
Expired activity
Please go to the PowerPak
homepage and select a course.
Optimizing Outcomes in Invasive Fungal Disease: Interdisciplinary Perspectives
OVERVIEW
The content in this activity is focused on practical aspects of antifungal management. Using a multidisciplinary approach, the activity focuses on diagnostics, practical pharmacologic aspects of antifungal therapies, as well as application in case examples. In addition to the stated faculty, the content is based on contributions from symposium faculty Drs Dimitrios Kontoyiannis and Thomas Patterson. We thank them for their contributions.
Antifungal Diagnostics Update
Reviewed by Luis Ostrosky-Zeichner, MD
This section provides an update on antifungal diagnostics, their importance in invasive fungal disease (IFD) management strategies, and their specific application in invasive candidiasis and invasive mold infections.
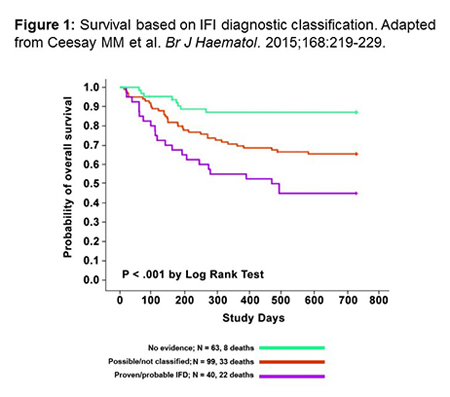 Why We Need Improved Diagnostics
Why We Need Improved Diagnostics
Improved diagnostics are needed to speed the use of appropriate antifungal therapy and improve outcomes.[Kontoyiannis 2015] A recent study by Ceesay and colleagues from the UK addressed diagnostic difficulties and differences in diagnostic utilization. In this study, conducted in 203 high-risk hematology patients, subjects were evaluated by a very strict diagnostic algorithm including CT scans on routine bases, weekly serum galactomannan, as well as Beta-D-glucan (BDG) at suspicion of infection. The investigators attempted to make a tissue diagnosis whenever possible. Figure 1 shows the impact of diagnosis of IFD on survival outcomes in this study. You can see that with this fairly regimented algorithm, outcomes were substantially better in those without evidence of IFD than in those with possible or non-classified disease, and significantly worse in patients who had proven or probable infections. The investigators were also able to show that if you only performed galactomannan (GM), the definitively diagnosed IFI rate was quite low, at 10.5% (95% CI 8.0 – 12.5). Rates were higher when BDG was used, and when GM and BDG were used together, the incidence rate increased to 19.6% (95% CI 17.5-22.4).[Ceesay 2015] So using the tests together provided useful diagnostic information.
Diagnostics for Invasive Candidiasis
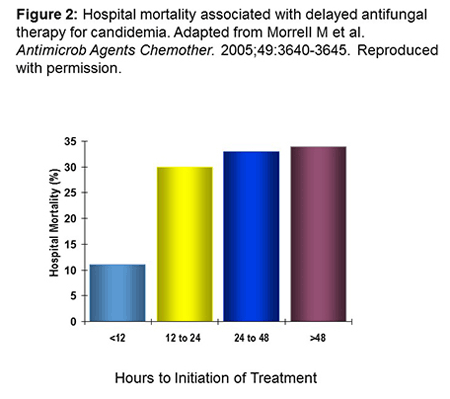 In terms of the impact of diagnostics specifically on IC outcomes, Morrell and colleagues showed that if you can initiate therapy within the first 12 hours of the presumed onset of candidemia based on high suspicion of disease or on a marker, mortality is dramatically lower than it is in patients for whom therapy was significantly delayed. Figure 2 shows results from this study: mortality rates vs the time difference between when the first blood sample that was culture positive for fungi was drawn and the time when antifungal treatment was first administered.[Morrell 2005] Despite the advantages of this early administration, the majority of patients (54.8%) received antifungal therapy 24 to 48 hours after the first blood culture to turn positive was drawn, presumably around the time that the culture results came back positive.[Morrell 2005]
In terms of the impact of diagnostics specifically on IC outcomes, Morrell and colleagues showed that if you can initiate therapy within the first 12 hours of the presumed onset of candidemia based on high suspicion of disease or on a marker, mortality is dramatically lower than it is in patients for whom therapy was significantly delayed. Figure 2 shows results from this study: mortality rates vs the time difference between when the first blood sample that was culture positive for fungi was drawn and the time when antifungal treatment was first administered.[Morrell 2005] Despite the advantages of this early administration, the majority of patients (54.8%) received antifungal therapy 24 to 48 hours after the first blood culture to turn positive was drawn, presumably around the time that the culture results came back positive.[Morrell 2005]
This study illustrates one of the challenges with blood cultures in IC. While blood culture testing has improved and we can get results back somewhat quicker, there is still a gap between the onset of candidemia and the culture results. Moreover, there are epidemiologic considerations—including the emergence of some difficult-to-treat and resistant non-albicans species—that are key considerations in terms of the choice of empiric therapy.[Redding 2004] It has been very difficult to conduct studies of empiric/pre-emptive therapy in a very regimented way. Therefore, nonculture-based validated markers, including BDG, are important factors to consider in deciding to initiate empiric therapy, as are known risk factors such as central catheters, co-morbid illness, and critical illness.[Ostrosky-Zeichner 2014]
Real-time PCR and BDG
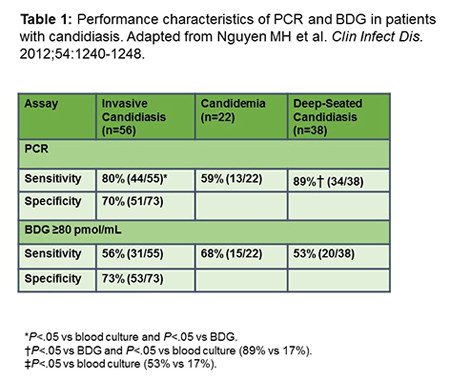 Advanced diagnostics such as PCR can provide species level determination as well as an evaluation of resistance mechanisms, which are useful outputs. Hong Nguyen and Neil Clancy from Pittsburgh compared real-time PCR with BDG and blood cultures for the diagnosis of IC. In this study, they used a PCR that detected the most common Candida spp., including C albicans, C tropicalis, C parapsilosis, C glabrata, and C krusei. They were able to evaluate patients with candidemia, obviously, with positive blood cultures, as well as those with other deep Candida infections, usually intra-abdominal infections. As shown in Table 1, interestingly, PCR by plasma or serum was more sensitive than whole blood for IC in general, but PCR and BDG were actually better than blood cultures for deep Candida spp. infections, with a significantly better sensitivity. It was, in fact, by combining either PCR or BDG with blood cultures, that the investigators were able to pick up many more of the cases, the candidemia cases as well as those with deep infections, for which cultures were negative. That same pattern has been seen potentially with other markers as well.[Nguyen 2012]
Advanced diagnostics such as PCR can provide species level determination as well as an evaluation of resistance mechanisms, which are useful outputs. Hong Nguyen and Neil Clancy from Pittsburgh compared real-time PCR with BDG and blood cultures for the diagnosis of IC. In this study, they used a PCR that detected the most common Candida spp., including C albicans, C tropicalis, C parapsilosis, C glabrata, and C krusei. They were able to evaluate patients with candidemia, obviously, with positive blood cultures, as well as those with other deep Candida infections, usually intra-abdominal infections. As shown in Table 1, interestingly, PCR by plasma or serum was more sensitive than whole blood for IC in general, but PCR and BDG were actually better than blood cultures for deep Candida spp. infections, with a significantly better sensitivity. It was, in fact, by combining either PCR or BDG with blood cultures, that the investigators were able to pick up many more of the cases, the candidemia cases as well as those with deep infections, for which cultures were negative. That same pattern has been seen potentially with other markers as well.[Nguyen 2012]
T2MR
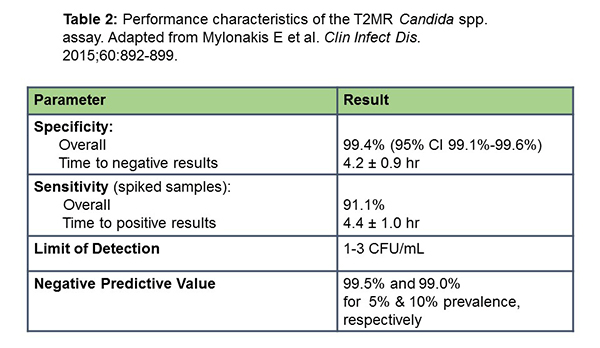
One of those other nonculture based diagnostic tests markers is the 2T magnetic resonance assay or T2MR for the diagnosis of candidiasis. In early studies, it was shown that in patients with candidemia, you were able to dramatically decrease the time to detection of these infections (from days for blood culture to 3 hours with T2MR).[Beyda 2013] The test works in the setting of antifungals, which can render blood cultures negative. Results from a recent study by Mylonakis and colleagues (which was used for the US FDA approval) are shown in Table 2. This study utilized samples from over 1800 hospitalized patients who had a blood culture ordered. The investigators spiked negative samples to see if they could be detected with this assay. Using those criteria, the sensitivity was actually good (about 90%), with time to positivity of only a few hours when compared to many hours for blood cultures. Sensitivity was reasonable for different Candida spp., including albicans/tropicalis (92.3%), parapsilosis (94.2%), and krusei and glabrata, the more resistant strains (88.1%). The limit of detection was just a few CFUs/mL, and the negative predictive value in a clinical setting was predicted to be very good.[Mylonakis 2015]
There are some caveats. If you look at the patients who actually had candidemia, there are very few in this series—only 6 prospectively collected specimens had positive blood culture results for Candida spp.There were 29 patients who had positive T2 tests who didn't have clear evidence of candidemia by blood culture. In these cases, a post-hoc analysis showed that 1 case was a later proven IC and 4 cases had Candida spp. isolated from other clinical samples, but these patients did not meet the criteria for IC.[Mylonakis 2015]How do you really know if those were false positives or if we just have a more sensitive assay with the T2MR? I think time will tell how that plays out and obviously it's going to take labs acquiring these instruments on a widespread basis to get more data in this setting.
