
Expired activity
Please go to the PowerPak
homepage and select a course.
Module 4. Overview of Non-Injectable Glucose-Lowering Agents
INTRODUCTION
Diabetes management requires a multifaceted approach, with the goals of treatment being to manage symptoms of hyperglycemia, prevent complications (acute and chronic), and improve the patient’s quality of life. The progression of diabetes is associated with many comorbid conditions, including but not limited to hypertension, hypercholesterolemia, cardiovascular disease, retinopathy, diabetic kidney disease, neuropathy, non-alcoholic fatty liver disease (NAFLD), gum disease, hearing loss, erectile dysfunction, and depression.1
Two widely utilized diabetes treatment guidelines are published by the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE). Both organizations published updated guideline documents in 2019.2,3 Table 1-1 illustrates the glycemic targets of each guideline. It is important to note that both guidelines recommend individualized targets depending on several patient factors. A more stringent hemoglobin A1c (A1C) target may be recommended for individuals that have a short diabetes duration, long life expectancy, type 2 diabetes (T2D) treated with lifestyle or metformin only and no significant cardiovascular complications. A less stringent target (such as < 8% as an example) may be appropriate for patients that have a history of severe hypoglycemia, limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbidities, or long-term diabetes in whom general A1C targets are difficult to attain.2,3 As an example, an elderly individual with kidney disease and neuropathy may be prone to falls from hypoglycemia. This patient would likely benefit from a less stringent A1C goal (such as < 8% for example). All therapy decisions need to come from the health care team after evaluation of the patient as a whole. In general, once an agent is added to the patient’s regimen, the A1C should be re-evaluated in about 3 months. If that number is still not at goal, the treatment approach should be re-evaluated and adjusted based on patient-specific considerations. In cases where a patient's A1C is substantially elevated at diagnosis, both guidelines recommend a more intensive initial approach to treatment. AACE/ACE recommends initial dual therapy when baseline A1C is greater than 7.5%, while ADA guidelines state that initial dual therapy can be considered when the baseline A1C is ≥1.5% above the individualized A1C goal.2,3 Guidelines and recommendations from ADA and AACE/ACE differ in approach, but are alike in more ways than they differ.
| Table 1-1: Glycemic Targets Recommended by Current ADA and AACE/ACE Guidelines2,3 |
| |
ADA |
AACE/ACE |
| A1C |
< 7.0% |
≤ 6.5% |
| Fasting (preprandial) plasma glucose |
80 – 130 mg/dL |
< 110 mg/dL |
| Peak postprandial glucose |
< 180 mg/dL |
< 140 mg/dL |
*Note: Goals represent appropriate goals for many nonpregnant adults. Goals should be individualized based on duration of diabetes, age/life expectancy, comorbid conditions, complications, hypoglycemia unawareness, etc.
ADA = American Diabetes Association; AACE/ACE = American Association of Clinical Endocrinologists/American College of Endocrinology; A1C = glycated hemoglobin |
Figure 1 provides a simplified representation of the 2019 ADA algorithm for use of glucose-lowering medications in T2D.2 As noted in the Figure, oral glucose-lowering medications play an important role in T2D management, as is also true in the AACE/ACE guidelines. In general, when choosing pharmacotherapy for a specific patient, one must consider patient- and medication-specific considerations including potential adverse effects, contraindications, drug interactions, ease of administration, comorbid conditions (such as comorbid atherosclerotic cardiovascular disease [ASCVD], heart failure [HF] and chronic kidney disease [CKD]), and cost. This module will explore the mechanisms of action, efficacy, adverse events, and contraindications of all major oral agents currently available for the management of T2D. A focus will be placed on patient- and medication-specific factors that should be considered within each oral glucose-lowering medication class.
Figure 1
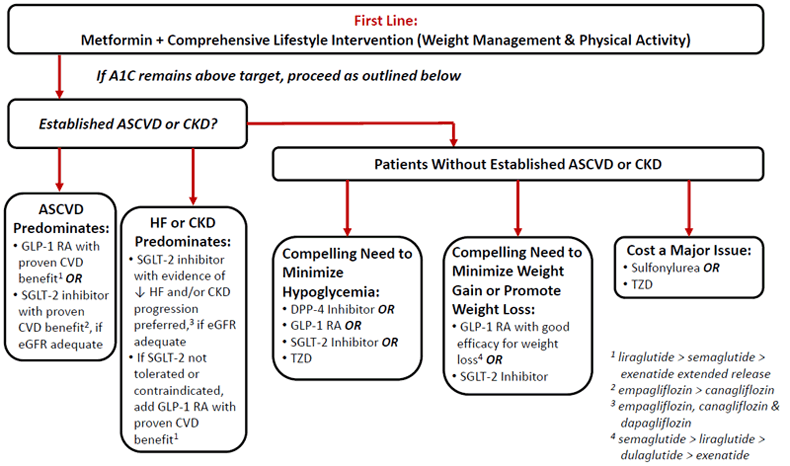 |
BIGUANIDES
Metformin, the only biguanide on the market, is the preferred first-line glucose-lowering agent for the treatment of patients with T2DM in both the ADA and AACE/ACE guidelines due to its effectiveness, long history of safety, and cost. It is also recommended by both organizations as a potential adjunct to lifestyle modification in people with prediabetes to prevent conversion to T2D.2,3 The ADA additionally recommends that once initiated, metformin should be continued as long as it is tolerated and not contraindicated. Metformin works primarily to reduce blood glucose concentrations by decreasing hepatic gluconeogenesis and increasing glucose uptake by muscle and adipose tissue.4 While the degree to which any glucose-lowering agent lowers A1C in a given patient can vary greatly, metformin is generally expected to reduce A1C by up to 1% to 1.5%.5 Metformin use also has the added benefit of reducing cholesterol and triglyceride levels and may even increase high-density lipoprotein (HDL) levels.6
