies depending on the degree of hyperglycemia.20-22 Monnier et al evaluated the contributions of FPG and PPG to overall hyperglycemia in patients with T2D and found that PPG contributes relatively more to A1C in patients who were fairly well controlled (e.g., lower A1C levels) and, as A1C levels rise, the contribution of FPG becomes more significant (Figure 3).21
| Figure 3: Relative contributions of fasting glucose and postprandial glucose levels to hyperglycemia in patients with type 2 diabetes21 |
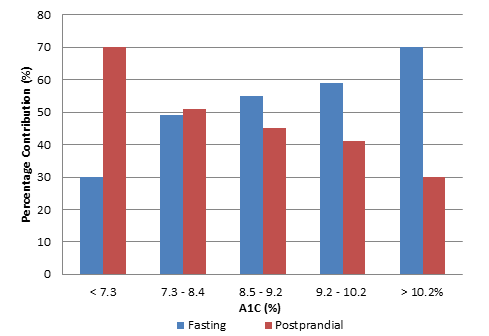 |
Munshi et al evaluated the relative contributions of FPG and PPG to total hyperglycemia in older and younger adults with T2D and found that the relative contribution of PPG to hyperglycemia was greater in older adults than in younger adults, even at higher A1C levels (Figure 4).22 These studies highlight the fact that PPG is an important component of overall hyperglycemia and may be the predominant component in patients who are closer to goal and in older adults.
| Figure 4: Relative contribution of postprandial glucose level to hyperglycemia in younger and older adults with type 2 diabetes22 |
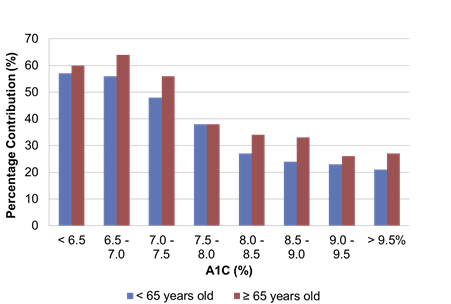 |
Some evidence suggests that postprandial hyperglycemia may be an independent risk factor for microvascular and macrovascular complications of diabetes. Increasing evidence confirms an independent association between PPG and overall mortality,23,24 cardiovascular mortality,23-25 heart disease,25-27 and microvascular complications.28 Additionally, data indicate that elevations in PPG may be a better predictor of cardiovascular mortality than FPG elevations.23,24 The proposed mechanism for this independent risk of postprandial hyperglycemia is oxidative stress caused by high mealtime glucose levels, as well as subsequent endothelial dysfunction and inflammation. There remains, however, a need for consistent evidence that lowering PPG levels reduces the risk of long term complications.29-30
General treatment recommendations
Both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE) have recently published updated guidelines for the management of diabetes.31-35 For T1D, both guidelines recommended strategies that mimic physiologic insulin secretion (e.g., basal insulin plus rapid-acting mealtime insulin or continuous subcutaneous insulin infusion). For T2D, both guidelines include many treatment options with different therapeutic approaches. The ADA guidelines recommend initial therapy with lifestyle intervention plus metformin. If glucose control is not achieved after 3 months of monotherapy, a medication from any one of the following classes of agents can be added: sulfonylureas, thiazolidinediones, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium glucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 receptor agonists (GLP-1 RA), or basal insulin. A third agent can be added if additional glucose-lowering effects are needed after 3 months of dual therapy. Metformin plus combination injectable therapy is recommended if 3 months of triple therapy fails to achieve glucose control. Options for combination injectable therapy include basal insulin plus mealtime insulin or basal insulin plus a GLP-1 RA. This approach is particularly recommended for patients who have achieved target FPG levels with basal insulin but still have elevated A1C levels. Combination injectable therapy can also be started as first-line therapy if patients have an A1C at baseline of 10% to 12% or higher, if baseline blood glucose is 300 to 350 mg/dL or higher, or if the patient is symptomatic.31-33
The AACE guidelines recommend initial therapy with lifestyle intervention plus metformin for patients with recent-onset T2D or mild hyperglycemia (A1C < 7.5%). Patients who present with A1C greater than 7.5% should be started on metformin plus another agent in addition to lifestyle therapy. The AACE preferentially recommends agents that do not increase the risk of hypoglycemia or weight gain; thus, the GLP-1 RAs, SGLT-2 inhibitors, and DPP-4 inhibitors are preferred, followed by thiazolidinediones, basal insulin, colesevelam, bromocriptine, alpha-glucosidase inhibitors (AGIs), sulfonylureas, and meglitinides. Additional therapy should be added if patients do not achieve glycemic goals after 3 months. A more intensive insulin-based approach is recommended for symptomatic patients with A1C greater than 9% or those that are not at goal on triple therapy. Options include basal insulin plus mealtime insulin or basal insulin plus a GLP-1 RA, SGLT-2 inhibitor, or DPP-4 inhibitor.34-35 A patient-centered, individualized approach to therapy is recommended by both guidelines with considerations for complimentary mechanisms of action, efficacy, risk of hypoglycemia, impact on weight, and side effect profiles.