If we summarize the data on Candida spp. diagnostics (Table 3), it's important still to think about risk factors for Candida spp. because you're going to have to use your clinical judgment in many of these cases before these markers really become positive. It's still very important to look at cultures and to look at susceptibility to help you guide therapy. The current recommendations are for azole susceptibility testing for most blood stream isolates. Echinocandin susceptibility is to be considered in certain settings: prior therapy, breakthrough infections, and Candida glabrata (when it's a serious blood stream infection). That’s what the new guidelines are more or less going to say. Clearly the role for new biomarkers and diagnostics is increasing and is going to help us as we move forward.
Diagnostics for Invasive Aspergillosis
What about Aspergillus spp. diagnostics? Clearly, with Aspergillus we're going to want to culture and confirm these isolates when possible. Galactomannan and BDG are increasingly utilized to facilitate diagnosis. PCR is not established, but it is making progress. And, as was the case with IC, resistance is increasingly noted. In general, as mentioned by Drs Kontoyiannis and Lewis, it is very important to understand an individual patient’s risk for invasive mold infection.[Kontoyiannis 2015]
Lateral Flow
An interesting and exciting technology has been the development of a lateral flow device, which was developed by Chris Thornton from the UK. It's commercialized in Europe. It's not available in the United States, but he's developed antibodies that are similar. This test is different than GM. It binds to an extracellular glycoprotein that is secreted during growth of Aspergillus spp. It functions like a pregnancy test, if you will.[Thornton 2008] It provides the potential for point-of-use testing, doesn't have to be refrigerated, is easy to perform, and pretty easy to read.
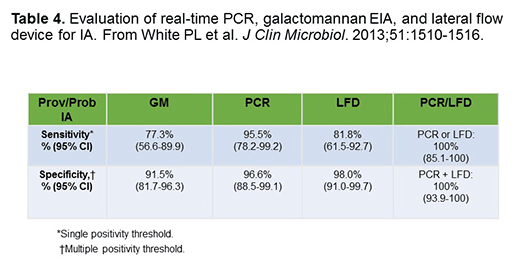 Table 4 shows data from Lewis White and Rosemary Barnes for a comparison of real-time PCR, GM-EIA, and the lateral flow device in 103 high-risk patients. The study involved 9 proven, 14 probable, and 22 possible invasive aspergillosis cases. You can see that the PCR had a very high sensitivity and specificity, and LFD has similar sensitivity as GM, with a specificity that was close to that of PCR as well.[White 2013] If you combine the tests, you really got excellent sensitivity and specificity. It’s really a potentially very useful test.
Table 4 shows data from Lewis White and Rosemary Barnes for a comparison of real-time PCR, GM-EIA, and the lateral flow device in 103 high-risk patients. The study involved 9 proven, 14 probable, and 22 possible invasive aspergillosis cases. You can see that the PCR had a very high sensitivity and specificity, and LFD has similar sensitivity as GM, with a specificity that was close to that of PCR as well.[White 2013] If you combine the tests, you really got excellent sensitivity and specificity. It’s really a potentially very useful test.
Galactomannan
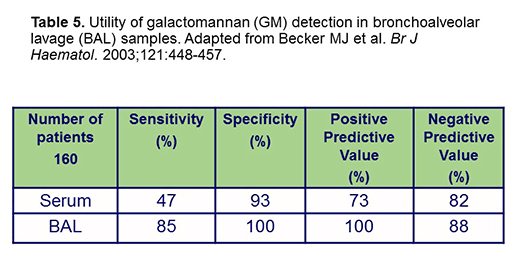
Regarding the use of the Aspergillus Platelia GM EIA, I think the caveats are well known. Certainly, limited sensitivity is much more likely to occur on mold-active therapy,[Marr 2005] and serial testing is needed for optimal results. However, it's not really recommended that you do GM testing on patients receiving mold-active therapy if they're not symptomatic because of the low positive predictive value of the test in this setting.[Duarte 2014; Marr 2015] In a symptomatic patient, it's probably reasonable to do. There was also concern regarding the potential for drug-related false-positive tests, especially when testing in patients receiving piperacillin-tazobactam,[Viscoli 2003] but that issue has probably been reduced. Moreover, as shown in Table 5, the use of BAL-GM is a very important and a very potentially useful tool with sensitivity that's dramatically better than serum GM. A negative GM BAL test is really pretty useful to exclude the diagnosis in high-risk patients.[Becker 2003] D’Haese and colleagues suggest that GM detection in CT-based BAL fluid has a high positive PPV for diagnosing IPA early in untreated patients. They suggest that a BAL GM index <0.5 is helpful to exclude diagnosis in high-risk patients.[D’Haese 2012]
PCR Testing
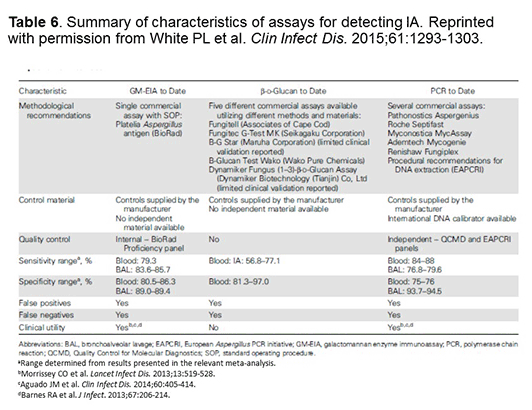
PCR testing for IA is still not approved in the United States. Concerns have been raised about the variable sensitivity/specificity, the limited per-test positivity, the lack of standardized targets/reagents, as well as the lack of external validation. However, as shown in Table 6, the EORTC/MSG IFI definitions group recently published a systematic review that basically shows the PCR data are really at least as good as the GM and BDG data that were used for inclusion of those assays in the EORTC/MSG definitions.[White 2015] The group has made methodological recommendations and has noted that commercial PCR assays can assist with standardization. We await the official publication of the revised IFI definitions criteria, as presented at the 2015 Trends in Medical Mycology Meeting, regarding PCR incorporation into the new IFI definitions.
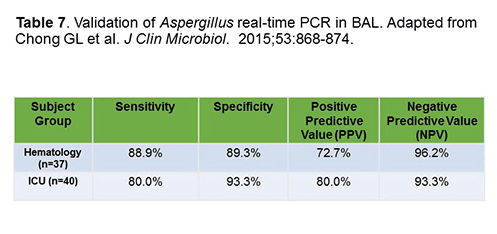 One evaluation of application of a real-time PCR system is shown in Table 7. This is a multiplex assay that detects relevant Aspergillus spp. and can also detect resistant strains as well as resistance mechanisms. The investigators implemented the test on 37 BAL samples from hematology patients and 40 patients from the ICU. A GM level ≥1.0 or a positive culture was taken as a gold standard for Aspergillus spp. diagnosis. It turns out that the signal is much stronger and much more useful to make a diagnosis with BAL vs serum. In this setting, this group from Belgium was actually able to detect resistance mechanisms in these strains as well.[Chong 2015]
One evaluation of application of a real-time PCR system is shown in Table 7. This is a multiplex assay that detects relevant Aspergillus spp. and can also detect resistant strains as well as resistance mechanisms. The investigators implemented the test on 37 BAL samples from hematology patients and 40 patients from the ICU. A GM level ≥1.0 or a positive culture was taken as a gold standard for Aspergillus spp. diagnosis. It turns out that the signal is much stronger and much more useful to make a diagnosis with BAL vs serum. In this setting, this group from Belgium was actually able to detect resistance mechanisms in these strains as well.[Chong 2015]
Beta-D-Glucan (BDG) Assay
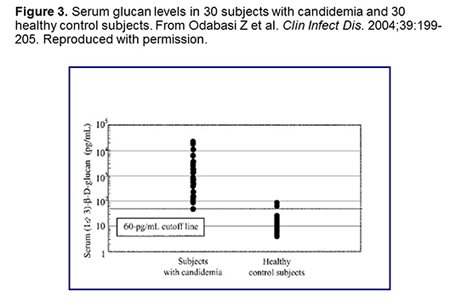 The BDG has been evaluated in a number of centers. Figure 3 shows data from 30 healthy adults vs 30 candidemic patients, and at a cutoff of 60 pg/mL, the test had reasonable sensitivity (97%) and specificity (93%).[Odabasi 2004] In a subsequent analysis in patients with AML or MDS who were receiving antifungal prophylaxis, the specificity was 90% for a single positive result and ≥96% for ≥2 sequential positive results. In a subsequent multicenter evaluation, conducted in 163 patients with documented IFIs (various genera) and 170 controls, the sensitivity of the assay was 70% and the specificity was 87%.[Ostrosky-Zeichner 2005] There is not a high volume of patients, and so doing this test in real-time is difficult. Tom Patterson reports that he has been working as part of an NIH contract group in San Antonio with Waco Life Science on an assay called a microfluidics-based BDG assay. Preliminary data for this assay suggests that it will provide rapid results and is relatively sensitive compared to traditional BDG assays.[Kapoor 2014]
The BDG has been evaluated in a number of centers. Figure 3 shows data from 30 healthy adults vs 30 candidemic patients, and at a cutoff of 60 pg/mL, the test had reasonable sensitivity (97%) and specificity (93%).[Odabasi 2004] In a subsequent analysis in patients with AML or MDS who were receiving antifungal prophylaxis, the specificity was 90% for a single positive result and ≥96% for ≥2 sequential positive results. In a subsequent multicenter evaluation, conducted in 163 patients with documented IFIs (various genera) and 170 controls, the sensitivity of the assay was 70% and the specificity was 87%.[Ostrosky-Zeichner 2005] There is not a high volume of patients, and so doing this test in real-time is difficult. Tom Patterson reports that he has been working as part of an NIH contract group in San Antonio with Waco Life Science on an assay called a microfluidics-based BDG assay. Preliminary data for this assay suggests that it will provide rapid results and is relatively sensitive compared to traditional BDG assays.[Kapoor 2014]
Aspergillus spp. Diagnostics-Summary
In summary regarding diagnosis of Aspergillus spp. and other molds, you can see it's really important to confirm positive results. As Drs Kontoyiannis and Lewis mentioned, a presentation and syndrome-oriented approach lacks sensitivity and specificity, so that confirmed diagnosis is important.[Kontoyiannis 2015] Because of the increasing impact of resistance, it is also important to identify to the species complex level, wherever possible. The role of susceptibility testing is in the guidelines. While it's not routinely recommended in the United States right now because we don't have that high level of resistance, resistance is becoming a more global problem. Moreover, you need to know the local resistance patterns.[Kontoyiannis 2015] Susceptibility testing is particularly helpful to consider in patients who have not responded or who have progressive infection. Clearly, these new assays—galactomannan, lateral flow, BDG, and hopefully in the future, PCR—will help improve outcomes.
