
Expired activity
Please go to the PowerPak
homepage and select a course.
Intensifying Therapy After Basal Insulin Optimization in Type 2 Diabetes (Article)
INTRODUCTION AND BACKGROUND
Diabetes mellitus is a serious, but controllable, disease. More than 29 million people in the United States (U.S.) have diabetes, but there are nearly triple as many people with pre-diabetes—more than 86 million. Type 2 diabetes mellitus (T2DM) accounts for 90% to 95% of new cases of diabetes. The complications and related conditions affiliated with diabetes continue to expand,1 and only 57% of adults with diabetes in the U.S. are meeting the American Diabetes Association (ADA) goal of a hemoglobin A1c (A1C) less than 7%; the proportion of patients meeting this goal is even lower in some minority populations.2 Therefore, experts support early and intensive intervention to best combat and manage diabetes.
It is estimated that, by the time a person is diagnosed with T2DM, he or she has actually had the disease for 9 or more years.3 It is well known that, in patients with pre-diabetes and T2DM, postprandial plasma glucose (PPG) is the first glycemic level to rise and stay elevated during the waking hours. Through the overnight fasting period, blood glucose levels return to normal by morning. The natural history of T2DM shows that, as PPG levels rise, so does pancreatic β-cell insulin secretion (Figure 1).3,4 However, as PPG levels remain elevated for longer than normal periods of time, the body cannot effectively or efficiently use the circulating blood glucose, despite an overabundance of available insulin. Patients often have hyperglycemia and hyperinsulinemia during the pre-diabetes and early stages of T2DM due to this insulin resistance.3,4 The pancreatic β-cells continue to work harder to produce more insulin but, eventually, become unable to maintain the extra workload and cannot functioning effectively. As a result, most individuals who are newly diagnosed with T2DM have already lost 50% to 80% of their pancreatic β-cell function.5
Figure 1. Natural History of Type 2 Diabetes (T2DM)3,4
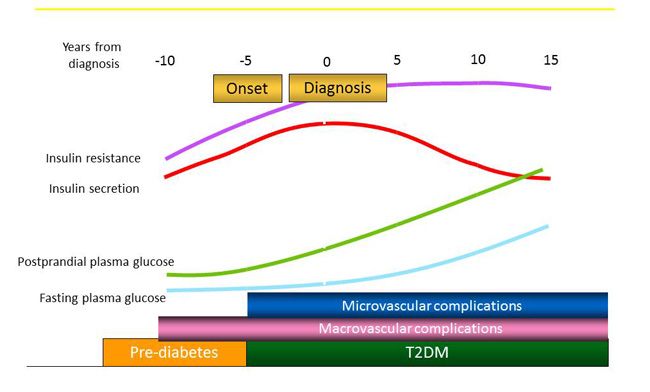
Contributing to the delay in diagnosis of T2DM is the fact that most people do not seek medical attention for signs of diabetes but, rather, for symptoms due to complications of diabetes. It is quite common for patients to schedule a medical visit or present to the emergency department because of vision problems, recurrent urinary tract infections, or cardiovascular complications, such as a myocardial infarction or stroke. These are often the situations in which patients are diagnosed with T2DM. It is estimated that 60% of people with T2DM have at least 1 complication of the disease and that nearly 50% of these complications are present at diagnosis.6 Therefore, the main goal of T2DM management is to preserve the remaining pancreatic β-cell function to prevent disease progression and minimize diabetes complications.