Recommended target ranges for A1C, FPG, and PPG are shown in Table 1.33,35 The issue of FPG versus PPG targets is complex, and how the treatment of these defects should be prioritized is not well established. Traditionally, the approach recommended by the ADA has been to target FPG goals first and then begin targeting PPG goals in individuals who have FPG levels within target but still have elevated A1C levels.33 The AACE guidelines do not clearly prioritize FPG or PPG initially but, instead, recommend that treatment decisions should be guided by the patient’s medical needs, and treatment goals and drug selection decisions should be based on a comprehensive patient assessment including a glycemic profile obtained by self-monitoring of blood glucose (SMBG) data. Patients will likely only achieve A1C targets if they achieve both FPG and PPG goals.35
| Table 1: Glycemic control targets |
| Organization |
A1C* |
FPG (mg/dL) |
PPG (mg/dL) |
| American Diabetes Association33 |
< 7% |
80 - 130 |
< 180
1-2 hours after meal |
| American Association of Clinical Endocrinologists34 |
< 6.5% |
< 110 |
< 140
2 hours after meal |
*These goals apply to patients without serious comorbidities and without risk factors for hypoglycemia and other adverse events. Goals should be individualized, and more or less stringent goals may be appropriate for certain patients.
A1C = glycosylated hemoglobin; FPG = fasting plasma glucose; PPG = postprandial glucose |
Recently, it has been suggested that the treatment of T2D should follow a pathophysiologic approach rather than a stepwise “treat to failure” approach.10,12 This new approach advocates the initial use of combinations of drugs with complementary mechanisms to correct multiple pathophysiologic defects present in T2D. Importantly, since many of the earliest defects in T2D result in increased PPG, these combinations would likely require a medication that targets PPG.10,12 Finally, considering that PPG may contribute more to hyperglycemia in elderly adults than in younger adults, glucose-lowering regimens that target PPG and have a low risk of hypoglycemia may be preferred in the elderly.22
Dietary considerations for PPG control
Both the quantity and type of carbohydrate in a food influences PPG levels, but total amount of carbohydrates consumed is the primary predictor of glycemic response.36 Monitoring carbohydrate intake and considering the blood glucose response to dietary carbohydrates are key diabetes self-management behaviors for improving PPG control.33 Modifying the types and amounts of dietary carbohydrates can improve PPG and A1C levels. Individuals with diabetes should be encouraged to replace refined carbohydrates and added sugars with whole grains, legumes, vegetables, and fruits. Sugar-sweetened beverages and low-fat or non-fat products with high amounts of added sugars should be discouraged.36
Patients taking meal-time insulin should be offered intensive instruction on the relationship between carbohydrate intake and insulin needs, as well as matching insulin administration and carbohydrate intake. Some patients may be able to have flexible dosing regimens and adjust insulin doses on the basis of planned carbohydrate intake while others may need a simple meal-planning approach using a fixed dose of insulin and emphasizing a fixed carbohydrate intake pattern.33,35
Therapeutic interventions for postprandial hyperglycemia
Uncontrolled PPG is common in diabetes. It contributes to overall hyperglycemia and is associated with poor outcomes. Treatment options that specifically target PPG are, therefore, critical components to achieving and sustaining glycemic control in patients with T1D and T2D. Table 2 lists currently available classes of diabetes medications and the respective impacts on glucose profile. Most traditional OADs, such as metformin and thiazolidinediones, primarily target FPG and have little direct impact on PPG levels. Sulfonylureas increase insulin secretion and, thus, lower both FPG and PPG indiscriminately. However, because of their slow onset of action, sulfonylureas do not impact first-phase insulin secretion. In addition, sulfonylureas lack durability and are associated with weight gain and hypoglycemia. This class also carries the highest risk of serious hypoglycemia of all non-insulin diabetes medications.34 The newest class of medications, the SGLT-2 inhibitors (canagliflozin, dapagliflozin, and empagliflozin), increase urinary glucose excretion whenever blood glucose levels are elevated. Like sulfonylureas, SGLT-2 inhibitors lower both FPG and PPG indiscriminately. The classes that primarily target PPG are summarized in Table 3 and are discussed below in more detail.