ANTIFUNGAL THERAPEUTICS UPDATE
Reviewed by Melissa D. Johnson, PharmD
This section provides an overview of the pharmacologic aspects of antifungal agents. When selecting an antifungal agent, there are a number of important considerations: the availability of clinical trials data to support the use of the agent in that infection; the spectrum of activity versus known suspected pathogens; the pharmacokinetics (PK) or penetration to the specific body sites of infection that you're worried about, drug side effects, drug/drug interactions, and other patient factors such as renal or hepatic dysfunction and concomitant medications.[Kontoyiannis 2015] What are the ideal properties of an antifungal agent? First, we want potent antifungal activity. We want fungicidal activity rather than fungistatic activity, a broad spectrum, low potential to induce resistance, the ability to overcome multi-drug resistance, and low toxicity.[Borowski 2000]
Clinical Trial Data for Antifungals
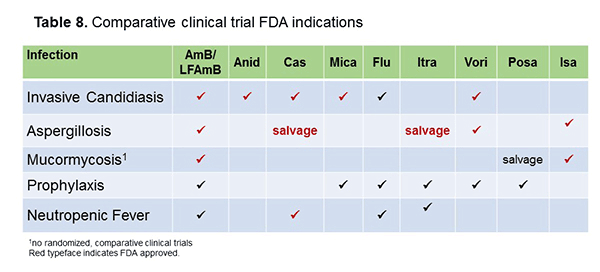 First, as shown in Table 8, looking at the clinical trials data for these antifungal agents for invasive candidiasis, we have a smattering of data across the echinocandin class, which also have FDA indications (in red). Amphotericin B has an indication and clinical trials as well. Fluconazole, surprisingly, actually doesn't have the FDA indication for candidemia, if you recall. It actually has it for esophageal candidiasis and oropharyngeal disease, but there's certainly a plethora of clinical trials looking at fluconazole for that infection, and it is certainly efficacious. I've given a checkbox for voriconazole, which has a clinical trial as well. Isavuconazole has a clinical trial that has been completed; their preliminary results have been provided. In that comparative study of isavuconazole versus caspofungin at the 10-day IV therapy comparison, isavuconazole was actually not non-inferior to caspofungin. The azole was less efficacious potentially by about 10% in raw numbers, about 60% versus 70% efficacy at the 10-day mark.[PR Newswire press release 2015]
First, as shown in Table 8, looking at the clinical trials data for these antifungal agents for invasive candidiasis, we have a smattering of data across the echinocandin class, which also have FDA indications (in red). Amphotericin B has an indication and clinical trials as well. Fluconazole, surprisingly, actually doesn't have the FDA indication for candidemia, if you recall. It actually has it for esophageal candidiasis and oropharyngeal disease, but there's certainly a plethora of clinical trials looking at fluconazole for that infection, and it is certainly efficacious. I've given a checkbox for voriconazole, which has a clinical trial as well. Isavuconazole has a clinical trial that has been completed; their preliminary results have been provided. In that comparative study of isavuconazole versus caspofungin at the 10-day IV therapy comparison, isavuconazole was actually not non-inferior to caspofungin. The azole was less efficacious potentially by about 10% in raw numbers, about 60% versus 70% efficacy at the 10-day mark.[PR Newswire press release 2015]
For aspergillosis, there aren't that many randomized controlled trials, recognizing the complexity of managing these patients and enrolling them in the trial setting. We certainly have the voriconazole versus amphotericin B trial that's well-known and showed a mortality benefit for voriconazole.[Herbrecht 2002] For isavuconazole, investigators recently also completed their aspergillosis trial showing favorable efficacy and getting an FDA indication for aspergillosis. Isavuconazole was comparable to voriconazole in that study, with a benefit in the adverse event profile.[Maertens 2015] Then we have salvage data for caspofungin and itraconazole, although these are not from randomized controlled trials.
Likewise, for mucormycosis, we don't really have randomized controlled trials, given the complexity of those infections, but some of the drugs do have indications, including isavuconazole, which did receive an FDA indication for that. There have also been some case series reported for posaconazole in the salvage setting.[Gonzalez-Ramos 2008] Prophylaxis is the main indication for posaconazole. We have also have data for some of the other agents for prophylaxis or for neutropenic fever.[Wingard 2010; Pfaller 2015; Winston 2000; Boogaerts 2001]
Clinical Spectrum of Activity
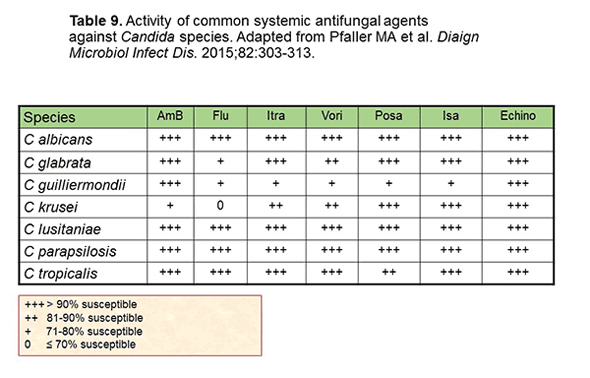
According to Drs Kontoyiannis and Lewis, understanding the spectrum of activity of antifungals is a key principle in their optimal use.[Kontoyiannis 2015] As shown in Table 9, for invasive candidiasis, the majority of agents are going to have pretty good activity across the species, but will fall down in glabrata, as we've mentioned. That always seem to be the downfall of these drugs. Then a little smattering of low values for guilliermondii, where there's some discrepant activity for the different azoles. There are some issues with krusei as well, where there's some lack of activity.
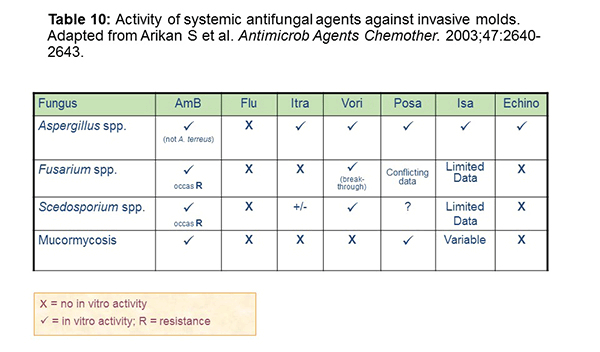
Activity against invasive molds is variable (Table 10). For Aspergillus species, it is important to recognize that fluconazole really lacks activity against these molds entirely. You'll see some differential activity for echinocandins in terms of Aspergillus, but this class lacks activity against some of these other molds. Voriconazole has holes as well with mucormycosis and maybe breakthroughs with Fusarium spp. infections. Itraconazole does not have great Mucor activity either.[Arikan 2003] Posaconazole has Aspergillus spp. and mucormycosis activity but variable activity against some of the other molds. Isavuconazole has variable activity across the molds, with limited data on its efficacy for some of the rarer molds.[Isavuconazonium FDA briefing document; Cresemba PI 2015]
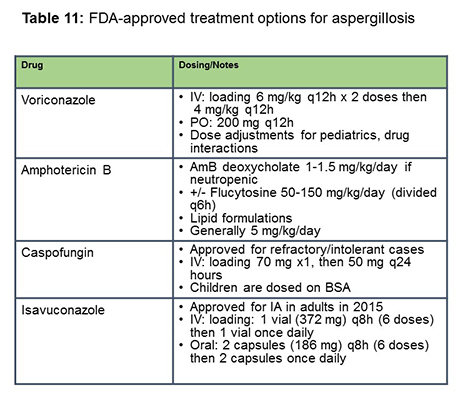
In terms of FDA-approved treatment options, for aspergillosis we're left with the treatment options of voriconazole, amphotericin B or lipid formulations, caspofungin, and isavuconazole (Table 11). These are the different dosing schemes that are recommended for these agents for aspergillosis. Posaconazole is not listed here. However, there is an ongoing trial of posaconazole versus voriconazole for invasive aspergillosis.