PATHOPHYSIOLOGY OF T2DM
T2DM is the result of at least 8 multisystem defects, including dysfunctions and alterations in the pancreatic α-cells, pancreatic β-cells, liver, skeletal muscle, adipose tissue, brain, gastrointestinal (GI) tract, and kidneys (Figure 2).5 The reduced sensitivity to insulin in liver, muscle, and adipose tissue and a progressive decline in pancreatic β-cell function lead to impaired insulin secretion. This eventually results in hyperglycemia, the distinctive feature of T2DM. In addition, the pancreas exhibits hyperglucagonemia (increased pancreatic α-cell glucagon secretion), which leads to increased hepatic gluconeogenesis. In the muscle, the glucose transporter 4, the major transporter involved in the uptake of glucose into skeletal tissue, is depleted, thereby resisting insulin action.5,7 Adipose tissue (visceral fat) shows reduced adiponectin and increased cytokines, including interleukin-6 and tumor necrosis factor-α. In T2DM, adipocytes are resistant to the antilipolytic effect of insulin, resulting in elevation of circulating free fatty acids, which stimulate gluconeogenesis, induce hepatic and muscle insulin resistance, and impair insulin secretion.5,7 The brain shows disruption of satiety signaling, leading to overeating, reduced dopamine, and impaired neurocircadian rhythm.5,7
Figure 2. The Ominous Octet: Multiple Pathophysiologic Defects in Type 2 Diabetes Mellitus (T2DM)5
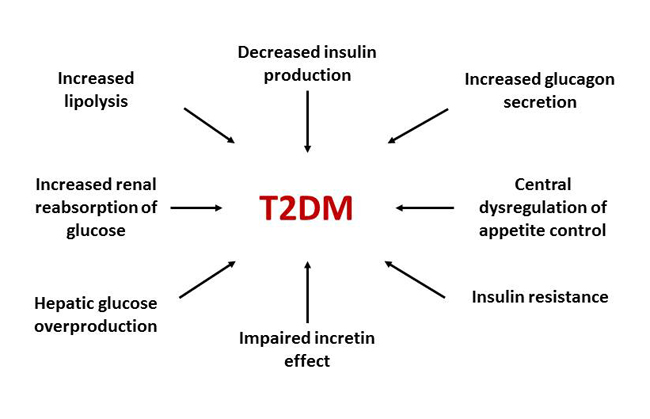
In the GI tract, glucagon-like peptide-1 (GLP-1), an incretin hormone released by the L-cells in the intestine in response to food ingestion, is reduced in T2DM. This results in increased speed of food movement from the stomach to the small intestine that leads to overeating, PPG elevations, decreased glucose-dependent insulin secretion, and increased glucagon secretion.7 Finally, in healthy individuals without diabetes, the kidneys reabsorb virtually all glucose from the renal filtrate. The sodium-glucose cotransporter-2 (SGLT-2), a high-capacity, low-affinity transporter expressed on the luminal surface of the proximal tubule, accounts for approximately 90% of renal glucose reabsorption. In patients with T2DM, SGLT-2 expression is increased. In turn, renal glucose reabsorption from the glomerular filtrate is increased, which can further contribute to hyperglycemia.7
KEY POINTS TO CONSIDER WHEN SELECTING PHARMACOTHERAPY
In addition to safety and effectiveness, there are several key considerations in the selection of T2DM pharmacotherapy. Since T2DM is a result of 8 or more system defects, drug therapy (monotherapy or combination therapy) that targets as many of the multiple defects as possible is best. Additionally, treatments with low to minimal risks of hypoglycemia should be implemented. Clinically significant hypoglycemia, defined as a blood glucose less than 54 mg/dL detected by a self-monitoring blood glucose meter, continuous glucose monitoring device, or laboratory measurement, is closely linked with cardiovascular concerns and cognitive decline, and, therefore, should be avoided as much as possible.8
Weight is another concern related to several diabetes pharmacotherapy agents. It is counterintuitive for a drug to cause weight gain in a population of patients who are usually trying to lose weight. Therefore, weight loss, or weight neutrality, should be considered in the drug selection process. Since diabetes is closely linked to cardiovascular disease, the cardiovascular safety of the T2DM pharmacotherapy must also be considered.8 Many of the older, traditional oral diabetes agents, such as sulfonylureas (SUs) and thiazolidinediones (TZDs) have cardiovascular risks associated with their use. Most of the newer oral and injectable non-insulin agents, such as dipeptidyl peptidase-4 (DPP-4) inhibitors, SGLT-2 inhibitors, and GLP-1 receptor agonists, have a positive or neutral cardiovascular profile.9
Another key consideration is the duration of T2DM. People with long-standing disease, especially if it is uncontrolled, will have minimal pancreatic β-cell function remaining and exogenous insulin may be the best or only option to gain adequate blood glucose control. In patients with a shorter duration of disease, or those who have had well-controlled glycemia, non-insulin agents can work successfully.10 Another critical point for individualizing pharmacotherapy is the degree of A1C-lowering required to achieve the glycemic goal. Most diabetes agents, other than insulin, lower A1C between 0.5% and 2% when used as monotherapy or in combination regimens. For patients with an A1C greater than 9%, and, therefore, a need for substantial A1C reduction, insulin should be included in the treatment regimen, since insulin is the only agent that can lower A1C more than 2%.10
The next consideration is which blood glucose index is not at the target level (i.e., fasting plasma glucose [FPG], PPG, or both). Monnier et al reported that A1C levels near normal (i.e., less than 7.3%) are more affected by PPG contributions than FPG contributions (e.g., approximately 70% PPG and 30% FPG). Higher A1C levels have a greater relative FPG contribution (e.g., an A1C of 10.2% is influenced by 30% PPG and 70% FPG) (Figure 3).11 This supports the reasoning of the "fix fasting first" treatment approach.11 Microvascular complications are the result of consistently elevated FPG levels, and macrovascular complications are directly linked to elevated PPG and food consumption.12
Figure 3. Fasting and Postprandial Contributions to Hemoglobin A1c Levels11
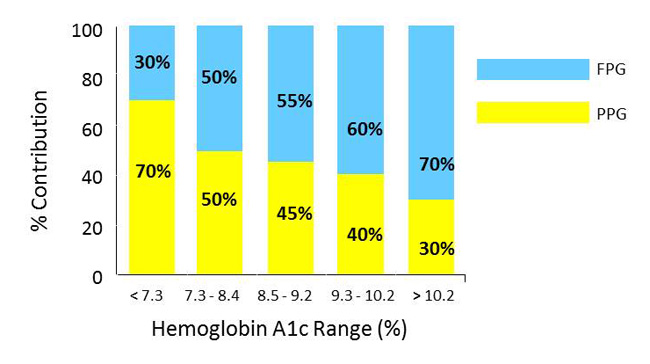
Abbreviations: FPG = fasting plasma glucose; PPG = postprandial plasma glucose.