| Table 2: Effects of diabetes drugs on the glucose profile35 |
| Class |
FPG-lowering ability |
PPG-lowering ability |
| Biguanides (metformin) |
Moderate |
Mild |
| Thiazolidinediones |
Moderate |
Mild |
| Sulfonylureas |
Moderate |
Moderate |
| Meglitinides |
Mild |
Moderate |
| Alpha-glucosidase inhibitors |
Neutral |
Moderate |
| GLP-1 RAs |
Mild to Moderate* |
Moderate to Marked |
| DPP-4 inhibitors |
Mild |
Moderate |
| SGLT-2 inhibitors |
Moderate |
Mild |
| Amylin agonist (pramlintide) |
Mild |
Moderate to Marked |
| Insulin |
Moderate to Marked (basal) |
Moderate to Marked (short or rapid acting) |
*Mild: albiglutide, exenatide, lixisenatide; Moderate: exenatide XR, liraglutide, and dulaglutide
DPP-4 = dipeptidylpeptidase-4; GLP-1 RAs = glucagon-like peptide-1 receptor agonists; SGLT-2 = sodium glucose cotransporter-2 |
| Table 3: Diabetes medications that target postprandial hyperglycemia32 |
| Class |
Agent(s) |
Administration |
Primary physiologic action(s) |
Main advantages |
Main disadvantages |
Meglitinides
|
Nateglinide
Repaglinide |
By mouth before each main meal; usually 3 times daily |
↑ insulin secretion |
Dosing flexibility |
Hypoglycemia
Weight gain
Frequent dosing |
| Alpha-glucosidase inhibitors |
Acarbose
Miglitol |
Oral, 3 times daily |
Slows intestinal carbohydrate digestion/absorption |
No hypoglycemia
Non-systemic action |
Generally modest A1C efficacy
GI side effects (e.g., flatulence, diarrhea)
Frequent dosing |
| GLP-1 RAs |
Albiglutide
Dulaglutide
Exenatide
Exenatide XR
Liraglutide |
Subcutaneous injection; twice daily, once daily, or once weekly |
↑ glucose-dependent insulin secretion
↓ glucose-dependent glucagon secretion
Slows gastric emptying
↑ satiety |
No hypoglycemia
Weight loss |
GI side effects (e.g., nausea, vomiting, diarrhea)
Injectable
Training requirements |
| DPP-4 inhibitors |
Alogliptin
Linagliptin
Saxagliptin
Sitagliptin |
By mouth; once daily |
↑ glucose-dependent insulin secretion
↓ glucose-dependent glucagon secretion |
No hypoglycemia
Well tolerated |
Generally modest A1C efficacy
May increase risk of heart failure |
| Amylin agonist |
Pramlintide |
Subcutaneous injection before each main meal; usually 3 times daily |
↓ glucagon secretion
Slows gastric emptying
↑ satiety |
Weight loss |
Generally modest effects on A1C
GI side effects (e.g., nausea, vomiting)
Hypoglycemia unless insulin dose is decreased
Frequent dosing
Injectable
Training requirements |
| Prandial Insulin |
Short-acting (human regular)
Rapid-acting insulin analogs (lispro, aspart, glulisine)
Inhaled insulin |
Subcutaneous injection or inhalation before each main meal; usually 3 times daily |
↑ glucose disposal
↓ hepatic glucose production
Suppresses ketogenesis |
Nearly universal response
Theoretical unlimited efficacy |
Hypoglycemia
Weight gain
Frequent dosing
Injectable (except inhaled insulin)
Training requirements |
| A1C = glycosylated hemoglobin; DPP-4 = dipeptidylpeptidase-4; GI = gastrointestinal; GLP-1 RAs = glucagon-like peptide-1 receptor agonists |
Meglitinides
The meglitinides are short-acting secretagogues that stimulate insulin release from pancreatic beta cells. They work through similar mechanisms as sulfonylureas but with a faster onset and shorter duration of action. Both repaglinide and nateglinide are absorbed within 30 to 60 minutes and have half-lives of 60 to 90 minutes. Due to the faster onset, meglitinides primarily increase first-phase insulin release and may be more effective at targeting PPG than sulfonylureas. Overall, however, the meglitinides have smaller A1C-lowering effects than sulfonylureas. Due to the shorter duration of action, meglitinides are less likely to cause hypoglycemia during the last postprandial phase than sulfonylureas.34 Although less common than with the sulfonylureas, the primary adverse effects (AEs) of meglitinides are hypoglycemia and weight gain. Patients should be educated about the timing of administration of meglitinides and the prevention, detection, and treatment of hypoglycemia while taking these drugs. Because of their fast onset of action, meglitinides need to be taken immediately (i.e., within 15 minutes) before meals, typically 3 times daily. To avoid hypoglycemia, patients should be instructed not to take a meglitinide if they skip a meal. Consequently, meglitinides provide some flexibility for patients with irregular meal schedules.37
Alpha-glucosidase inhibitors
AGIs effectively lower PPG levels by blocking the alpha-glucosidase enzyme and slowing the rate of carbohydrate absorption in the small intestine. They have modest effects on A1C levels and negligible effects on FPG. The STOP-NIDDM study found that treating PPG elevations with acarbose (an AGI) significantly lowered the risk of cardiovascular events in patients with impaired glucose tolerance.38 A meta-analysis of 7 randomized controlled trials also found that acarbose reduced the risk of myocardial infarction and any cardiovascular event in patients with T2D.39 Despite this, the AGIs are used infrequently in the U.S. due to the common occurrence of gastrointestinal (GI) AEs such as flatulence, bloating, and abdominal discomfort. The AGIs require 3-times-daily dosing; the doses of AGIs should be started at low doses and titrated slowly to help decrease the occurrence of GI AEs.