To minimize side effects, metformin is generally initiated at either 500 mg twice daily or 850 mg once daily to be taken with food; even lower initial doses may be warranted if adverse gastrointestinal (GI) events are a concern.6 The usual maintenance dose is 500 to 1000 mg twice daily.7 Renal function should be assessed prior to and periodically during metformin therapy. Initiation of therapy is not recommended for patients with a baseline estimated glomerular filtration rate (eGFR) of 30-45 mL/min/1.73 m2. If a patient's eGFR falls below 45 mL/min/1.73 m2 during therapy, an assessment of metformin's risks and benefits is warranted, and a dose reduction should be considered. Metformin is contraindicated in patients with an eGFR below 30 mL/minute/1.73 m2 at any time.6
Metformin carries a black box warning related to the risk of lactic acidosis, though a Cochrane review of 347 studies found no difference in risk between metformin therapy and either placebo or non-biguanide therapies.8 Although lactic acidosis is rare with metformin, renal impairment, advanced age, radiological contrast dye, hypoxic states (e.g., acute congestive heart failure), hepatic impairment, and excessive alcohol intake increases this risk. Co-administration with cationic agents, such as dofetilide may result in altered levels of either drug, so caution and appropriate monitoring is warranted with coadministration.6
The most common adverse effects associated with metformin use include diarrhea, nausea, abdominal discomfort, metallic taste, flatulence, and anorexia. Diarrhea is the most common complaint and may be avoided by using an extended-release formulation. Metformin may also decrease the absorption of vitamin B12, so it may be wise to monitor B12 levels for certain patients on metformin therapy and to add vitamin supplementation to the individual’s regimen, if necessary. The ADA specifically recommends that B12 levels should be periodically measured, especially in those with symptoms of anemia or peripheral neuropathy.2 The risk of hypoglycemia with metformin is quite low when used as monotherapy, but the risk rises when utilized in combination with agents that can contribute to hypoglycemia, such as insulin and insulin secretatogues.6 As noted above, metformin is contraindicated in people with severe renal impairment (eGFR below 30 mL/min/1.73 m2), a known hypersensitivity to metformin, or acute or chronic metabolic acidosis, including diabetic ketoacidosis (DKA).6
SODIUM-GLUCOSE COTRANSPORTER 2 (SGLT2) INHIBITORS
Medications in this class are dapagliflozin (Farxiga), canagliflozin (Invokana), empagliflozin (Jardiance), and ertugliflozin (Steglatro). SGLT2 inhibitors work by reducing reabsorption of glucose in the kidney, which results in greater urinary excretion of glucose.9 In addition to lowering A1C, they also may promote weight loss, increase HDL, and decrease blood pressure.10 The role of SGLT2 inhibitors in the management of T2D and its complications is expanding as we learn more about the cardiovascular and renal benefits of use in certain patient populations. As outlined in Figure 1, the ADA currently recommends SGLT2 inhibitors (specifically empagliflozin or canagliflozin) as an option for add-on to metformin in patients with established ASCVD or CKD.2 SGLT2 inhibitors (empagliflozin, canagliflozin, or dapagliflozin) are additionally preferred in patients where HF or CKD predominates, provided that they have adequate renal function for use.2
Dapagliflozin is available as 5- and 10-mg tablets, with an initiation dose of 5 mg daily in the morning, with or without food. The maximum dose is 10 mg once per day. Dapagliflozin is not recommended for use in patients with an eGFR of less than 45 mL/min/1.73 m2.11 Canagliflozin is available in 100- and 300-mg tablets. The recommended initiation dose is 100 mg once daily before the day’s first meal. The maximum dose is 300 mg once daily for patients with an eGFR of greater than 60 mL/min/1.73 m2 and 100 mg once a day for individuals with an eGFR of 45 to 60 mL/min/1.73 m2. This agent is currently not recommended for use in patients with an eGFR of less than 45 mL/min/1.73 m2.12 Empagliflozin is available as 10- and 25-mg tablets, with a recommended initiation dose of 10 mg once daily in the morning, with or without food. The maximum dose is 25 mg once a day. Empagliflozin is not recommended for use in patients with an eGFR of less than 45 mL/min/1.73 m2.13 Ertugliflozin is available in 5- and 15-mg tablets and is recommended for initiation at 5-mg once daily, taken in the morning with or without food. The dose can be increased to 15-mg as tolerated and needed for glycemic control.14 Ertugliflozin use is contraindicated in people with an eGFR less than 30 mL/min/1.73 m2 and the manufacturer does not recommend starting the drug in people with an eGFR less than 60 mL/min/1.73 m2.