PK/PD
In terms of the PK/PD principles, we have to remember that we're looking not only at absorption, distribution, metabolism, and elimination, but also the concentration at the site of infection and body fluids or tissues as well as pharmacodynamic parameters. These include time above the MIC, or area-under-the-concentration (AUC) curve over the MIC of the organism. As mentioned by Drs Kontoyiannis and Lewis, addressing tissue penetration is a key strategy to optimize antifungals.[Kontoyiannis 2015] We'd want to try to maximize those pharmacodynamic parameters to get the best bang for your buck with your antifungal drug.
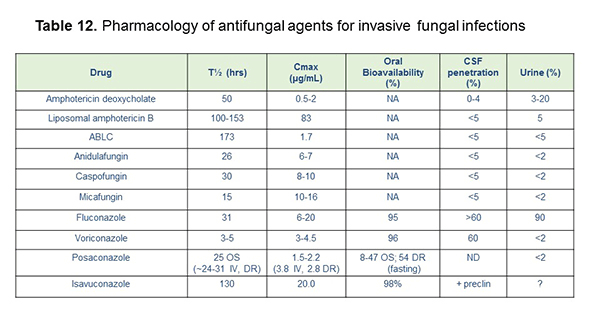 Table 12 shows a summary of some of the differential pharmacokinetics of the antifungal drugs, and we've picked out just a few features here to discuss. The half-lives of these really range from something with a shorter half-life like voriconazole to very long half-lives with amphotericin B. The newer agent isavuconazole has an extremely long half-life after loading. It can be up to 130 hours.[Dodds 2000; Lewis 2008; Lewis 2011; Kraft 2014; Maertens 2015; Aranda-Rajah 2015] The peak serum concentrations are relevant in the sense that if you have a peak-to-MIC type of target, that could be relevant, and so you're looking at your Cmax relative to what the MICs for traditional organisms are. The CSF penetration varies for these, and although amphotericin B has very low CSF penetration, we know it has immunomodulatory properties in the CNS that may lead to good activity even for infections like cryptococcal meningitis. Fluconazole has the best CSF penetration, as well as voriconazole among azoles, and we're awaiting additional data for isavuconazole and posaconazole there. Urine penetration is best, again, for fluconazole, although we know that echinocandins do penetrate into the renal parenchyma. If you have a tissue-based infection in the kidney, they could be helpful there, but they don't have great urine concentrations.
Table 12 shows a summary of some of the differential pharmacokinetics of the antifungal drugs, and we've picked out just a few features here to discuss. The half-lives of these really range from something with a shorter half-life like voriconazole to very long half-lives with amphotericin B. The newer agent isavuconazole has an extremely long half-life after loading. It can be up to 130 hours.[Dodds 2000; Lewis 2008; Lewis 2011; Kraft 2014; Maertens 2015; Aranda-Rajah 2015] The peak serum concentrations are relevant in the sense that if you have a peak-to-MIC type of target, that could be relevant, and so you're looking at your Cmax relative to what the MICs for traditional organisms are. The CSF penetration varies for these, and although amphotericin B has very low CSF penetration, we know it has immunomodulatory properties in the CNS that may lead to good activity even for infections like cryptococcal meningitis. Fluconazole has the best CSF penetration, as well as voriconazole among azoles, and we're awaiting additional data for isavuconazole and posaconazole there. Urine penetration is best, again, for fluconazole, although we know that echinocandins do penetrate into the renal parenchyma. If you have a tissue-based infection in the kidney, they could be helpful there, but they don't have great urine concentrations.
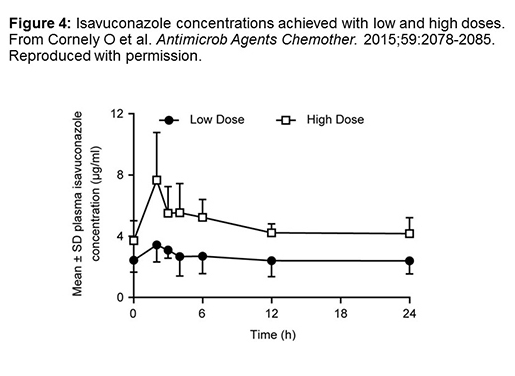
Figure 4 provides some information about concentrations that you would see with isavuconazole. This is one of the PK studies I could find comparing a low-dose to a high-dose regimen in hematology-oncology patients. This was a small number of patients: they were looking at the standard 200 milligram dose, which is the approved dose, versus a doubling of the dose at 400 milligrams daily.[Cornely 2015] As you can see, there's a pretty linear effect, so that the approximate trough concentration would go from about 2.5 mcg/mL to around 5 mcg/mL if you double the dose. It's thought that the linear kinetics for isavuconazole are one of the things that will make it stand out as opposed to some of the non-linear kinetics we'll talk about with some of the other azoles. The concentrations reached, however, are not all that different than the required MIC reached for some organisms. It depends on the organism that you're treating. For Candida, you're going to have a very low MIC. You'll be able to readily achieve that, but with some of the molds, you might get into a little bit of trouble.
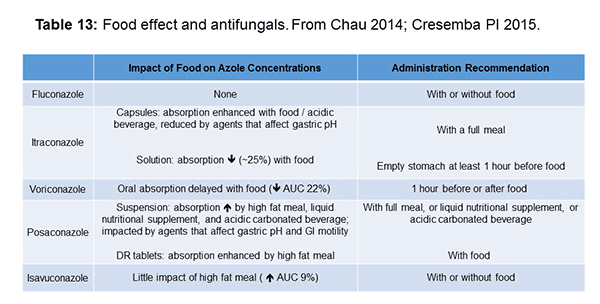 As shown in Table 13, azoles also have a number of food interactions, and we’re focusing here on azoles because they’re the only drugs administered orally, so with the IV echinocandins or amphotericin, you don’t have to worry about food. They have other problems you have to worry about. But the food interactions can be challenging, particularly for posaconazole where a high-fat meal increases the absorption. It depends on the type of formulation you’re using. This is for the suspension and the delayed-release tablets. The DR tablets just say you can give it with food, but if you are having difficulty achieving your target concentrations, you could consider increasing with the fatty content of the meal or administering it with an acidic beverage. Isavuconazole, from the information we have so far, can be administered with or without food and voriconazole can be given 1 hour before or after food.[Chau 2014; Cresemba PI 2015]
As shown in Table 13, azoles also have a number of food interactions, and we’re focusing here on azoles because they’re the only drugs administered orally, so with the IV echinocandins or amphotericin, you don’t have to worry about food. They have other problems you have to worry about. But the food interactions can be challenging, particularly for posaconazole where a high-fat meal increases the absorption. It depends on the type of formulation you’re using. This is for the suspension and the delayed-release tablets. The DR tablets just say you can give it with food, but if you are having difficulty achieving your target concentrations, you could consider increasing with the fatty content of the meal or administering it with an acidic beverage. Isavuconazole, from the information we have so far, can be administered with or without food and voriconazole can be given 1 hour before or after food.[Chau 2014; Cresemba PI 2015]
Drug Interactions
Drug interactions are more limited with the IV preparations of polyenes and echinocandins. Polyenes, because they’re basically eliminated through a path that does not involve the liver, are not going to have a lot of specific drug interactions but they will have issues with drugs that have overlapping toxicities or compatibility issues. Echinocandins have slight interactions through the cytochrome P450 system, and caspofungin levels will be reduced slightly by P450 inhibitors. Micafungin is also a weak inhibitor of 3A4 and that can lead to some interactions, particularly with transplant medications.[Dodds Ashley 2006]
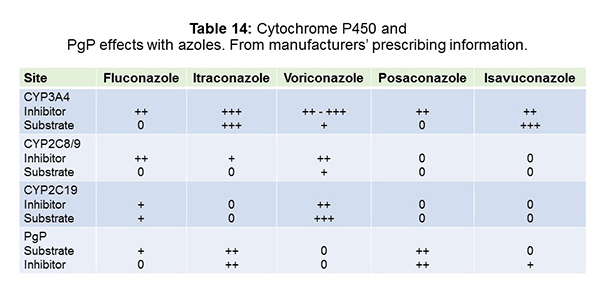 As shown in Table 14, looking specifically at the azoles, this is where you run into a lot of your P450 issues, and voriconazole is kind of notorious for this. It’s an inhibitor and substrate at 3A4, 2C8 and 9, and 2C19. You can really run into a lot of problems here. Isavuconazole, the new kid on the block, is primarily 3A4 involved and thought to perhaps not be as bad as some others. Posaconazole has fewer interactions as well, but it does have an interaction with p glycoprotein (PgP), which is a transporter across cell membranes in several deep tissues like liver, gut, and kidney, as well as the blood/brain barrier. We’re recognizing these p glycoprotein interactions more and more and it’s just important to keep in mind that it may lead to some unanticipated effects. Itraconazole, likewise, has 3A4 and PgP interactions and fluconazole has a smattering of these.[Chau 2014; Dodds Ashley 2006; Cresemba 2015]
As shown in Table 14, looking specifically at the azoles, this is where you run into a lot of your P450 issues, and voriconazole is kind of notorious for this. It’s an inhibitor and substrate at 3A4, 2C8 and 9, and 2C19. You can really run into a lot of problems here. Isavuconazole, the new kid on the block, is primarily 3A4 involved and thought to perhaps not be as bad as some others. Posaconazole has fewer interactions as well, but it does have an interaction with p glycoprotein (PgP), which is a transporter across cell membranes in several deep tissues like liver, gut, and kidney, as well as the blood/brain barrier. We’re recognizing these p glycoprotein interactions more and more and it’s just important to keep in mind that it may lead to some unanticipated effects. Itraconazole, likewise, has 3A4 and PgP interactions and fluconazole has a smattering of these.[Chau 2014; Dodds Ashley 2006; Cresemba 2015]