Glucagon-like peptide-1 receptor agonists
GLP-1 RAs stimulate glucose-dependent insulin secretion, inhibit inappropriate glucagon secretion after meals, slow gastric emptying, and increase satiety. Through these mechanisms, the GLP-1 RAs provide improvements in both PPG and FPG levels.18 There are currently 5 GLP-1 RAs approved for use in the U.S. (Table 3). Additionally, lixisenatide is approved in Europe and has been submitted for approval to the U.S. Food and Drug Administration (FDA). The pharmacokinetics, dynamics, dosing, and clinical effects differ among the GLP-1 RAs. The shorter-acting agents (exenatide and lixisenatide) are associated with more significant delays in gastric emptying and targeted reductions in PPG. Compared to short-acting GLP-1 RAs, the longer-acting agents (albiglutide, dulaglutide, exenatide XR, and liraglutide) have less of an effect on gastric emptying and rely more on the insulin secretion mechanism, which leads to reductions in both FPG and PPG.40,41
The GLP-1 RAs offer several advantages, including excellent A1C-lowering efficacy, weight loss, and a low risk of hypoglycemia.31 They are recommended as second-line treatment options to be added to metformin by the ADA and are preferred treatment options by the AACE.31,34 The GLP-1 RAs require subcutaneous injection twice daily (exenatide), once daily (lixisentide and liraglutide), or once weekly (albiglutide, dulaglutide, and exenatide XR). All GLP-1 RAs are available in pen devices. The most common AEs of GLP-1 RAs are GI-related events (e.g., nausea, vomiting, and diarrhea).
Recently, attention has been on the addition of a GLP-1 RA to basal insulin in T2D. GLP-1 RAs and insulin have complementary mechanisms of action and glucose profile targets: basal insulin lowers FPG and GLP-1 RAs can lower PPG. Additionally, combining the 2 classes may allow patients to achieve glycemic control with lower doses, which could result in fewer AEs than when the individual drugs are used independently. A meta-analysis of 15 studies found that the combination therapy yielded a mean reduction in A1C of 0.44% (95% CI -0.60, -0.29; p < 0.001) and weight loss of 3.22 kg (95% CI -4.90, -1.54; p < 0.001); the combination did not increase the relative risk of hypoglycemia (RR = 0.99; 95% CI 0.76, 1.29; p < 0.001).42
Finally, adding a GLP-1 RA to basal insulin offers a potentially safer and easier approach to achieving glycemic control compared to adding prandial or meal-time insulin. Five clinical trials have compared GLP-1 RAs to prandial insulin in patients with T2D on basal insulin therapy. Overall, the studies demonstrated A1C lowering with basal insulin plus GLP-1 RA therapy that was either non-inferior or superior to basal insulin plus prandial insulin therapy. GLP-1 RA therapy resulted in weight loss and prandial insulin led to weight gain. Patients who were treated with a GLP-1 RA experienced minimal hypoglycemia; the most common AEs were predictably GI-related events.43-47 These studies suggest that basal insulin plus a GLP-1 RA provides a safe and effective alternative to basal-bolus insulin with a smaller treatment burden. Thus, both the ADA and AACE guidelines recommend GLP-1 RAs as alternatives to prandial insulin to treat postprandial hyperglycemia in patients with A1C levels that are not at goal despite reaching FPG goals with basal insulin.32,34 Patients should be educated about storage, proper injection technique, dosing, and AEs when starting a GLP-1 RA.
Two fixed-ratio combinations (FRC) containing a GLP-1 RA and basal insulin in a single injection are in development: insulin degludec/liraglutide (iDegLira) and insulin glargine/lixisenatide (iGlarLixi).48,49 The FRC iDegLira has been approved in Europe under the trade name Xultophy and is supplied as a 3-ml pen device containing 100 unit/ml insulin degludec and 3.6 mg/ml liraglutide.50 Each dose step contains 1 unit of insulin degludec and 0.036 mg of liraglutide with a maximum dose of 50 units of insulin degludec and 1.8 mg of liraglutide. Buse et al found that iDegLira decreased A1C significantly more than insulin degludec alone (-1.9% vs -0.9%; p < 0.001); it also led to weight loss and had similar rates of hypoglycemia compared to insulin.51 The slow titration of the combination product led to lower rates of GI AEs than typically expected with the liraglutide component of the combination. FRC products will be available in pen devices that are similar in design to current basal insulin products, but they will have some unique education points, including dose titration and dose adjustments when switching to an FRC from basal insulin or a GLP-1 RA. Both iDegLira and iGlarLixi will be administered subcutaneously once a day and the dose will be titrated based on the basal insulin component.