There are 12 pharmacotherapy classes of drugs available for T2DM, but no single class fixes all 8 of the dysfunctional defects in T2DM. This is the reason monotherapy is rarely sustainable in controlling blood glucose. Combination therapy—using 2 or 3 different classes of drugs with distinct mechanisms of action that target several of the 8 dysfunctional defects—provides the best chance of controlling blood glucose, delaying the progression of diabetes, and reducing the risk of developing complications and related conditions.7
The 2017 consensus statement from the ADA and the European Association for the Study of Diabetes guidelines continues to endorse a step-wise approach to glycemic control that is based on the gradual intensification of diabetes drug therapy (Figure 4).8 Metformin is recommended as first-choice monotherapy for most patients with T2DM, provided there are no contraindications to its use. If adequate glycemic control is not achieved with metformin within 3 months or is subsequently lost despite titration to optimal dose, then a second glucose-lowering therapy should be added with a complementary mode of action.8
Figure 4. General Recommendations for Antihyperglycemic Therapy in Type 2 Diabetes Mellitus8
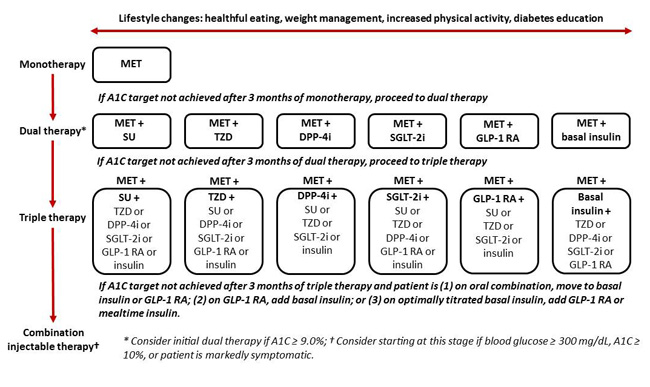
Abbreviations: A1C = hemoglobin A1c; DPP-4i = dipeptidyl peptidase-4 inhibitor;
GLP-1 RA = glucagon-like peptide-1 receptor agonist; MET = metformin;
SGLT-2i = sodium-glucose cotransporter-2 inhibitor; SU = sulfonylurea; TZD = thiazolidinedione.
Diabetes medications recommended for second-line treatment include SUs, TZDs, DPP-4 inhibitors, SGLT-2 inhibitors, GLP-1 receptor agonists, and basal insulin. It is important to note these are not listed in any order of preference and the ADA suggests that selection should be based on potency/efficacy, hypoglycemia risk, proclivity to induce weight gain, side effects, and cost. If a patient is unable to achieve or sustain glycemic control after 3 months on recommended dual therapy, the guidelines then recommend further intensification to triple therapy, which may include any of the aforementioned agents.8
INJECTABLE THERAPY OPTIONS AFTER BASAL INSULIN
Basal insulin alone is the most convenient initial insulin regimen for T2DM and it targets defects in 5 locations: pancreatic α-cells, pancreatic β-cells, liver, skeletal muscle, and adipose tissue.7 Patients can start with low doses of insulin, such as 0.1 to 0.2 units/kg/day or 10 units/day, to become comfortable and familiar with injection technique and insulin effects. In addition, clinicians can provide patients with a simple algorithm to self-titrate basal insulin doses to meet blood glucose goals. For example, patients can increase the basal insulin dose by 2 to 4 units (or 10% to 15%) every 3 to 4 days until the FPG target is reached. If hypoglycemia occurs, patients should decrease the dose by 4 units (or 10% to 15%).8,13
In general, the newer, longer-acting basal insulin analogs (glargine, detemir, degludec) have lower risks of hypoglycemia than the older, intermediate-acting Neutral Protamine Hagedorn (NPH) insulin. However, cost may be an issue, since the newer agents may be more expensive than older insulin products.8,14
Once basal insulin has been optimized, the question clinicians must consider is what to add next. The time to consider combination injectable therapy is when the total daily dose of basal insulin reaches more than 0.5 units/kg/day and the A1C remains above target.13 Metformin may be continued if there is no contraindication; however, if other oral agents are included in a patients' current regimen, discontinuation may be warranted to reduce complexity, adverse effects, or cost.