Dapagliflozin is a weak substrate of P-gp, so levels of this drug may be affected by strong inhibitors or inducers of P-gp. In addition, dapagliflozin levels may be reduced by coadministration with rifampin and increased by combining it with mefenamic acid.11 Likewise, canagliflozin levels may be lessened by coadministration with rifampin or other inducers. Canagliflozin may increase levels of digoxin, potentially leading to digoxin toxicity, so increased monitoring of digoxin levels is recommended.12 Neither empagliflozin or ertugliflozin have demonstrated any meaningful pharmacokinetic drug interactions at this time.13,14 All SGLT2 inhibitors may reduce blood pressure (BP).11-14 Therefore, doses of BP medications, especially diuretics, may have to be decreased when an SGLT2 inhibitor is initiated.
The most commonly reported adverse effects associated with SGLT2 inhibitors are urinary tract infections (UTIs) and female genital mycotic infections caused by increased glucose exposure in the genitourinary tract. SGLT2 inhibitors are contraindicated for use in patients with hypersensitivity to the product or severe renal impairment or end-stage renal disease.11-14 Dapagliflozin has also been associated with an increased risk of bladder cancer and should be avoided in people with a history of bladder cancer.11 The U.S. Food and Drug Administration (FDA) issued a safety announcement in December 2015 warning patients and providers about an increased risk of ketoacidosis and serious, potentially life-threatening UTIs associated with SGLT2 inhibitors. Be sure to advise patients taking SGLT2 inhibitors of the symptoms of ketoacidosis and UTIs so they know when to seek medical attention. Symptoms of ketoacidosis include nausea, vomiting, abdominal pain, tiredness, and trouble breathing. UTI symptoms include a feeling of burning when urinating, increased frequency or urgency of urination, lower abdominal or pelvic pain, fever, or blood in the urine. The FDA is now requiring that manufacturers of these medications conduct a 5-year pharmacovigilance study to collect additional information on the link between SGLT2 inhibitors and ketoacidosis.15 All SGLT2 inhibitors also now carry a warning about the increased risk of acute kidney injury. Risk factors for this adverse effect include decreased blood volume; chronic kidney insufficiency; congestive HF; and taking other medications that affect the kidneys (e.g., blood pressure medications and non-steroidal anti-inflammatory drugs).16 In post-marketing studies, canagliflozin has also demonstrated an increased risk of bone fractures and an increased risk of foot and leg amputation.17,18 Canagliflozin now carries black-box warnings related to amputations and fractures. Studies have not yet demonstrated these adverse effects as class effects for SGLT2 inhibitors.
DIPEPTIDYL PEPTIDASE-4 (DPP-4) INHIBITORS
Medications in this class include sitagliptin (Januvia), saxagliptin (Onglyza), linagliptin (Tradjenta), and alogliptin (Nesina). The ADA recommends DPP-4 inhibitors as an option for add-on to metformin in patients who have a compelling need to minimize hypoglycemia.2 The AACE/ACE guidelines list DPP-4 inhibitors as potential first-line agents and they are fourth in order of preference.3 These medications work by inhibiting the DPP-4 enzyme that is responsible for degradation of the endogenous incretins gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1), hormones acting to increase insulin secretion, decrease glucagon secretion, slow gastric emptying, and increase satiety.4,19 When compared to other glucose-lowering agents, DPP-4 inhibitors have a relatively modest impact on A1C but can be beneficial in people who are close to A1C goal but still have postprandial glycemic excursions. The benefits of DPP-4 inhibitors include their oral administration, low risk of hypoglycemia, excellent general tolerability profile, and neutral effect on weight.
Sitagliptin is available as 25-, 50-, and 100-mg tablets. The initiation and maintenance dose for most adults is 100 mg once daily, but that should be reduced to 50 mg per day in patients with a creatinine clearance (CrCl) of 30 to 50 mL/min and 25 mg daily in patients with a CrCl of less than 30 mL/min.20 Saxagliptin is available as 2.5- and 5-mg tablets. The initiation and maintenance dose may be either 2.5 or 5 mg once daily and should not exceed 2.5 mg a day in patients with a CrCl of less than 50 mL/min.21 Alogliptin is available as 6.25-, 12.5-, and 25-mg tablets. The initiation and maintenance dose for most adults is 25 mg once daily and should be reduced to 12.5 mg daily for patients with a CrCl 30 to 60 mL/min and to 6.25 mg once per day for those with a CrCl of less than 30 mL/min.22 Linagliptin is available in 5-mg tablets, with an initiation and maintenance dose of 5 mg once daily. It is the only medication in this class that does not require dose adjustment for those with renal impairment.23 All medications in this class can be taken with or without food. Importantly, if any of these agents are added to the regimen of a patient currently taking an insulin secretagogue (sulfonylurea or meglitinide), the dose of the insulin secretagogue may have to be decreased to reduce the risk of hypoglycemia.20-23
Sitagliptin coadministration with digoxin results in increased digoxin levels, so monitoring of the digoxin level may be necessary.20 Saxagliptin is a substrate of CYP 3A4/5. Coadministration with strong CYP 3A4/5 inhibitors will increase the concentration of saxagliptin. It is recommended that the dose of saxagliptin be limited to 2.5 mg daily for patients taking strong CYP 3A4/5 inhibitors.21 It is also logical that strong inducers of CYP 3A4/5 would decrease saxagliptin concentrations, though specific interactions have not been identified. Linagliptin is a substrate of P-glycoprotein pumps (P-gp) and CYP 3A4. Concurrent administration with strong inducers (e.g., rifampin) will decrease levels of linagliptin.23 Also logical is that strong inhibitors of P-gp and/or CYP 3A4 tend to increase concentrations of linagliptin. No clinically meaningful drug interaction with alogliptin has been identified.22 Given the mutual effects of DPP-4 inhibitors and GLP-1 receptor antagonists on the incretin system, drugs from the two classes should not be used in combination with each other.