DPP-4 inhibitors
DPP-4 inhibitors inhibit the degradation of GLP-1 and GIP by inhibiting the enzyme DPP-4. Increased physiologic levels of GLP-1 and GIP increase glucose-dependent insulin secretion and suppress glucose-dependent glucagon secretion, which decreases PPG in patients with T2D.18 The DPP-4 inhibitors differ from GLP-1 RAs in that DPP-4 inhibitors extend the elimination half-life of endogenous GLP-1 and GIP while the GLP-1 RAs provide higher pharmacologic levels of the GLP-1 RAs.18 Unlike the GLP-1 RAs, the DPP-4 inhibitors have little effect on gastric emptying and satiety and, therefore, do not lead to significant weight loss, but are, instead, considered weight neutral. There are currently 4 DPP-4 inhibitors approved for use in the U.S. (Table 3). The DPP-4 inhibitors have smaller A1C-lowering effects than other second-line treatment options, but they do offer convenient once-daily oral dosing and are well tolerated with low risks of AEs, including hypoglycemia.32 An FDA drug safety communication warns that saxagliptin and alogliptin may increase the risk of heart failure, particularly in patients who already have heart or kidney disease and health care providers should consider discontinuing these agents in patients who develop heart failure.52
Insulin
Insulin products are commonly categorized on the basis of their pharmacokinetic and pharmacodynamic profiles. Long-acting, basal insulin analogs (glargine, detemir, and degludec) and intermediate-acting insulin (human NPH) primarily lower FPG and have minimal to no effect on PPG. Insulin agents that specifically target PPG include rapid-acting insulin analogs (RAIAs; lispro, aspart, and glulisine), short-acting insulin (regular human), and inhaled insulin.
Patients with T1D require intensive, physiologic insulin therapy, or the use of both basal and prandial insulin in an effort to mimic the normal secretion of insulin by the pancreas. This can be accomplished by multiple daily injections (MDI) of insulin (both long-acting and rapid-acting formulations) or an insulin pump. A typical MDI regimen might include a long-acting basal insulin administered once daily to target FPG plus 3 injections of an RAIA (1 injection before each meal) to manage postprandial hyperglycemia.
The goal of prandial insulin therapy is to reduce PPG by mimicking endogenous insulin secretion patterns. This requires a quick onset of action to minimize the quick rise in glucose and a short duration of action to avoid late postprandial hypoglycemia.37
The majority of patients with T2D requiring insulin therapy can often be successfully treated with basal insulin alone. But, some patients whose glycemia remains uncontrolled while receiving basal insulin or patients with symptomatic, uncontrolled hyperglycemia may require combined basal plus prandial insulin. There are several approaches to initiating prandial insulin in T2D, the simplest of which is to add 1 injection of prandial insulin before the largest meal, titrate the dose slowly until the SMBG target is reached and then add additional mealtime insulin later, if needed. A more aggressive approach is to start prandial insulin before each meal and titrate the doses slowly until the SMBG target is reached. For either approach, the basal dose should be evaluated and possibly reduced by the amount of prandial insulin being added.32 Although less like physiologic insulin production, pre-mixed insulin given twice daily might be a more convenient option for some patients.
Regular human insulin is less costly than insulin analogs, but both the ADA and AACE guidelines state that RAIAs are preferred over regular human insulin because RAIAs have a more rapid onset and a shorter duration of action; they are also associated with better PPG control and fewer episodes of hypoglycemia than regular insulin.31,34 A meta-analysis of 50 trials in T1D and 30 trials in T2D comparing RAIAs to regular human insulin found that RAIAs reduced A1C and reduced the risk of nocturnal hypoglycemia in patients with T1D. These benefits were not consistently observed across the studies in the T2D population.53
The most common AEs of subcutaneous prandial insulin are hypoglycemia and weight gain. Intensifying treatment from a basal insulin regimen to a more intensive regimen including basal insulin plus prandial insulin will likely increase the risk of hypoglycemia and weight gain.54
For patients taking subcutaneous prandial insulin, comprehensive education is required regarding storage, administration, dosing, injection technique, site rotation, dose titration, and disposal. The dose of prandial insulin varies and is patient-specific. Matching the dose of prandial insulin to the estimated carbohydrate intake should be considered, especially for patients with T1D. However, this approach requires extensive education about carbohydrate counting and is not feasible for all patients.33 Timing of prandial insulin administration is another important education point. Regular human insulin should be administered within 30 minutes of a meal. Since RAIAs start to take effect within 15 minutes, patients should be instructed to take these insulins within 15 minutes before a meal. If there are concerns about hypoglycemia due to delays in consuming the meal, patients can inject with the first bite of the meal or immediately after the meal. Insulin pens and smaller, shorter pen needles have made subcutaneous prandial insulin practical and easy to use.