The 3 general combination injectable therapy options for patients with T2DM already using basal insulin are: 1) add a bolus (prandial) insulin, 2) add a GLP-1 receptor agonist, or 3) switch to a premixed basal/bolus combination insulin product. Consideration for each of these options should be discussed with the patient and the patient's views and preferences should be respected (Figure 5).8
Figure 5. Approach to Starting and Adjusting Insulin in Type 2 Diabetes Mellitus8
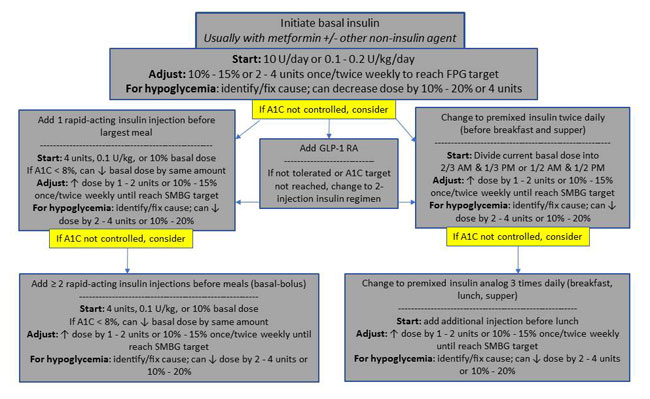
Abbreviations: A1C = hemoglobin A1c; GLP-1 RA = glucagon-like peptide-1 receptor agonist;
SMBG = self-monitoring of blood glucose.
A primary benefit of adding bolus insulin is that it is a tried and true therapy. Insulin is the oldest agent available for treating diabetes and its use has markedly improved over the past century since it first became available in 1922. Patients can start with a simple regimen, such as the addition of 1 bolus dose daily given 10 to 15 minutes prior to the largest meal of the day (which is usually supper for most people). The guidelines recommend starting with 4 units (0.1 unit/kg or 10% of the current basal insulin dose) of a rapid-acting insulin analog (lispro, aspart, glulisine).8,13 Rapid-acting insulins are preferred over short-acting (regular) insulin due to the prompt onset of action after dosing. It should be noted that, for patients with an A1C less than 8% when initiating bolus insulin, it is recommended to decrease the basal insulin by the same amount (4 units or 10%) in an effort to minimize the risk of hypoglycemia.8 The concept of "start low and titrate slow" allows patients to become comfortable with multiple insulin injections while minimizing side effects.13,15
For patients on basal insulin, clinicians can guide patients to self-titrate bolus insulin to meet blood glucose goals. The recommendation is to increase the rapid-acting insulin dose by 1 to 2 units (or 10% to 15%) once to twice weekly until premeal and/or PPG targets are achieved. If hypoglycemia occurs, the dose should be decreased by 2 to 4 units (or 10% to 20%).8,13 It is also important to identify and correct the source of hypoglycemia, such as skipping a meal, inadvertently taking the wrong insulin, or administering too much insulin.
The disadvantage of bolus insulin is that it has the highest risk of hypoglycemia compared to all other treatment options. In addition, it is notorious for causing weight gain, which is not a side effect that is well received by patients or clinicians. Medical nutrition therapy provided by a registered dietitian with expertise in diabetes is highly recommended for patients on insulin therapy.13 This will allow patients to learn and understand healthy eating, match their carbohydrate intake with their insulin dose, and, hopefully, minimize weight gain while maintaining appropriate blood glucose levels throughout the day.