As noted previously, these agents are generally well-tolerated, but adverse events may include headache, nasopharyngitis, and hypoglycemia, though the risk of hypoglycemia is relatively low with these medications.7,20-23 There have been some reports of pancreatitis associated with the use of DPP-4 inhibitors, but causality has not been demonstrated.7 The FDA has additionally issued two recent safety communications related to DPP-4 inhibitors.24,25 All available DPP-4 inhibitors now carry a warning related to joint pain that can be severe and disabling. This warning was based on 33 cases of severe arthralgia reported to the FDA over a period of about 7 years as well as 7 published case reports. Reports indicated that joint pain was typically relieved in less than a month following discontinuation of the DPP-4 inhibitor.26 A warning has also been added to the labels of saxagliptin and alogliptin related to an increased risk of HF, particularly in patients with existing heart or kidney disease.27 The FDA reviewed two large cardiovascular outcomes trials in patients with heart disease. The saxagliptin trial found that 3.5% of patients in the saxagliptin group were hospitalized for HF compared to 2.8% of patients in the placebo group. Similarly, the alogliptin trial found that 3.9% of alogliptin patients were hospitalized for HF compared to 3.3% of placebo patients.
The only contraindication to employing DPP-4 inhibitors is hypersensitivity to the product.20-23 These agents are weight-neutral, making a DPP-4 inhibitor a compelling choice for patients who are overweight or obese. When considering the addition of a DPP-4 inhibitor to a person’s regimen, also give thought to the patient’s renal function as well as the anticipated A1C lowering effect.
THIAZOLIDINEDIONES (TZDS)
Medications in this class are pioglitazone (Actos) and rosiglitazone (Avandia). TZDs are noted as options within the ADA guidelines for add-on to metformin when there is a compelling need to minimize hypoglycemia or when cost is a major consideration.2 The AACE/ACE guidelines list TZDs as potential agents for use as monotherapy, dual therapy or triple therapy, but they are given relatively low preference and the guidelines recommend caution when prescribing these medications.3 That said, there is growing interest in TZD use due to evidence that pioglitazone can reduce/improve nonalcoholic steatohepatitis.28
The TZDs are considered insulin sensitizers, meaning that they enhance the responsiveness of adipose and muscle tissues to the action of insulin. This is primarily facilitated by interaction of TZDs with peroxisome proliferator-activated receptor-γ (PPAR-γ). Activation of this receptor results in increased transcription of a glucose transporter called GLUT-4. The increased number of GLUT-4 transporters on the cell surface results in increased glucose uptake by these cells in response to insulin.29 Because the mechanism of action relies on the transcription of new transporters, the maximal glycemic effects of the TZDs may not be seen for 4 to 8 weeks. Interaction of these agents with the PPAR family of receptors also provides the beneficial effects of raising HDL levels and lowering triglycerides.30 This positive effect on the lipid profile became a subject for debate when it was announced that rosiglitazone was associated with an increased risk of myocardial infarction (MI).31 These findings led to the institution of a risk evaluation and mitigation strategy (REMS), which severely restricted the distribution of rosiglitazone-containing products. Subsequent study, however, has confirmed that there is no increased risk of MI with rosiglitazone use and the dispensing restrictions were lifted.32 The REMS program was removed entirely in December 2015.33 Pioglitazone has been associated with an elevated risk of bladder cancer, though results of epidemiological studies and controlled trials have been mixed. The FDA warns against use of pioglitazone in patients with active bladder cancer and advises caution in patients with a history of bladder cancer.34
Pioglitazone is available as 15-, 30-, and 45-mg tablets and is typically initiated at a dose of 15 or 30 mg once daily. The maximum dose per day is 45 mg, for most patients, or 15 mg for those taking strong CYP 2C8 inhibitors such as gemfibrozil.35 Lower doses are likewise recommended in patients also taking insulin due to an increased risk of fluid retention and edema. Rosiglitazone is available as 2-, 4-, and 8-mg tablets and is customarily initiated at a dose of 4 mg daily. The maximum dose per day is 8 mg.36
Both pioglitazone and rosiglitazone are substrates of CYP 2C8. Thus, coadministration of either medication with inducers (e.g., phenobarbital) or inhibitors (e.g., gemfibrozil) of CYP 2C8 will decrease or increase levels of the TZD, respectively.35,36
The most commonly reported adverse effects associated with TZD therapy are weight gain and edema.4,7,29 Consider the weight-gain potential of any drug used to treat diabetes prior to recommending it. Weight loss and maintenance are quite important for glycemic control as well as management of many other comorbid conditions. Medications that are weight-neutral or promote weight loss are generally considered preferable to those agents that cause weight gain in patients with diabetes. There have also been postmarketing reports linking TZD use to macular edema and an increased risk of fractures. TZDs are contraindicated in patients with New York Heart Association (NYHA) class III or IV HF or hypersensitivity to the product.35,36 Those with NYHA class I or II HF may still experience edema, which may be worsened by the administration of TZDs. For this reason, TZDs should be avoided or used with extreme caution in any patient with HF.