Education regarding proper SMBG testing and interpretation is also required for patients and should include proper use of a glucometer, timing of SMBG, expected goals, and interpreting or using SMBG data. The ADA guidelines recommend that most patients on intensive insulin regimens should test their own blood glucose levels prior to meals, occasionally postprandially, at bedtime, prior to exercise, when they suspect they have low blood glucose, after treating low blood glucose levels, and prior to critical tasks such as driving.33 SMBG data can be used to calculate a correction dose of prandial insulin to be used in combination with the normal prandial dose to correct a high glucose level before a meal. Measuring glucose levels also helps patients understand how food and insulin affect glucose levels, which is crucial for self-management of diabetes.33 Finally, educating patients about the detection, prevention, and treatment of hypoglycemia is critically important.
Inhaled insulin is the newest prandial insulin option with a rapid-acting profile; it offers an alternative to pre-meal injections of insulin. Inhaled insulin (Afrezza) was approved by the FDA in 2014 for glycemic control in adults with T1D or T2D. Pharmacodynamic studies have demonstrated that inhaled insulin has a faster onset of action and shorter duration of action than a subcutaneous RAIA, insulin lispro.55 These potential advantages, however, have not translated to significantly improved glycemic outcomes compared to RAIAs. Bode et al compared inhaled insulin to subcutaneous insulin aspart; both were added to basal insulin in patients with T1D. Change in A1C from baseline was non-inferior with inhaled insulin compared to insulin aspart (-0.21 vs -0.4; 95% CI 0.02, 0.36). Patients who used inhaled insulin experienced fewer episodes of hypoglycemia and less weight gain.56 Rosenstock et al evaluated inhaled insulin compared to inhaled placebo added to OADs in patients with T2D. After 24 weeks, inhaled insulin significantly reduced A1C compared to inhaled placebo (-0.8% vs -0.4%, p < 0.001). Hypoglycemia was more common in the inhaled insulin group than in the placebo group.57 Inhaled insulin plus basal insulin was also found to be non-inferior to a pre-mixed insulin analog in patients with T2D. The A1C reduction from baseline was -0.59 with inhaled insulin plus basal insulin, and the A1C reduction was -0.71 with pre-mixed insulin. Hypoglycemia occurred less frequently with inhaled insulin.58
Inhaled insulin has some unique patient education points. First, it is a dry powder inhaler system that uses an inhaler delivery device with pre-filled, unit-dose cartridges. The cartridges (available in 4-, 8-, and 12-unit strengths) should be stored in the refrigerator before use and at room temperature while in use. For a patient with T2D who is new to insulin, the recommendation is to start with a single 4-unit cartridge with each meal. Patients who have previously used RAIAs and are switching to inhaled insulin should round up to nearest 4-unit interval. For example, a patient using 10 units of insulin aspart before meals should round up to 12 units and use one 4-unit and one 8-unit cartridge before each meal. Inhaled insulin should be administered at the beginning of a meal and patients should be educated on proper inhalation technique (Figure 5). Like other insulin products, inhaled insulin can cause hypoglycemia and weight gain. Additionally, the most common AE of inhaled insulin is transient, dry cough. Inhaled insulin is contraindicated in patients with chronic lung disease such as asthma or chronic obstructive pulmonary disease, and it should not be used in individuals who smoke. The use of inhaled insulin requires lung function tests before and after starting therapy.55
| Figure 5: How to use inhaled insulin55 |
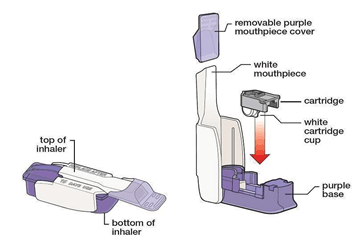 |
Step 1: Select the correct cartridge for your dose.
Step 2: Open the inhaler and load the cartridge. Keep the inhaler level to avoid spilling the powder. Close the inhaler.
Step 3: Hold the inhaler away from your mouth and exhale deeply. Do not exhale into the device. Place the mouthpiece in your mouth and create a seal with your lips around the mouthpiece. Inhale deeply and hold your breath for several seconds before exhaling.
Step 4: Put the mouthpiece cover back onto the inhaler. Open the inhaler, remove the cartridge, and discard in the trash.