If A1C and blood glucose goals are not achieved with the 1 bolus plus 1 basal insulin regimen, a patient can increase to 2 bolus insulin injections given prior to breakfast and supper plus 1 basal injection daily. Adjustments for hyperglycemia and hypoglycemia can be self-titrated, as previously mentioned. Finally, if A1C and blood glucose goals are not met with the 2 bolus plus 1 basal insulin regimen, increasing to a 3 bolus plus 1 basal insulin regimen is warranted.15 The 3 bolus plus 1 basal therapy is the closest match to endogenous insulin production that a patient can achieve, other than by using a continuous insulin infusion system (also known as an insulin pump).
Another option that can be added to optimized basal insulin therapy for patients requiring additional postprandial coverage is a GLP-1 receptor agonist. This combination (basal insulin plus a GLP-1 receptor agonist) is growing in popularity due to the many benefits and synergy of this dynamic duo. The GLP-1 receptor agonists target dysfunctional defects in 6 locations: pancreatic α-cells, pancreatic β-cells, liver, skeletal muscle, brain, and GI tract.7
However, there are notable differences among the available agents. Simply explained, there are short- acting and long-acting GLP-1 receptor agonists. The short-acting GLP-1 receptor agonists (exenatide twice daily and lixisenatide once daily) target PPG only, lower A1C by 0.8% to 1.5%, and must be dosed within 60 minutes of the morning meal.12,13 The long-acting GLP-1 receptor agonists (liraglutide once daily, exenatide once weekly, albiglutide once weekly, and dulaglutide once weekly) target FPG with some residual effect on PPG, resulting in a slightly larger decrease in A1C (0.8% to 1.8%). These agents can be dosed any time of the day, without regard to meals (Table 1).8,12,13
| Table 1: Dosing Chart for Select Non-insulin Therapies8,13 |
| GLP-1 receptor agonists |
| |
Exenatide (Byetta) |
Lixisenatide (Adlyxin) |
Liraglutide (Victoza) |
Exenatide extended-release (Bydureon) |
Albiglutide (Tanzeum) |
Dulaglutide (Trulicity) |
| Dose |
5 or 10 mcg BID (within 30 to 60 min of morning and evening meals) |
10 or 20 mcg once daily (within 60 min of same meal each day) |
Initiate at 0.6 mg daily for 1 week, then increase to 1.2 mg daily; may increase to 1.8 mg daily, if needed; can be taken any time of the day |
2 mg once weekly |
30 or 50 mg once weekly |
0.75 or 1.5 mg once weekly |
| Max dose |
10 mcg BID |
20 mcg daily |
1.8 mg daily |
2 mg weekly |
50 mg weekly |
1.5 mg weekly |
| FPG or PPG effects |
PPG |
PPG |
FPG and PPG |
FPG and PPG |
FPG and PPG |
FPG and PPG |
| DPP-4 inhibitors |
| |
Sitagliptin (Januvia) |
Saxagliptin (Onglyza) |
Linagliptin (Tradjenta) |
Alogliptin (Nesina) |
| Dose |
25 to 100 mg once daily |
2.5 to 5 mg once daily |
5 mg once daily |
6.25 to 25 mg once daily |
| Renal impairment dose adjustment |
CrCl ≥ 30 to ≤ 50 mL/min: 50 mg daily CrCl < 30 mL/min: 25 mg daily |
CrCl ≤ 50 mL/min: 2.5 mg daily |
No adjustment required |
CrCl ≥ 30 to < 60 mL/min: 12.5 mg daily CrCl ≥ 15 to < 30 mL/min: 6.25 mg daily |
| SGLT-2 inhibitors |
| |
Canagliflozin (Invokana) |
Dapagliflozin (Farxiga) |
Empagliflozin (Jardiance) |
| Dose |
100 mg once daily before first meal of the day; can increase to 300 mg once daily if eGFR ≥ 60 mL/min/1.73 m2 |
5 mg once daily before the first meal of the day; can increase to 10 mg once daily |
10 mg once daily before the first meal of the day; can increase to 25 mg once daily |
| Renal impairment considerations |
eGFR < 45 mL/min/1.73 m2: not recommended |
eGFR < 60 mL/min/1.73 m2: not recommended eGFR < 30 mL/min/1.73 m2: contraindicated |
eGFR < 45 mL/min/1.73 m2: not recommended eGFR < 30 mL/min/1.73 m2: contraindicated |
| Abbreviations: BID = twice daily; CrCl = creatinine clearance; DPP-4 = dipeptidyl peptidase-4; eGFR = estimated glomerular filtration rate; FPG = fasting plasma glucose; GLP-1 = glucagon like peptide-1; PPG = postprandial plasma glucose; SGLT-2 = sodium-glucose cotransporter-2. |
As noted, most GLP-1 receptor agonists are dosed once daily or once weekly, which minimizes the number of daily injections needed. The weight loss benefit commonly associated with all GLP-1 receptor agonists can counteract weight gain from basal insulin. Also, there is minimal risk of hypoglycemia associated with GLP-1 receptor agonists.12 Finally, on the basis of recent results of cardiovascular outcomes trials, a GLP-1 receptor agonist, liraglutide, has a favorable ADA review for patients with known cardiovascular risk factors.8