SULFONYLUREAS
Medications in this class are divided into first- and second-generation agents, of which the second-generation agents are used almost exclusively. First-generation sulfonylureas include chlorpropamide (Diabinese), tolazamide (Tolinase), and tolbutamide (Orinase). Second-generation agents encompass glyburide (Diabeta, Glynase PresTab), glimepiride (Amaryl), and glipizide (Glucotrol). The ADA guidelines include sulfonylureas as an option for add-on to metformin when cost is a major consideration.2 The AACE/ACE guidelines give the sulfonylureas the lowest recommendation for use for monotherapy, dual therapy, and triple therapy in consideration of hypoglycemia risk, risk of weight gain, and a lack of durability of efficacy over time.3
Sulfonylureas act as potassium channel blockers on the β cells of the pancreas. This causes the cells to carry a more positive membrane potential at rest, easing the ability of the cells to depolarize. This leads to enhanced β-cell sensitivity to glucose and increased insulin secretion.37 Sulfonylureas can have substantial impact on A1C, with a potential A1C reduction of 1% to 1.5%.5 Because these medications primarily improve hyperglycemia by enhanced pancreatic β-cells secretion of insulin, they become less effective as diabetes progresses and pancreatic β-cell function decreases.7
Generally speaking, sulfonylureas are initiated at a low dose and slowly titrated upward based on effect and tolerability. These medications should be taken with meals. Administration on an empty stomach will increase the risk of hypoglycemia. Glimepiride is available as 1-, 2-, and 4-mg tablets. The usual starting dose is 1 or 2 mg once daily. The usual maintenance dose is 1 to 4 mg per day, with a maximum dose of 8 mg daily.38 The immediate-release form of glipizide is available as 5- and 10-mg tablets. The usual starting dose is 5 mg daily, though doses of 2.5 mg may be necessary to prevent hypoglycemia. The maximum dose per day is 40 mg, but individual doses should not exceed 15 mg.39 Glipizide is also available as an extended-release formulation for once-daily dosing. Glyburide is manufactured in regular and micronized formulations. The two formulations are not bioequivalent and, as such, are not therapeutically interchangeable. If a patient is switched from one formulation to the other, re-titration is likely necessary.40 Depending on the formulation of glyburide used and patient tolerability, the dose may range from 1.25 to 20 mg a day.5 The maximum daily dose for the micronized formulation is 12 mg.40 All second-generation sulfonylureas may be dosed once or twice daily.
Sulfonylureas are highly protein-bound. As such, coadministration of sulfonylureas with other highly protein-bound drugs may lead to hypoglycemia.38-40 Aspirin may elevate levels of glimepiride.38 Ciprofloxacin could potentiate the hypoglycemic action of glyburide.40 Sulfonylureas are also CYP 2C9 substrates; thus, coadministration with inhibitors (such as metronidazole and fluconazole) or inducers of CYP 2C9 will increase or decrease the levels of sulfonylureas, respectively.41
The most common adverse effects associated with sulfonylureas are hypoglycemia and weight gain.4,7 Hypoglycemia is more common with this drug class than with many of the other oral agents that are available. Sulfonylureas should be avoided in patients with a concerning history of hypoglycemic events, especially in those with hypoglycemia unawareness. Also consider that weight gain is among the most common comorbid conditions in individuals with diabetes. Given that weight management is an especially important lifestyle modification in diabetes, considering alternative agents that are weight-neutral or promote weight loss may be wise. That said, sulfonylureas are available generically and are considerably more affordable than many glucose-lowering agents. Their use may be necessary in people with T2D who cannot afford more expensive options. All sulfonylureas are contraindicated in patients with hypersensitivity to the product or who have DKA.38-40
MEGLITINIDES
Non-sulfonylurea insulin secretagogues, or meglitinides, include repaglinide (Prandin) and nateglinide (Starlix). These agents carry the same general cautions as the sulfonylureas. Like sulfonylureas, these medications work by blocking potassium channels on β cells in the pancreas, simplifying the depolarization of cells and, thereby, leading to increased insulin secretion. The drugs have a more rapid onset of action and a shorter duration of action than sulfonylureas. For this reason, these agents are taken at mealtime to target postprandial blood glucose excursions.47
Because of their rapid onset of action, meglitinides are taken within 30 minutes of starting a meal and generally 3 times daily. If a meal is skipped, then that meglitinide dose should also be skipped because taking a meglitinide on an empty stomach may result in hypoglycemia. Nateglinide is available as 60- and 120-mg tablets. The usual initiation and maintenance dose of nateglinide is 120 mg 3 times daily, although a dose of 60 mg 3 times per day may be used in patients who are close to meeting their A1C goal at initiation.52 Repaglinide is available as 0.5-, 1-, and 2-mg tablets, with the initiation dose depending on the patient’s A1C. If that number is less than 8%, the recommended initiation dose is 0.5 mg 3 times daily; if the A1C is 8% or higher, the recommended initiation dose is 1 or 2 mg 3 times a day. The maximum total daily dose is 16 mg.43
Nateglinide is a CYP 2C9 substrate.42 Thus, coadministration of nateglinide with inhibitors or inducers of CYP 2C9 may increase or decrease levels of nateglinide, respectively. Repaglinide is a substrate of both CYP 2C8 and 3A4. Coadministration of repaglinide with gemfibrozil causes dangerously elevated levels of repaglinide. Itraconazole, ketoconazole, trimethoprim, cyclosporine, simvastatin, and clarithromycin are examples of medications that may also increase repaglinide levels, though not as much as gemfibrozil. Repaglinide concentrations are decreased by coadministration with rifampin.43