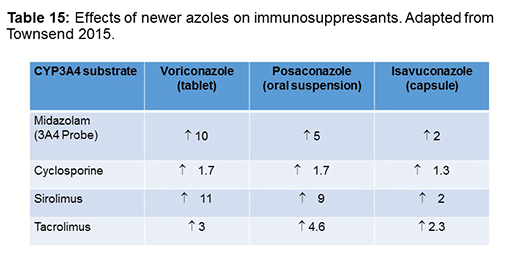 I specifically wanted to focus on 3A4 and the transplant population. Table 15 shows results from a nice study that looked at midazolam, which is a cytochrome P450 3A4 inhibitor type of probe, and you can use that to see the potential interactions with azoles and how they might impact other drugs that are metabolized by the P450 3A4 system. As you see, voriconazole increased midazolam by more than 10-fold, while isavuconazole only increased it 2-fold, and posaconazole was in the middle. This translated similarly into sirolimus concentrations that were elevated 11-fold and 2-fold with voriconazole and isavuconazole, respectively. We still have to worry a little bit with isavuconazole with some of these transplant medications but maybe not as much as we’ve had to with voriconazole.[Townsend 2015]
I specifically wanted to focus on 3A4 and the transplant population. Table 15 shows results from a nice study that looked at midazolam, which is a cytochrome P450 3A4 inhibitor type of probe, and you can use that to see the potential interactions with azoles and how they might impact other drugs that are metabolized by the P450 3A4 system. As you see, voriconazole increased midazolam by more than 10-fold, while isavuconazole only increased it 2-fold, and posaconazole was in the middle. This translated similarly into sirolimus concentrations that were elevated 11-fold and 2-fold with voriconazole and isavuconazole, respectively. We still have to worry a little bit with isavuconazole with some of these transplant medications but maybe not as much as we’ve had to with voriconazole.[Townsend 2015]
There are other factors affecting the PK/PD of these drugs. These include things like age, gender, severity of illness, renal and hepatic function; states that affect oral absorption like high gastric pH or high upper achlorhydria or GVHD; limited IV access; genetic components related to polymorphisms that can affect voriconazole concentrations; and drug interactions. We’ll talk a little bit more about some of these.
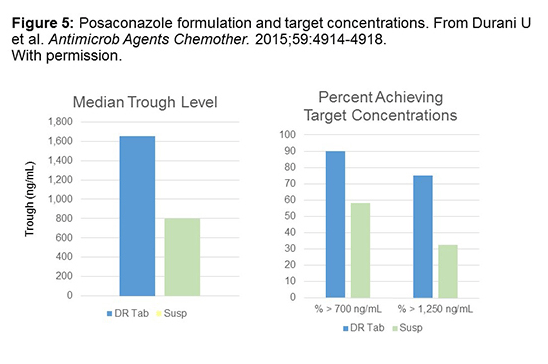
A lot of people are interested in the new delayed-release tablets of posaconazole and whether or not we’re achieving target concentrations with this new formulation. As you can see here in Figure 5, in green, these are the concentrations achieved with the suspension in a study that included a substantial number of solid organ transplant and oncology patients receiving posaconazole for prophylaxis as well as treatment. The mean trough concentration was around 800 ng/mL with the suspension, and that was about doubled when patients were given the DR tablets. You’ll see that giving the DR tablets, on average, in difficult-to-treat patients, can achieve target concentrations that are about twice as much as that for the suspension.[Durani 2015] Looking at the percent that achieved target concentrations, 90% of patients on the DR tablet achieved a target greater than 700 ng/mL, which could be considered a target for prophylaxis and more than 75% achieved a target of 1250 ng/mL. This, again, is about double that achieved on the suspension.
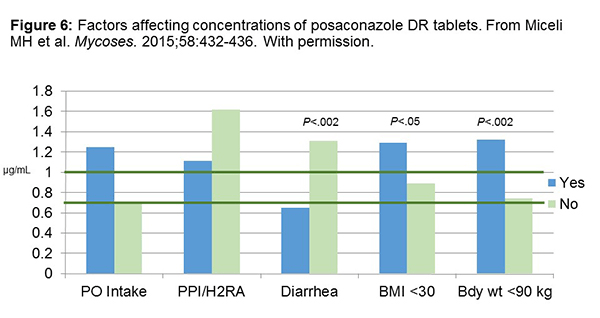
Looking at factors, however, that would affect the DR tablet concentrations, we can see the new formulation is still not perfect. As shown in Figure 6, patients with diarrhea, depicted here in blue, have about half the levels vs people that do not have diarrhea (green). Similarly, people who have larger BMI (>30), have about half the levels of people who have BMIs <30. People who are obese, with large body mass or body weight, will have lower concentrations than people with low body weight (<90 kg). On average, 70% or 71% of patients in this particular study achieved target concentrations of 0.7 mcg/mL.[Miceli 2015] If you look at a target concentration closer to 1, as you’ll see, a lower proportion of patients actually achieve that. There are patients that you will be treating that still are not at optimal posaconazole concentrations with the DR tablets, and you have to definitely take that into consideration with your TDM.[Miceli 2015]
Overall, the challenges of optimizing dosing in the population still persist. We have challenges with poor absorption for itraconazole, posaconazole, or voriconazole. We also have high inter- and intra-patient variability, which still seems to be a concern for itraconazole and voriconazole (we’ll go over next), and the lack of an oral dosage form for candins and amphotericin B.
Voriconazole does not show a great dose response, so if you increase the dose, the concentrations don’t proportionally increase as well, so its kinetics are non-linear.[Trifilio 2007] One of the problems with giving voriconazole in these patients is that if you repeat the concentration a week after you did your last concentration, it could be different. We don’t necessarily know why that happens. There may be changes in clinical parameters, or their metabolism gets revved up. There have been reports of induction or self-induction. It is not really known why this variability occurs, but it’s highly variable even within a patient. Individual patient levels vary on different weeks, and levels from patient to patient (on the same dose) can be dramatically different.[Trifilio 2007]
Target Levels and TDM
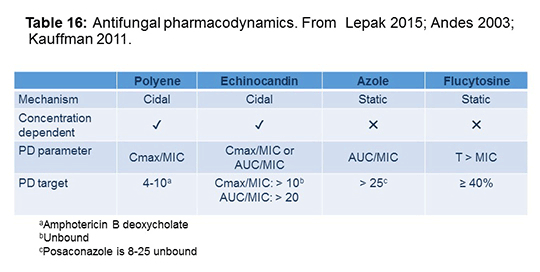
How do we target our dosing for these drugs? Some of it has to do with the pharmacodynamics, where we know a drug is cidal and other drugs are static. We have these PK parameters, that at least in animal models or in some clinical models, have been shown to be relevant. As shown in Table 16, for polyenes, peak to MIC is the parameter of interest whereas for candins, it’s peak to MIC or AUC/MIC. For azoles, it’s AUC/MIC and for flucytosine, it’s time above the MIC. These are the relevant targets. These are still hotly debated in the literature. I still don’t think that after 20 years of doing this we have the final answer on this issue, but this is what we have to go by at this time.[Lepak 2015; Andes 2003; Kauffmann 2011]
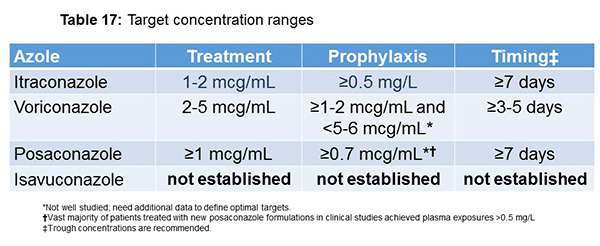
Recently, the Australian experts got together and published some target concentration ranges for azoles, as did the Canadian group. Table 17 is basically a compilation of that work. For voriconazole, in the setting of treatment, they recommend 2 to 5 mcg/mL as a target for trough concentration. For posaconazole, the target is at least 1 mcg/mL for treatment, and for isavuconazole, we still don’t know. For prophylaxis, they recommend a target between 1 to 2 and 5 to 6 mcg/mL for voriconazole, and for posaconazole, it’s greater than 0.7 mcg/mL. I mentioned earlier, regarding the timing of testing, again, it is still not necessarily perfect, but for voriconazole, they’re saying at least get your first level after 3 to 5 days. For posaconazole, they say at least 7 days. Many of the posaconazole studies that I showed you for DR tablets used a 5-day cut point. Take it as you will.[Chau 2014; Laverdiere 2014; Jung 2014; Noxafil briefing document]
For isavuconazole, we’re still getting more information about the exposure-response relationships expected with this drug. There’s not much out there yet but in a small study, a subgroup analysis of the larger SECURE Aspergillus trial that was presented at ECCMID last spring, they looked at 36 patients that had paired MICs from their Aspergillus isolates as well as PK samples. They put together the parameters and evaluate whether they reached any kind of pharmacodynamic target in each patient. They compared the MICs, which were between 0.5 and 16 mg/L and the wide range of AUC/MIC exposures in these patients, which were in the 37 to 196 range, which is actually pretty good. They’re above that AUC/MIC target of 25 that we talked about earlier. Given this, they found no statistically significant relationships between any one pharmacokinetic parameter and outcome for clinical outcome in that study. The only thing that was predictive of outcome was duration of treatment, which to me meant the patient was still alive to receive treatment. They were not able to identify any specific pharmacodynamic targets in that study. However, this was a very small study though and limited by power and so hopefully as the drug evolves, we’ll get more information.[Desai 2015]
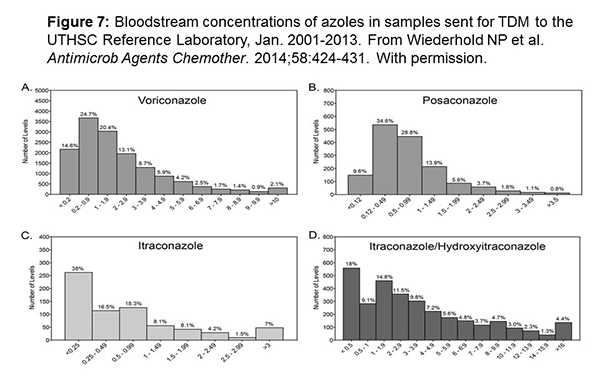
Figure 7 shows some data about how we’re doing in the real world setting. Again, credit to Nathan Wiederhold who runs the reference laboratory at the University of Texas Health Science Center. His group shows that about 30% to 50% of all levels sent to his lab are subtherapeutic. Despite the fact that we know what our targets are, we know what standard doses are, we’re still not achieving our target concentrations 30% to 50% of the time depending on what you put that target level at.[Wiederhold 2014] It would be a good idea if we did better from the beginning and weren’t chasing our tail on these infections.