Note: If using more than 1 cartridge, the process should be repeated until the proper dose has been inhaled. The inhaler device should not be washed. The device should be discarded and replaced every 15 days. The medication guide outlines step-by-step instructions for use. |
There are several new insulins and insulin products in development that seek to achieve ultra-rapid time to onset and peak activity, which would more closely mimic the quick secretion of insulin that normally occurs in response to meals. New products in development include agents or technologies that achieve ultra-rapid pharmacokinetic parameters by increasing insulin absorption rates within the subcutaneous tissue, increasing local blood flow, or increasing insulin diffusion. A new insulin formulation in development creates an ultra-rapid action by including ethylenediaminetetraacetic acid, more absorption. Injecting RAIAs with hyaluronidase also appears to accelerate the absorption of RAIAs and produce a faster onset and shorter duration of action.59
Amylin agonist
Pramlintide is an agonist of the hormone amylin. Pramlintide inhibits glucagon secretion, slows gastric emptying, and increases satiety. This agent is typically reserved for patients treated with intensive insulin therapy, often patients with T1D, to decrease postprandial hyperglycemia, minimize glucose variability, and improve weight. Pramlintide is administered via subcutaneous injection before meals and concurrent reduction of prandial insulin dosing is required to reduce the risk of hypoglycemia.31
Summary
Postprandial hyperglycemia is common in patients with diabetes. It contributes to elevated A1C levels and is associated with diabetes-related complications. Treatment options that target PPG are, therefore, critical components to achieving and sustaining glycemic control in patients with T1D and T2D. Targeting PPG should be considered when selecting treatment options. Specifically, treatments that lower PPG differ in efficacy, tolerability, dosing, administration, and ease of use. A patient-centered, individualized approach to diabetes management is recommended and should consider complimentary mechanisms of action, efficacy, risk of hypoglycemia, impact on weight, and side effect profiles.
REFERENCES
- Centers for Disease Control and Prevention. 2014 National Diabetes Statistics Report. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 18, 2015.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-86.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-53.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854-65.
- Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-89.
- Ismail-Beigi F, Craven T, Banerji MA, et al. ACCORD Trial Group. Effect of intensive treatment of hyperglycemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomized trial. Lancet. 2010;376(9739):419-30.
- Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560-72.
- Stark Casagrande S, Fridkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care. 2013;36(8):2271-9.
- Khunti K, Wolden ML, Thorsted BL, et al. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36(11):3411-7.
- Schwartz SS, Epstein S, Corkey BE, et al. The time is right for a new classification system for diabetes: rationale and implications of the beta-cell-centric classification schema. Diabetes Care. 2016;39:179-86.
- DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773-95.
- DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care. 2013;36(2):S127-38.
- Nicolaus M, Brodl J, Linke R, et al. Endogenous GLP-1 regulates postprandial glycemia in humans: relative contributions of insulin, glucagon, and gastric emptying. J Clin Endocrinol Metab. 2011;96(1):229-36.
- Gerich JE. Pathogenesis and treatment of type 2 (non-insulin dependent) diabetes mellitus (NIDDM). Horm Metab Res. 1996;28(9):404-12.
- Monnier L, Colette C, Dunseath GJ, Owens DR. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care. 2007;30:263-9.
- Ceriello A. The glucose triad and its role in comprehensive glycemic control: current status, future management. Int J Clin Pract. 2010;64(12):1705-11.
- Meah F, Juneja R. Insulin tactics in type 2 diabetes. Med Clin North Am. 2015;99(1):157-86.
- Gerich J. Pathogenesis and management of postprandial hyperglycemia: role of incretin-based therapies. Int J Gen Med. 2013;6:877-95.
- American Diabetes Association. Postprandial blood glucose. Diabetes Care. 2001;24(4):775-8.
- Rodbard HW, Karolicki B. Management of type 2 diabetes – methods for addition of prandial insulin to basal insulin. European Endocrinology. 2014;10(2):124-30.
- Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients; variations with increasing HbA1c. Diabetes Care. 2003;26:881-5.
- Munshi MN, Pandya N, Umpierrez GE, et al. Contributions of basal and prandial hyperglycemia to total hyperglycemia in older and younger adults with type 2 diabetes mellitus. J Am Geriatr Soc. 2013;61:535-41.
- Balkau B, Shipley M, Jarrett RJ, et al. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men. 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policeman Study. Diabetes Care. 1998;21(3):360-67.
- DECODE Study Group; the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161(3):397-405.
- Barrett-Connor E, Ferrara A. Isolated post-challenge hyperglycemia and the risk of fatal cardiovascular disease in older women and men. The Rancho Bernardo study. Diabetes Care. 1998;21(8):1236-9.
- Donahue RP, Abbott RD, Reed DM, Yano K. Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry. Honolulu Heart Program. Diabetes. 1987;36(6):689-92.
- Temelkova-Kurktschiev TS, Koehler C, Henkel E, et al. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1C level. Diabetes Care. 2000;23:1830-4.
- Gabir MM, Hanson RL, Dabelea D, et al. Plasma glucose and prediction of microvascular disease and mortality: evaluation of 1997 American Diabetes Association and 1999 World Health Organization criteria for diagnosis of diabetes. Diabetes Care. 2000;23(8):1113-8.