The most common adverse events are hypoglycemia and weight gain.4 Thus, like sulfonylureas, these medications would not be ideal choices for those with concerning hypoglycemia or obesity. The frequency of dosing required with meglitinides may also be a barrier for some patients. Meglitinides are contraindicated for use in patients with hypersensitivity to the product, T1D, or DKA.42,43 Additionally, repaglinide is contraindicated for coadministration with gemfibrozil.43
ALPHA-GLUCOSIDASE INHIBITORS
Alpha-glucosidase inhibitors include acarbose (Precose) and miglitol (Glyset). 2 Agents in this class are rarely used in the U.S. due to their GI intolerance. They work by inhibiting alpha-glucosidase, an enzyme located in the small intestine. This enzyme breaks down polysaccharides and disaccharides into absorbable monosaccharides such as glucose.44
Both agents in this class are available as 25-, 50-, and 100-mg tablets. The recommended initiation dose for both agents is 25 mg dosed 3 times daily with meals; but patients who experience intolerable GI side effects may benefit from an even lower initiation dose of 25 mg once per day. As tolerated, and as necessary for glycemic control, the dose may be increased to a maximum of 100 mg 3 times daily with meals.44,45 The maximum dose of acarbose is lowered to 50 mg 3 times a day for individuals who weigh less than 132 lb (60 kg). This is traceable to the risk of elevated serum transaminase (AST and ALT) in these patients.44
Since these agents work by delaying the absorption of glucose, it is possible that they will also slow the absorption of other drugs during coadministration. For instance, both acarbose and miglitol have been shown to reduce levels of digoxin after they are used in tandem. Appropriate monitoring and dose adjustment of digoxin may be necessary. In addition, the effect of alpha-glucosidase inhibitors may be lessened by coadministration with adsorbents, such as charcoal or digestive enzyme preparations.44,45
The most common adverse events are flatulence, diarrhea, and abdominal bloating, which may be minimized by initiating at a low dose and titrating upward slowly.4,7 Hypoglycemia risk is low, but if it occurs in a patient taking an alpha-glucosidase inhibitor, the episode must be treated with glucose (e.g., glucose tablets), not sucrose (e.g., table sugar) because alpha-glucosidase is not available to convert other sugars to the rapidly absorbable glucose. Both agents are contraindicated in those with hypersensitivity to the product, DKA, inflammatory bowel disease, or other chronic intestinal diseases affecting digestion or absorption, or conditions that may deteriorate as the result of gas formation in the intestine.44,45 These medications are not commonly used because of the burdensome side-effect profile and relatively minor effect on A1C. Alpha-glucosidase inhibitors may still be beneficial for some patients, but consider the expected A1C reduction and weigh that against the person’s quality of life while taking the medication.
COLESEVELAM
Colesevelam (Welchol) is a bile acid sequestrant that is approved for the treatment of T2D. Colesevelam is thought to work by reducing hepatic glucose production, increasing incretin levels, and decreasing glucose absorption in the GI tract.4 Colesevelam is available as 625-mg tablets or as 3.75-g packets for reconstitution as a suspension in water, fruit juice, or a soft drink. Regardless of dosage form, colesevelam should be taken with meals. The initiation and maintenance dose is 3.75 g once daily or in 2 divided doses.46
Colesevelam may reduce the absorption of some other drugs, including other oral hypoglycemic medications. The manufacturer recommends taking other medications 4 hours before colesevelam. The drug may also cause increased levels of metformin when co-administered with extended-release formulations.46
The most common adverse effects are constipation, nausea, and elevated triglycerides.4 The drug is contraindicated in patients with a history of bowel obstruction, serum triglycerides greater than 500 mg/dL, or a history of hypertriglyceridemia-induced pancreatitis.46 The use of colesevelam for the treatment of diabetes has been quite limited, perhaps because of the high pill burden, the large size of the tablets, and the comparatively small effect on A1C.
BROMOCRIPTINE
Bromocriptine (Cycloset) is a dopamine receptor agonist. It is listed as a second-line agent in the AACE/ACE guidelines, but it is given low preference.2 This formulation is not interchangeable with Parlodel or its generic bromocriptine formulations. The agent is not included in the ADA-guidelines treatment algorithm and it is unclear how bromocriptine improves glycemic control. The drug is thought to act centrally to regulate metabolism and increase insulin sensitivity.4 Bromocriptine is available as 0.8-mg tablets for glycemic control, with an initial dose of 0.8 mg daily. That dose is increased weekly by 1 tablet per day to the maximum tolerated dose of between 1.6 and 4.8 mg a day in the morning.47
Bromocriptine is highly protein-bound. Therefore, coadministration with other highly protein-bound drugs may result in elevated levels of those drugs. Combining this medication with ergot derivatives may reduce the effectiveness of these drugs while increasing such side effects as nausea, vomiting, and fatigue. The manufacturer recommends not using ergot derivatives within 6 hours of bromocriptine, which is metabolized by CYP 3A4. Thusly, coadministration with inducers or inhibitors of CYP 3A4 will decrease or increase levels of bromocriptine, respectively. In addition, the concurrent taking of bromocriptine with sympathomimetic drugs may result in hypertension and tachycardia, so simultaneous use should be limited and not exceed 10 days’ duration.47
The most common negative side effects are dizziness, syncope, and nausea.6 Bromocriptine should be recommended with food to help reduce the adverse GI events. The agent is contraindicated in patients with hypersensitivity to the product or syncopal migraines, as well as in women who are nursing.47 In practice, the use of bromocriptine to improve glycemic control is very limited because there are many more effective options available.