Adverse Events
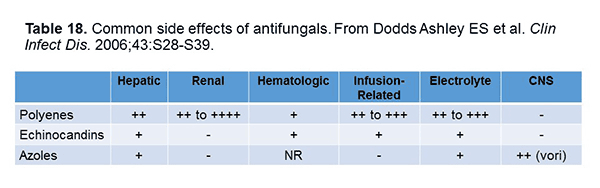
In terms of side effects, I think we’re all pretty well aware of the class side effects of the antifungals (Table 18).[Dodds Ashley 2006] As Drs Kontoyiannis and Lewis mention, addressing adverse events is an important consideration, and TDM is an important tool in this process.[Kontoyiannis 2015] Indeed, some of the side effects can be attributed to high concentrations that can be managed by your TDM.
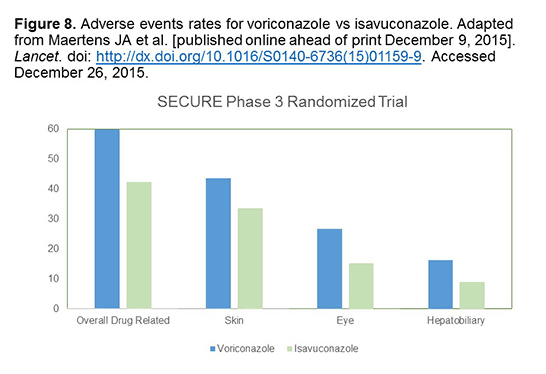
In terms of adverse events for isavuconazole, what really stands out is that overall, in terms of overall drug-related reactions, there was about 20% less toxicity with isavuconazole here in the green vs voriconazole in the IA study (Figure 8). This is the big splash for isavuconazole. It’s a drug that has more linear kinetics, more predictable levels, and about 20% less toxicity with maybe efficacy that is similar for IA vs voriconazole. When you break it down into the individual adverse events, it’s a real smattering so it’s something like 10% less in terms of skin toxicity and eye toxicity and maybe 5% or 10% less than hepatobiliary toxicity. It’s a little bit of everything so it’s not just one toxicity that’s better. It’s just a little bit here and there and it all adds up to about a 20% difference.[Maertens 2015]
Real-World Strategies to Optimize PK/PD
What does this all mean to us on the wards? What I’m out there doing every day and getting calls about pharmaco-optimizing PK, I live in the zone of: “My patient has been on this drug for 5 to 6 days and the drug is still undetectable. What do I do?” This is the constant call that I get. For low levels, we think about things like are they absorbing it, are they hypermetabolic, are they metabolizing it quicker than you ever thought they would because they have an underlying condition like cystic fibrosis? We look at the dosing, and consider their body weight. Are they a large body-weight or a small body-weight person? Are they adherent? Of course, that’s the first thing you go to if you’re treating a patient with HIV. Age or timing of the level are also important. Young children and adolescents metabolize things differently than adults, and that’s always a consideration here. Was the level drawn too early based on the duration of therapy or was it wrongly timed in relation to the dose?
If there are high levels, think about: Is it leading to toxicity, how are they being dosed, are they mistakenly taking it too often or not in a way that they should? We had a guy crushing the posaconazole DR tablets and putting them in a G tube. His trough concentrations were like 30 mcg/mL. People do crazy things. This can lead to erratic concentrations or levels that may be high.
For patients with low levels, specifically with voriconazole, you may need to make some changes. For example, if you do have a patient who might be on day 5 of IV therapy and still has undetectable levels, you might consider adding omeprazole, using that cytochrome P450 interaction to your advantage, and elevating the voriconazole level. There are case reports of that in the literature. We do not do it routinely, but I’ve seen a couple really hard-to-treat cases where it was helpful. We also will consider increasing the frequency of dosing of a mold-active azole if they’re a hypermetabolizer, and we can’t get a measurable concentration even after multiple days of standard dosing. Instead of giving voriconazole or posaconazole (as suspension) 2 times a day, you might be giving it 3 or 4 times a day. That may be better than giving just an elevated dose fewer times a day, because they are eliminating it as fast as you give it. Obviously, consider covering with an alternate agent or consider an alternate agent until you can get a therapeutic concentration in that patient. For posaconazole, you’ve got to go back to thinking about the formulation. Are you using the suspension, are you using the DR tablets, or are you using IV? If using a suspension, you can think about the acidic beverage, and get rid of the PPIs or H2 blockers if you can. Think about your dosing options. Do you want to do more than once a day or do you want to keep it at once a day if the patient is on DR tablets? Consider IV therapy or an alternate agent as well.
If you’ve got high levels, again, think about the timing. Was the level draw right at the peak, out of the same line the IV was infusing through? That might explain a ridiculously high level if your patient is not having any problems. As for toxicity, think about your drug interactions and if they’ll continue on those interacting drugs. If you’re really concerned about it, particularly with voriconazole, if you get a level back that’s ridiculously high, I would go ahead and hold the dose, let it come down, and then restart using a proportion of the dose you were using previously, and then recheck your levels.
Summary
In summary, we have yet to develop the optimal antifungal agent. The available agents do differ in spectrum, their pharmacokinetics and pharmacodynamics, and drug/drug, drug/food interactions. We do have some data available to help guide us with a TDM but we definitely need more data in this area. Overall, you should use these available data and accepted principles we’ve discussed to individualize therapy for your patients.
Interdisciplinary Case Application
As Drs Kontoyiannis and Lewis advised, multidisciplinary care is an important component of management of IFIs.[Kontoyiannis 2015] The following case illustrates some of the aspects of care described above.
Case 1: Patient with a Hematologic Malignancy
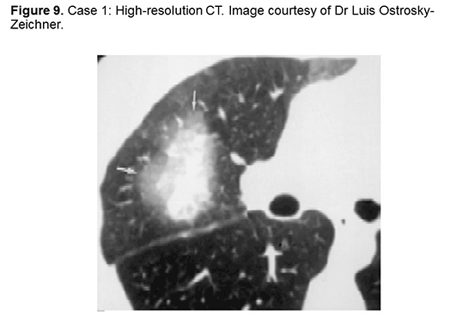 The case we’re going to discuss is a 27-year-old Hispanic woman with ALL who is undergoing induction chemotherapy. It’s important to know that her regimen includes vincristine. She has persistently increased ALT and AST. She has been persistently neutropenic for more than 10 days. She’s having just an unusually prolonged neutropenia. She is complaining of chills, malaise, and mild nonproductive cough. You do a high-resolution CT and you find this little fellow (Figure 9). What is the best next diagnostic test to be done for this patient?