- Peter R, Rees A. Postprandial glycemia and cardiovascular risk. Br J Diabetes Vasc Dis. 2008;8:8-14.
- Raz I, Wilson PW, Strojek J, et al. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care. 2009;32:381-6.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: a position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364-79.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2015;38(1):140-9.
- American Diabetes Association. Standards of Medical Care in Diabetes – 2016. Diabetes Care. 2016;39(Suppl 1):S1-112.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2016 executive summary. Endocr Pract. 2016;22(1):84-113.
- Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for Developing a Diabetes Mellitus Comprehensive Care Plan – 2015. Endocr Pract. 2015;21(Suppl 1):1-87.
- Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2014;37(Suppl 1):S120-43.
- Tibaldi J. Importance of postprandial glucose levels as a target for glycemic control in type 2 diabetes. Southern Medical Journal. 2009;102(1):60-6.
- Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486-94.
- Hanefeld M, Cagatay M, Petrowitsch T, et al. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: a meta-analysis of seven long-term studies. Eur Hart J. 2004;25:10-6.
- Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728-42.
- Meier JJ, Rosenstock J, Hincelin-Mery A, et al. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open-label trial. Diabetes Care. 2015;38(7):1263-73.
- Eng C, Kramer CK, Zinman B, Retnakaran. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228-34.
- Diamant M, Nauck MA, Shaginian R, et al. Glucagon-like peptide-1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care. 2014;37(10):2763-73.
- Shao N, Kuang HY, Hao M, et al. Effects of exenatide on obesity and NAFLD with elevated liver enzymes in patients with type 2 diabetes. Diabetes Metab Res Rev. 2014;30(6):521-9.
- Mathieu C, Rodbard HW, Cariou B, et al. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD-ON). Diabetes Obes Metab. 2014;16:636-44.
- Roy-Duval C, Hanefeld M, Gentile S, et al. Advancing basal insulin glargine with prandial lixisenatide QD vs insulin glulisine QD or TID in type 2 diabetes: the GetGoal-Duo2 evidence-based trial. Diabetologia. 2015;58(Suppl 1):S39.
- Rosenstock, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care. 2014;37(8):2317-25.
- Novo-Nordisk. Xultophy® (NN9068) Type 2 diabetes: filed for registration. http://www.novonordisk.com/rnd/pipelinedetails.1428300648863_2.html. Accessed March 23, 2016.
- Zealand announces that Sanofi has submitted LixiLan for regulatory review in the US, triggering a USD 20 million milestone payment [Press release]. Copenhagen, Denmark: Zealand Pharmaceuticals; December 23, 2015. https://newsclient.omxgroup.com/cdsPublic/viewDisclosure.action?disclosureId=690565&lang=en. Accessed March 23, 2016.
- Xultophy. Annex I: Summary of product characteristics. Bagsvaerd, Denmark: Novo Nordisk A/S; 2014. http://ec.europa.eu/health/documents/community-register/2014/20140918129550/anx_129550_en.pdf. Accessed March 23, 2016.
- Buse JB, Visbøll T, Thurman J, et al. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37(11):2926-33.
- U.S. Food and Drug Administration Drug Safety Communication. FDA adds warnings about heart failure risk to labels for type 2 diabetes medicines containing saxagliptin and alogliptin. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM493965.pdf. Accessed April 18, 2016.
- Canadian Agency for Drugs and Technologies in Health. Rapid-acting insulin analogues for the treatment of diabetes mellitus: meta-analyses of clinical outcomes. CADTH Technology Overviews. 2010;1(1):1-7.
- Liu SC, Tu YK, Chien MN, Chien KL. Effect of antidiabetic agents added to metformin on glycemic control, hypoglycemia and weight change in patients with type 2 diabetes: a network meta-analysis. Diabetes Obes Metab. 2012;14:810-20.
- Afrezza [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2016.
- Bode BW, McGill JB, Lorber DL, et al. Inhaled Technosphere insulin compared with injected prandial insulin in Type 1 diabetes: a randomized 24-week trial. Diabetes Care. 2015;38(12):2266-73.
- Rosenstock J, Franco D, Korpachev V, et al. Inhaled technosphere insulin versus inhaled technosphere placebo in insulin-naïve subjects with type 2 diabetes inadequately controlled on oral antidiabetes agents. Diabetes Care. 2015;38:2274-81.
- Rosenstock J, Lorber DL, Gnudi L, et al. Prandial inhaled insulin plus basal insulin glargine versus twice daily biaspart insulin for type 2 diabetes: a multicenter randomized trial. Lancet. 2010;375(9733):2244-53.
- Cahn A, Miccoli R, Dardano A, Del Prato S. New forms of insulin and insulin therapies for the treatment of type 2 diabetes. Lancet Diabetes Endocrinol. 2015;3(8):638-52.
Back to Top