COMBINATION THERAPY
Because the use of multiple glucose-lowering medications is often required to meet individualized glycemic goals, numerous products containing fixed-dose combinations of oral diabetes medications are now available to decrease pill burden and simplify medication regimen management. Pharmacists should be aware of the individual components of each combination product and ensure that patients are not over-prescribed any one active ingredient. While fixed-dose products may have a benefit in terms of convenience, the cost of combination products should be considered against the cost of taking the components separately for those with poor insurance coverage.
CONCLUSION
There are many oral agents currently available in the U.S. marketplace to help manage diabetes. Choosing the appropriate therapy for a specific patient may feel like a daunting task, but there are 2 major sets of treatment guidelines to turn to for help. The ADA and the AACE/ACE have both recently released updated treatment guidelines. Metformin is the preferred first-line agent in each set of guidelines and is a good place to start for all patients with T2D who do not have a contraindication.2,3 For patients not reaching individualized goals with metformin alone, the ADA recommends adding a second glucose-lowering agent in consideration of comorbidities (such as ASCVD, HF and CKD), hypoglycemia risk, desire to minimize weight gain or promote weight loss, and cost (see Figure 1).2 AACE/ACE also provides multiple options for add-on therapy with consideration of patient- and medication-specific factors.3 Keep in mind that no matter which medications a patient may use to help manage diabetes, all of them are considered to be adjuncts to diet and exercise. Lifestyle modification is really the key to success in managing diabetes.
When choosing medication therapy for a specific patient, it is important to consider the individual as a whole. One must consider a medication’s place in the treatment guidelines, adverse effects, contraindications, drug interactions, and ease of administration as well as the patient's personal preferences. Remember that most oral agents will reduce a patient’s A1C by less than 1%; so if a person requires significant A1C reduction, combination therapy or therapy with injectable medications may be necessary. Under the care of a multidisciplinary, collaborative treatment team, including effective counseling from pharmacists, patients can successfully manage their diabetes and prevent or delay complications.
| Potential Counseling Tips for Pharmacists |
| Category |
Potential Counseling Tips |
| Disease State Knowledge |
- Diabetes is associated with many complications and comorbidities, including eye conditions, high blood pressure, high cholesterol, heart disease, kidney disease, fatty liver disease, and gum disease, as well as weakness, numbness, or pain in the hands and feet, hearing loss, erectile dysfunction, and depression.
- Lifestyle modifications, such as diet and exercise, are key to successfully managing diabetes.
|
| Proper Follow-up and Care for People with Diabetes |
- If you are started on a new diabetes medication, your glycosylated hemoglobin (A1C) should be re-evaluated in about 3 months. If your A1C is not at goal, a second medication may be added. The American Diabetes Association recommends that people with diabetes should also have a comprehensive medical evaluation annually when possible.
|
| The Pharmacist's Role in Diabetes Care |
- When helping a prescriber decide which diabetes medication is best for a patient, it is important to consider patient- and medication-specific factors including potential adverse effects, contraindications (or reasons a particular drug may not be the best choice for your patient), drug interactions, and ease of use, as well as the specific comorbidities of the patient. Completing a CMR for patients with diabetes is particularly helpful when attempting to identify potential issues and when developing solutions.
|
| Medication tips |
- Metformin should be taken with food to prevent stomach problems. Let your pharmacist know if you experience any side effects when you start the medication, such as diarrhea, because a change in formulation may help alleviate symptoms if they occur.
- When taking metformin your provider may want to test your vitamin B12 levels because metformin use may cause your vitamin B12 levels to decline over time.
- Sulfonylureas and meglitinides should generally be taken with meals to prevent low blood sugars. If you experience low blood sugars while taking these medications you should let your pharmacist or provider know.
- You may not realize the full effects of a TZD medication for up to 8 weeks after starting the medication or increasing the dose. Let your pharmacist know if you experience water retention while on these medications.
- If you are taking a drug in the SGLT2 inhibitor class, let your pharmacist or provider know if you develop a feeling of burning when urinating, increased frequency or urgency of urination, lower abdominal or pelvic pain, fever, or blood in the urine. While rarely associated with SGLT2 inhibitor use, you should also report any symptoms of nausea, vomiting, abdominal pain, tiredness, or trouble breathing.
- If you are taking acarbose or miglitol, let your pharmacist or provider know if you are experiencing flatulence, diarrhea, or abdominal pain because these symptoms can often be helped by decreasing the dose.
|
REFERENCES
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Estimates of diabetes and its burden in the United States. 2017.
- American Diabetes Association. Standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Suppl. 1):S1-S193.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2019 executive summary. Endocr Pract. 2019;25(1):69-100.
- O'Mara NB. PL detail-document, drugs for type 2 diabetes;2015.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: A patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364-1379.