One study evaluated GLP-1 receptor agonists, instead of bolus insulin, in combination with basal insulin: the short-acting GLP-1 receptor agonist, exenatide, was compared to bolus insulin lispro. The results of the study revealed A1C reductions for both agents were nearly identical and exenatide was non-inferior to lispro.16,17 However, FPG was significantly lower (p = 0.002) in the exenatide group (117 mg/dL) than in the lispro group (130 mg/dL), and the exenatide group experienced significant weight loss (p = 0.001) while the lispro group experienced weight gain. It was also noted that the exenatide group experienced more GI effects than the lispro group (47% vs. 13%). The lispro group had more episodes of minor hypoglycemia (41% vs. 30%) and more confirmed nonnocturnal hypoglycemia (34% vs. 15%) than the exenatide group. More studies are currently underway regarding GLP-1 receptor agonist use in place of bolus insulin for T2DM.16
In November 2016, 2 different once-daily, fixed-dose GLP-1 receptor agonist plus basal insulin combination injectable products were approved by the U.S. Food and Drug Administration (FDA): insulin glargine U-100 plus lixisenatide (Soliqua 100/30) and insulin degludec U-100 plus liraglutide (Xultophy 100/3.6). Both combinations have been extensively studied and have demonstrated greater A1C reductions in combination than with either individual agent alone. In addition, because of the fixed-dose combination and gradual titration of the GLP-1 receptor agonist, GI side effects were markedly less frequent with the combination product than with the individual GLP-1 receptor agonist as monotherapy.
When switching from basal insulin to a fixed-dose combination, each product has specific starting dose instructions that are based on the current daily dose of basal insulin. For insulin glargine/lixisenatide, patients taking fewer than 30 units of insulin should be started on 15 units of the combination product. (The dose is based on the insulin component of the combination.) Patients taking 30 to 60 units of basal insulin daily should initiate insulin glargine/lixisenatide with a dose of 30 units.18,19 Patients can self- titrate 2 to 4 units once weekly on the basis of FPG. It is important to note that, since lixisenatide is a short-acting GLP-1 receptor agonist, it must be dosed 60 minutes before the first meal of the day. The insulin degludec/liraglutide starting dose is 16 units once daily, regardless of a patient's current basal insulin dose.20 (This starting dose is also based on the insulin component of the combination.) The combination can be self-titrated by 2 units every 3 to 4 days on the basis of FPG and can de dosed any time of day, with or without food. However, it is recommended that the injection be given at the same time every day.21,22 The main disadvantage of GLP-1 receptor agonists, as monotherapy or in fixed-dose combinations, is that these agents tend to be costly, especially with third party formulary plans.
The third option in the ADA combination injectable therapy guideline is to change the basal-only regimen to a premixed basal/bolus insulin (such as NPH/regular 70/30, NPH/lispro 75/25, or NPH/aspart 70/30). The advantage of premixed insulin is that it minimizes the number of injections a patient needs to self-administer daily. (Traditional basal plus bolus dosing requires 4 total daily injections.) Premixed insulin injections are typically given twice daily, before breakfast and prior to supper.15 The major disadvantage of this regimen is the increased risk of hypoglycemia from the intermediate-acting NPH. The pharmacokinetic profile of NPH has a peak time of action 6 to 8 hours after injection. For patients who may have inconsistent eating habits or who skip meals and snacks, there is an increased risk of hypoglycemia, usually in the late afternoon and nocturnal hours of the morning when NPH is peaking.13
The somogyi effect is pronounced in patients who use NPH insulin. This occurs when the NPH insulin exerts its effect during the early morning hours (e.g., 2 am or 3 am), resulting in a nocturnal low blood glucose level (e.g., less than 70 mg/dL). The body responds with increased glucagon release and gluconeogenesis, resulting in significantly elevated blood glucose that leads to an FPG that is well above target (e.g., higher than 200 mg/dL).13 To minimize the risk of the somogyi effect, patients can change the twice-daily, premixed regimen to a 3-injection schedule, with 1 premixed injection before breakfast, a short-acting or rapid-acting insulin injection before supper, and an NPH injection at bedtime.13,15 By moving the NPH to bedtime, its peak will occur during the waking hours, not during the night. However, it is imperative for a patient on this regimen to wake up and eat breakfast on schedule and not sleep late or skip breakfast to avoid morning hypoglycemia.