The case we’re going to discuss is a 27-year-old Hispanic woman with ALL who is undergoing induction chemotherapy. It’s important to know that her regimen includes vincristine. She has persistently increased ALT and AST. She has been persistently neutropenic for more than 10 days. She’s having just an unusually prolonged neutropenia. She is complaining of chills, malaise, and mild nonproductive cough. You do a high-resolution CT and you find this little fellow (Figure 9). What is the best next diagnostic test to be done for this patient?
The next diagnostic test should be:
- BAL
- Serum GM
- BAL GM
- Serum BDG
- Fine-needle transthoracic biopsy
Expert commentary:
- It is important to emphasize that you send the BAL for these markers like GM because you’ll just increase your yield a lot, and so I think it’s really important to get the whole team on board—pulmonary doctors, the micro lab, whoever does the GM testing, so it’s really a team effort.
- For some patients with very peripheral lesions, that fine needle is probably not as bad an option as is generally thought, so sometimes that can be a useful test as well. This procedure is underutilized but has a good yield. Moreover, some individuals worry about bleeding complications because of low platelets, but it is a prolonged bleeding time that is the issue, not low platelets.
- Beyond the BAL GM, it is important to send a regular BAL for culture and microscopy. You want to try to recover the organism, because you may want to do susceptibilities. Not everything is Aspergillus
- There may be some complementary information with serum GM in this patient. Experience tells us that some of these patients, for some reason, have an invasive disease that is spilling over to the blood stream, and you capture it. While serum galactomannan can be affected by several factors, both tests can be complementary, both diagnostically and prognostically. It’s pretty clear now that routine serial sampling in asymptomatic patients on mold active therapy gives a very low yield of true positive results. In a symptomatic patient, the yield won’t be zero.
We’re going to move onto the next decision point for this patient. The patient is started on vancomycin, cefepime, and metronidazole. You do send the BAL for culture and cytology. The serum BDG comes back at 70 pcg/mL.
Do you start an antifungal?
- NO, BDG is negative and it has 99% NPV
- Yes, voriconazole
- Yes, L-AmB
- Yes, isavuconazole
- Yes, voriconazole + anidulafungin
- Yes, posaconazole
Any commentary on the BDG being 70 in this patient?
Expert commentary:
- That’s kind of close to a cut off. You’re going to be very anxious about that. You can’t be 100% sure that you don’t have an infection. There’s certainly organisms that this could be where beta glucan results are going to be negative, like Mucor or crypto even. I think you’ve got to be careful about that. Unfortunately, the therapeutic choices are really hard, because they’re not really a good one, frankly, because you’ve got to take into account that vincristine.
- Liposomal amphotericin is probably going to be a better choice in this setting, I would say, but again, you already have elevated LFTs, and L-AmB is certainly associated with increased liver toxicity as well. Moreover, because of the potential drug interaction with vincristine, you would avoid voriconazole or posaconazole. LAmB is a good choice for that reason, and it has better mold activity. You don’t know what you’re dealing with at this point, it could be Mucor, so at least you would cover both with that.
- We didn’t put an echinocandin on the list, which you’d be really cautious about that option because of the limited activity for molds other than Aspergillus. You don’t have a diagnosis here.
- An opposing viewpoint is that we don’t really know that much about the toxicity between vincristine and azoles. Most of the data comes from a single institution study. Peripheral neuropathy, grade I or II, you can tolerate it depending on how sick the patient is. Vincristine is metabolized by the 3A4 system. Isavuconazole also is metabolized so theoretically, you can have a problem with all azoles, but maybe the problem is over-emphasized. The neurotoxicity propensity of azoles with vincristine has been described,[Moriyama 2012] but most of the studies are not very good. Investigators are looking more closely at the impact of these interactions and how serious the interactions are.
The patient continues to deteriorate and now requires intubation. You repeat the CT, and the CT is showing cavitation. You do a serum GM, and that is 0.5, the BAL GM is 0.4, and the cytology is showing broad non-septate hyphae.
Would you give/change antifungals at this time?
- No, BG and GM are negative, cytology=contaminant
- Yes, posaconazole
- Yes, L-AmB + caspofungin
- Yes, isavuconazole
- Yes, voriconazole + anidulafungin
We have a patient that is deteriorating, negative BDG essentially, negative GM in serum and in BAL, and broad, non-septate hyphae. I think everybody should be thinking about Mucor in this case at this point in time, and the question is what is the ideal therapy for mucormycosis today in October of 2015?
Expert Commentary:
- Some experts would use L-AmB plus caspofungin, with the caveat that they’d like to see more isavuconazole data on this and certainly having some comparative data would be nice. We try to add on posaconazole at some point and transition them to that if they survive long enough.
- Others suggest they would individualize based on the patient. If you had a patient whose creatinine was really elevated, then you’re probably going to be more inclined to choose one agent or the other. Liver function, kind of the same way. That might sway you one way or the other. I think this is a time where you’re going to have multiple agents on your formulary, hopefully, and you’re going to be able to choose based on the pharmacokinetic principles outlined. That’s going to keep our jobs secure as ID clinicians, because I think we can be helpful in that setting to really help guide the right choices.
- The experts also provided expertise on combination therapy for mucormycosis. Their perspective was that combination therapy may not be as important as early initiation of therapy.
Case 2: Woman Post MVA with Abdominal Trauma
The second case is a 39-year-old white woman, hospitalized for a motor vehicle accident with extensive abdominal trauma. She is in day 10 of a surgical ICU state, status post splenectomy. She has a colostomy and multiple fracture repairs. She is febrile at 38.9°C on vancomycin and piperacillin/tazobactam. She has high fluid requirements, is maintaining blood pressure, has multiple lines including a left femoral catheter, which was inserted 10 days ago, and she has negative blood cultures. Is this a patient at high risk or low risk for fungal infection?
Which of the following are known risk factors for IFIs in this patient:
- Prolonged ICU stay
- Asplenia
- High fluid requirements
- All of the above
- None of the above
Expert Commentary:
- The faculty suggested that the main risk factor is the central venous catheter that has been in place for over a week. The ICU stay is also important. Formally, of the risk factors this patient has, the only one that has been described in the literature as an actual risk factor for candidemia is prolonged ICU stay.[Ostrosky-Zeichner 2011] There’s no real literature pointing to asplenia or high fluid requirements or a septic patient being at particularly high risk. This was kind of a trick question. The only one really described in the literature is A (prolonged ICU stay).
Would you provide antifungals?
- Yes, with fluconazole
- Yes, with an echinocandin
- Yes, with amphotericin B
- No, the blood culture is negative
My general thought people might start on an echinocandin in this case. Do you want to comment on these options?
Expert Commentary:
- I think it’s interesting because she doesn’t necessarily have specific risk factors for azole-resistant Candida, but she’s got a valve that is a potential source as well as the femoral catheter we talked about. She had a colostomy, I think, and some other bowel trauma and you always worry a little bit about glabrata in that setting, maybe even tropicalis living in the gut or If she’s not gotten fluconazole in the past, I know there are some hospitals out there that would go with fluconazole, particularly community hospitals.
- The opposing view is that some experts would use a lot of fluconazole in a person like this, frankly. It depends a little on how sick she was. If she’s really very septic, of course, she’s going to get an echinocandin but if she’s mostly febrile, has leukocytosis or just not doing well, we’re going to probably start fluconazole really early and do the work-up. We’re going to look really close for glabrata to make sure she is not colonized any place with resistant glabrata, then we’re going to be ready to move on to echinocandin pretty quick.
- Experts referenced the Kontoyiannis and Lewis article about the importance of knowing the local epidemiology.[Kontoyiannis 2015] One of the experts has 60% non-albicans in her institution, so she would typically start with echinocandin until proven otherwise. Then if it comes back as something susceptible to fluconazole, would consider switching. At a tertiary academic medical center, that’s more often what she sees.
- Another expert indicated that if you’re going to start fluconazole, it’s very important not to underdose the drug because of the obesity epidemic in the United States and the dosing: a 130-kg person shouldn’t get 400 mg of fluconazole.
- While there is some question about what to do, in every meta-analysis, the echinocandin looks better than azoles and the classic diagram for studies in candidemia show that if patients are very stable (based on APACHE II score), all drugs look good. If their APACHE II score is low, they do well no matter what you do; if it is above 20, all drugs look bad and in between, an echinocandin has an edge. This again is another indication that the host is an important factor to consider.[Kontoyiannis 2015]
Do we need to make a dose adjustment for echinocandins in critically ill people?
- That’s a good question. I think it’s an evolving area. People have started to look at this but we don’t routinely at this point dose escalate. That said, some people decrease the caspofungin dose if the patient has hepatic dysfunction (according to package insert recommendations) but we tend to not do that. We don’t give 35 mg daily to patients just because they have a slight elevation in LFTs but certainly if they have fulminant liver failure, then the package insert would say to decrease the dose.
- I would definitely agree. We never decrease the dose of the echinocandin. We don’t give those higher doses of micafungin though. We give a standard dose.
- We would sometimes dose escalate micafungin more for concern about resistance, for instance, in somebody who has been on micafungin in the past and has a resistant isolate but there’s no data to support this. It’s unknown whether that would ever overcome any mutation, honestly. People are just desperate.
The case progresses, the patient is started on caspofungin. The blood culture remains negative. We send out a BDG and the BDG comes back at 989. We’re very lucky in that we have the T2 test, and the T2 comes back positive for C parapsilosis. The patient continues to be febrile.
What do we do now?