- Glucophage and glucophage XR [package insert]. New York, NY: Bristol-Myers Squibb Co.;2017.
- George CM, Brujin LL, Will K, Howard-Thompson A. Management of blood glucose with noninsulin therapies in type 2 diabetes. Am Fam Physician. 2015;92(1):27-34.
- Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;(1):CD002967.
- Lajara R. The potential role of sodium glucose co-transporter 2 inhibitors in combination therapy for type 2 diabetes mellitus. Expert Opin Pharmacother. 2014;15(17):2565-2585.
- Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes ObesMetab. 2014;16(5):457-466.
- Farxiga [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP;2019.
- Invokana [package insert]. Titusville, NJ: Jannsen Pharmaceuticals, Inc.;2018.
- Jardiance [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.;2018.
- Steglatro [package insert]. Whitehouse Station, NJ: Merck and Co., Inc.; 2018.
- S. Food and Drug Administration. FDA drug safety communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. FDA Web site. http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Published 2015. Updated 2015. Accessed April 9, 2019.
- S. Food and Drug Administration. FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR). Retrieved from https://www.fda.gov/Drugs/DrugSafety/ucm505860.htm. Accessed May 19, 2018.
- S. Food and Drug Administration. FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density. Retrieved from https://www.fda.gov/Drugs/DrugSafety/ucm461449.htm. Accessed May 19, 2018.
- S. Food and Drug Administration. FDA confirms increased risk of leg and foot amputations with the diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR). Retrieved from https://www.fda.gov/Drugs/DrugSafety/ucm557507.htm. Accessed May 19, 2018.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. 2006;368(9548):1696-1705.
- Januvia [package insert]. Whitehouse Station, NJ: Merck and Co. Inc.;2019.
- Onglyza [package insert]. New York, NY: Bristol-Myers Squibb Co.;2018.
- Nesina [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc.;2016.
- Tradjenta [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.;2017.
- S. Food and Drug Administration. FDA warns that DPP-4 inhibitors for type 2 diabetes may cause severe joint pain. Retrieved from https://www.fda.gov/Drugs/DrugSafety/ucm459579.htm. Accessed April 9, 2019.
- S. Food and Drug Administration. FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Retrieved from https://www.fda.gov/Drugs/DrugSafety/ucm486096.htm. Accessed April 9, 2019.
- S. Food and Drug Administration. FDA warns that DPP-4 inhibitors for type 2 diabetes may cause severe joint pain. Retrieved from https://www.fda.gov/Drugs/DrugSafety/ucm459579.htm. Accessed April 9, 2019.
- S. Food and Drug Administration. FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Retrieved from https://www.fda.gov/Drugs/DrugSafety/ucm486096.htm. Accessed April 9, 2019.
- Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with non-alcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165:305-315.
- Yki-Jarvinen H. Thiazolidinediones. N Engl J Med.2004;351(11):1106-1118.
- Orasanu G, Ziouzenkova O, Devchand PR, et al. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone represses inflammation in a peroxisome proliferator-activated receptor-alpha-dependent manner in vitro and in vivo in mice. J Am Coll Cardiol. 2008;52(10):869-881.
- Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med.2010;170(14):1191-1201.
- Mahaffey KW, Hafley G, Dickerson S, et al. Results of a reevaluation of cardiovascular outcomes in the RECORD trial. Am Heart J.2013;166(2):240-249.e1.
- S. Food and Drug Administration. Rosiglitazone-containing diabetes medicines: Drug safety communication - FDA eliminates the risk evaluation and mitigation strategy (REMS). FDA Website Web site. https://www.fda.gov/Drugs/DrugSafety/ucm476466.htm. Published 2015. Updated 2015. Accessed April 9, 2019.
- S. Food and Drug Administration. Updated FDA review concludes that use of type 2 diabetes medicine pioglitazone may be linked to an increased risk of bladder cancer. Retrieved from https://www.fda.gov/Drugs/DrugSafety/ucm519616.htm. Accessed April 9, 2019.
- Actos [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc.;2017.
- Avandia [package insert]. Research Triangle Park, NC: GlaxoSmithKline;2019.
- Nolte MS. Pancreatic hormones and antidiabetic drugs. In: Katzung BG, Masters SB, Trevor AJ, eds. Basic and Clinical Pharmacology.11th ed. New York, NY: Lange Medical Books/McGraw-Hill; 2009:727.
- Amaryl [package insert]. Bridgewater, NJ: Sanofi-aventis U.S. LLC;2009.
- Glucotrol [package insert]. New York, NY: Pfizer, Inc.;2013.
- Glynase prestab [package insert]. New York, NY: Pfizer, Inc.;2009.
- Scheen AJ. Drug interactions of clinical importance with antihyperglycaemic agents: an update. Drug Saf. 2005;28(7):601-631.
- Starlix [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corp.;2013.
- Prandin [package insert]. Princeton, NJ: Novo Nordisk, Inc.;2011.
- Precose [package insert]. Wayne, NJ: Bayer Healthcare Pharmaceuticals;2011.
- Glyset [package insert]. New York, NY: Pfizer, Inc.;2012.
- Welchol [package insert]. Parsippany, NJ: Daiichi Sankyo, Inc.;2019.
- Cycloset [package insert]. Tiverton, RI: VeroScience, LLC.;2015.
Always use the free online Word to HTML converter to avoid messy code on your websites!
Back to Top