If a patient is still above the A1C target on a twice-daily premixed regimen, consider switching to premixed analog insulin (NPH/lispro 75/25, NPH/aspart 70/30) 3 times daily.8 In general, the 3-times- daily premixed analog regimen has been found to be non-inferior in terms of hypoglycemia rates to basal-bolus regimens, possibly due to further dividing the twice-daily dose into a 3-times-daily dose.
With any of the premixed regimens, patients must understand the importance of eating on schedule, as these regimens do not allow mealtime flexibility. For these reasons, the newer, long-acting basal insulins are preferred to the older NPH insulin. However, the cost of NPH tends to be significantly lower than the insulin analogs and, sometimes, clinicians need to work with patients on how to optimize treatment with NPH to get the best possible effects with the least amount of side effects.14
Medication and device use errors are prevalent among patients with T2DM. For all patients using injectable devices, it is critical for pharmacists to provide frequent medication and device checkups to ensure proper use and medication delivery. Patients should be instructed how to use injectable devices and then demonstrate back to the pharmacist the appropriate technique before using the device for the first time. Additionally, pharmacists should routinely assess patients' injection techniques during medical visits or when picking up refills to ensure correct use.
ORAL THERAPY OPTIONS AFTER BASAL INSULIN
Though the ADA guidelines do not provide a preference for which pharmacotherapy agent to add for dual or triple therapy, the clear message is that, if the A1C target is not achieved after 3 months of therapy, a change in the drug regimen is warranted.8 It is also noteworthy that the American Association of Clinical Endocrinologists/American College of Endocrinology guidelines recommend consideration of the use of 1 of 3 non-insulin options instead of bolus insulin as add-on to basal insulin therapy in T2DM: GLP-1 receptor agonists, SGLT-2 inhibitors, and DPP-4 inhibitors.23
When reviewing second-line or third-line therapy options, clinicians should recognize that most drugs target PPG, some drugs target FPG, and a few drugs target both.10 As previously described, the A1C level can indicate which blood glucose level (FPG or PPG) needs lowering, thereby guiding appropriate drug therapy selection.11
SUs "fix" only 1 dysfunctional defect in the pancreatic β-cells.7 Although SUs provide a notable A1C reduction (1.5% to 2.0%) and target both FPG and PPG, the elevated risk of hypoglycemia and weight gain associated with these agents do not make them an attractive option, especially in combination with basal insulin.24,25 In fact, the ADA guidelines support the discontinuation of SUs when starting insulin therapy in order to reduce the risk of hypoglycemia.
TZDs "fix" 3 dysfunctional defects in liver, skeletal muscle, and adipose tissue.7 TZDs target FPG and PPG with a notable A1C lowering (1.0% to 1.5%) and have a negligible risk of hypoglycemia. However, weight gain is a common side effect, as is edema and an increased risk of heart failure, especially when combined with insulin.24
DPP-4 inhibitors "fix" 4 of the dysfunctional defects in pancreatic α-cells, pancreatic β-cells, liver, and the incretin system in the GI tract.7 DPP-4 inhibitors primarily target PPG; therefore, the A1C reduction (0.4% to 0.7%) is not as robust as with agents that target FPG. DDP-4 inhibitors can be most effective when used in combination with drugs that target FPG, such as metformin and basal insulin.24 Table 1 reviews dosing recommendations and renal dose adjustments for DPP-4 inhibitors.8,13 The best outcomes for these agents are achieved in patients who do not require substantial A1C lowering. Key benefits of the DPP-4 inhibitors include weight neutrality and a relatively benign side effect profile of headache, nasopharyngitis, and upper respiratory infections.26
SGLT-2 inhibitors "fix" 1 defect in the kidney and, possibly, a second in adipose tissue.7 Since these agents work in the kidneys throughout the day, they continuously lower blood glucose and, therefore, target both FPG and PPG with a moderate reduction in A1C (0.7% to 1.1%). Table 1 reviews dosing recommendations and renal impairment considerations for SGLT-2 inhibitors.8,13 These agents have a weight loss benefit, and they are ideal as an add-on to basal insulin to counteract weight gain.27 Increased urination is a side effect of this class of drugs, so caution should be used in patients with a history of urinary tract infections, genital-urinary infections, and dehydration. Patients who are taking medications or have lifestyles that put them at increased risk of dehydration or fluid volume changes should avoid SGLT-2 inhibitors. Recently, an SGLT-2 inhibitor, empagliflozin, was granted an FDA cardiovascular safety indication for use in patients with diabetes who have known cardiovascular disease.28 Whether this is a class effect or drug-specific finding is yet to be determined.
SUMMARY
The keys to optimal glucose control and improved health outcomes in T2DM are early diagnosis and treatment with therapy that addresses as many of the underlying pathophysiologic abnormalities as possible. Despite the advances in diabetes treatment and care, many patients are still not meeting their therapeutic goals. Monotherapy treatment approaches are seldom successful and are rarely sustainable. Use of combination therapy is often the best approach to managing T2DM. The use of newer, non- insulin medications in combination with basal insulin are proving beneficial for controlling A1C, FPG, and PPG while, at the same time, minimizing hypoglycemia and providing weight loss or neutrality.
REFERENCES
- Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Published 2014. Accessed February 1, 2017.
- American Association of Clinical Endocrinologists. State of Diabetes Complications in America. http://multivu.prnewswire.com/mnr/AACE/2007/docs/Diabetes_Complications_Report.pdf. Published 2007. Accessed February 1, 2017.
- Ramlo-Halsted BA, Edelman SV. The natural history of type 2 diabetes: practical points to consider in developing prevention and treatment strategies. Clin Diabetes. 2000;18(2):80-85.
- Nathan DM. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347(17):1342-1349.
- DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773-795.
- Spijkerman AM, Dekker JM, Nijpels G, et al. Microvascular complications at time of diagnosis of type 2 diabetes are similar among diabetic patients detected by targeted screening and patients newly diagnosed in general practice: the hoorn screening study. Diabetes Care. 2003;26(9):2604-2608.
- DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care. 2013;36(Suppl 2):S127-138.
- American Diabetes Association. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2017;40(Suppl 1):S64-S74.
- Bailey CJ. Safety of antidiabetes medications: an update. Clin Pharmacol Ther. 2015;98(2):185-195.
- Burke S, Cornell S. Medication management in type 2 diabetes. Clinician Reviews. 2008;18(3):28-34.
- Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26(3):881-885.
- Cornell S, Dorsey VJ. Diabetes pharmacotherapy in 2012: considerations in medication selection. Postgrad Med. 2012;124(4):84-94.
- Sisson EM, Zimmerman KM. Pharmacotherapy for glucose management. In: The Art and Science of Diabetes Self-Management Education. 4th ed. American Association of Diabetes Educators. 2017;18:469-516.
- Hirsch IB. Insulin in America: a right or a privilege? Diabetes Spectr. 2016;29(3):130-132.
- Mayfield JA, White RD. Insulin therapy for type 2 diabetes: rescue, augmentation, and replacement of beta-cell function. Am Fam Physician. 2004;70(3):489-500.
- Diamant M, Nauck MA, Shaginian R, et al; 4B Study Group. Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care. 2014;37(10):2763-2773.
- Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384(9961):2228-2234.
- Soliqua 100/33 [prescribing information]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2016.
- Rosenstock J, Diamant M, Aroda VR, et al; LixiLan PoC Study Group. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of lixisenatide and insulin glargine, versus insulin glargine in type 2 diabetes inadequately controlled on metformin monotherapy: the LixiLan proof-of-concept randomized trial. Diabetes Care. 2016;39(9):1579-1586.
- Xultophy 100/3.6 [prescribing information]. Bagsvaerd, Denmark: Novo Nordisk A/S; 2016.
- Gough SC, Bode B, Woo V, et al; NN9068-3697 (DUAL-I) Trial Investigators. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885-893.
- Rodbard HW, Buse JB, Woo V, et al. Benefits of combination of insulin degludec and liraglutide are independent of baseline glycated haemoglobin level and duration of type 2 diabetes. Diabetes Obes Metab. 2016;18(1):40-48.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2017 executive summary. Endocr Pract. 2017;23(2):207-238.
- Bergenstal RM, Bailey CJ, Kendall DM. Type 2 diabetes: assessing the relative risks and benefits of glucose-lowering medications. Am J Med. 2010;123(4):374.e9-e18.
- Nathan DM, Buse JB, Kahn SE, et al; GRADE Study Research Group. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36(8):2254-2261.
- White WB, Pratley R, Fleck P, et al. Cardiovascular safety of the dipetidyl peptidase-4 inhibitor alogliptin in type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15(7):668-673.
- List JF, Woo V, Morales E, et al. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32(4):650-657.
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG Outcome Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128.
Back to Top