- Stop antifungals, BDG and T2 may be false +, no response and negative blood cultures
- Switch caspofungin to fluconazole
- Switch caspofungin to L-AmB
- Remove central line
What’s your level of comfort in treating C parapsilosis with echinocandins?
Expert Commentary:
- It's not too bad, but the comfort level in treating Candida parapsilosis with a line in place is zero. You can make the argument in some patients that the gut is really the source (like in an oncology/hematology setting), but in this setting, with the line, you think P for plastic, P for parapsilosis, and P for persistent. It's just not going to go away if you don't pull that line out. We've probably overplayed the resistance of echinocandins for this pathogen, since C parapsilosis did not fall out as a factor in studies, C tropicalis did.
- There are data for caspofungin showing successful treatment of a severe infection with C parapsilosis in conjunction with hyperbaric oxygen.[Deshpande 2003] Most of the literature suggests source control in this situation is more important than the theoretical resistance with echinocandins. Moreover, parapsilosis is a complex of organisms. Sometimes you see some resistance in some of the parapsilosis isolates, but common things being common things, in this patient, you should remove the line and reassess.
- In terms of activity, liposomal AmB is similar to caspofungin. Both are pretty good against biofilm.[Iñigo 2012] Azoles are less active against biofilms. An azole would probably not be your choice if you had to leave the line in.
REFERENCES
- Andes D. In vivo pharmacodynamics of antifungal drugs in treatment of candidiasis. Antimicrob Agents Chemother. 2003;47:1179-1186.
- Aranda-Rajah MR, Kontoyiannis D. Isavuconazole: a new extended spectrum triazole for invasive mold diseases. Future Microbiol. 2015;10:693-708.
- Arikan S, Yurdakul P, Hascelik G. Comparison of two methods and three end points in determination of in vitro activity of micafungin against Aspergillus spp. Antimicrob Agents Chemother. 2003;47:2640-2643.
- Becker MJ, Lugtenburg EJ, Cornelissen JJ, Van Der Schee C, Hoogsteden HC, De Marie S. Galactomannan detection in computerized tomography-based broncho-alveolar lavage fluid and serum in haematological patients at risk for invasive pulmonary aspergillosis. Br J Haematol. 2003;121:448-457.
- Beyda ND, Alam MJ, Garey KW. Comparison of the T2Dx instrument with T2Candida assay and automated blood culture in the detection of Candida species using seeded blood samples. Diagn Microbiol Infect Dis. 2013;77:324-326.
- Boogaerts M, Winston DJ, Bow EJ, et al; Itraconazole Neutropenia Study Group. Intravenous and oral itraconazole versus intravenous amphotericin B deoxycholate as empirical antifungal therapy for persistent fever in neutropenic patients with cancer who are receiving broad-spectrum antibacterial therapy: a randomized, controlled trial. Ann Intern Med. 2001;135:412-422.
- Borowski E. Novel approaches in the rational design of antifungal agents of low toxicity. Farmaco. 2000;55:206-208.
- Ceesay MM, Desai SR, Berry L, et al. A comprehensive diagnostic approach using galactomannan, targeted β-d-glucan, baseline computerized tomography and biopsy yields a significant burden of invasive fungal disease in at risk haematology patients. Br J Haematol. 2015;168:219-229.
- Chau MM, Kong DC, van Hal SJ, et al. Consensus guidelines for optimising antifungal drug delivery and monitoring to avoid toxicity and improve outcomes in patients with haematological malignancy, 2014. Intern Med J. 2014;44:1364-1388.
- Chong GL, van de Sande WW, Dingemans GJ, et al. Validation of a new Aspergillus real-time PCR assay for direct detection of Aspergillus and azole resistance of Aspergillus fumigatus on bronchoalveolar lavage fluid. J Clin Microbiol. 2015;53:868-874.
- Cornely OA, Böhme A, Schmitt-Hoffmann A, Ullmann AJ. Safety and pharmacokinetics of isavuconazole as antifungal prophylaxis in acute myeloid leukemia patients with neutropenia: results of a phase 2, dose escalation study. Antimicrob Agents Chemother. 2015;59:2078-2085.
- Cresemba Prescribing Information. Northbrook, IL: Astellas Pharma US; 2015.
- Desai AV, Kovanda L, Hope W, et al. Exposure response analysis of isavuconazole in patients with disease caused by Aspergillus species or other filamentous fungi. Poster presented at 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID); Copenhagen, Denmark; 25–28 April, 2015. P0217.
- Deshpande K. Candida parapsilosis fungaemia treated unsuccessfully with amphotericin B and fluconazole but eliminated with caspofungin: a case report. Crit Care Resusc. 2003;5:20-23.
- D'Haese J, Theunissen K, Vermeulen E, et al. Detection of galactomannan in bronchoalveolar lavage fluid samples of patients at risk for invasive pulmonary aspergillosis: analytical and clinical validity. J Clin Microbiol. 2012;50:1258-1263.
- Dodds ES, Drew RH, Perfect JR. Antifungal pharmacodynamics: review of the literature and clinical applications. Pharmacotherapy. 2000;20:1335-1355.
- Dodds Ashley ES, Lewis R, Lewis JS, Martin C, Andes D. Pharmacology of systemic antifungal agents. Clin Infect Dis. 2006;43:S28-S39.
- Duarte RF, Sánchez-Ortega I, Cuesta I, et al. Serum galactomannan-based early detection of invasive aspergillosis in hematology patients receiving effective antimold prophylaxis. Clin Infect Dis. 2014;59:1696-1702.
- Durani U, Tosh PK, Barreto JN, Estes LL, Jannetto PJ, Tande AJ. Retrospective comparison of posaconazole levels in patients taking the delayed-release tablet versus the oral suspension. Antimicrob Agents Chemother. 2015;59:4914-4918.
- González-Ramos MM, Bertrán-Pasarell J, Guiot H, et al. Clinical experience with posaconazole in patients with invasive mucormycosis: a case series. P R Health Sci J. 2008;27:328-332.
- Herbrecht R, Denning DW, Patterson TF, et al. Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408-415.
- Iñigo M, Pemán J, Del Pozo JL. Antifungal activity against Candida biofilms. Int J Artif Organs. 2012;35:780-91.
- Jung DS, Tverdek FP, Kontoyiannis DP. Switching from posaconazole suspension to tablets increases serum drug levels in leukemia patients without clinically relevant hepatotoxicity. Antimicrob Agents Chemother. 2014;58:6993-6995.
- Kapoor R, Yamaguchi I, Chang W. A fast and sensitive (1→3) - β-D-glucan microfluidic assay for the diagnosis and treatment monitoring of invasive fungal infections. American Association for Clinical Chemistry Annual Meeting and Clinical Lab Expo. July 27-31, 2014; Chicago, IL.
- Kauffmann CA, Pappas PG, Sobrl JD, Dismukes WE, eds. Essentials of Clinical Mycology. 2d ed. New York, NY: Springer; 2011.
- Kontoyiannis DP, Lewis RE. Treatment principles for the management of mold infections. Cold Spring Harb Perspect Med. April 2015; 5:a019737.
- Kraft WK, Chang PS, van Iersel ML, Waskin H, Krishna G, Kersemaekers WM. Posaconazole tablet pharmacokinetics: lack of effect of concomitant medications altering gastric pH and gastric motility in healthy subjects. Antimicrob Agents Chemother. 2014;58:4020-4025.
- Laverdiere M, Bow EJ, Rotstein C, et al. Therapeutic drug monitoring for triazoles: a needs assessment review and recommendations from a Canadian perspective . Can J Infect Dis Med Microbiol. 2014;25:327-343.
- Lepak AJ, Andes DR. Antifungal pharmacokinetics and pharmacodynamics. Cold Spring Harb Perspect Med. May 2015;5:a019153.
- Lewis RE. Pharmacokinetic considerations for the use of newer antifungal agents. Curr Fungal Infect Rep. 2008;2:5-11.
- Lewis RE. Current concepts in antifungal pharmacology. Mayo Clin Proc. 2011;86:805-817.
- Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomized-controlled, non-inferiority trial [published online ahead of print December 9, 2015]. Lancet. doi: http://dx.doi.org/10.1016/S0140-6736(15)01159-9
- Marr KA, Laverdiere M, Gugel A, Leisenring W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin Infect Dis. 2005;40:1762–1769.
- Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162:81-89.
- Miceli MH, Perissinotti AJ, Kauffman CA, Couriel DR. Serum posaconazole levels among haematological cancer patients taking extended release tablets is affected by body weight and diarrhoea: single centre retrospective analysis. Mycoses. 2015;58:432-436.
- Moriyama B, Henning SA, Leung J, et al. Adverse interactions between antifungal azoles and vincristine: review and analysis of cases. Mycoses. 2012;55:290-297.
- Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49:3640-3645.
- Mylonakis E, Clancy CJ, Ostrosky-Zeichner L, et al. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: a clinical trial. Clin Infect Dis. 2015;60:892-899.
- Nguyen MH, Wissel MC, Shields RK, et al. Performance of Candida real-time polymerase chain reaction, β-D-glucan assay, and blood cultures in the diagnosis of invasive candidiasis. Clin Infect Dis. 2012;54:1240-1248.
- Noxafil FDA Briefing Document.
- Odabasi Z, Mattiuzzi G, Estey E, et al. β-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis. 2004;39:199-205.
- Ostrosky-Zeichner L, Alexander BD, Kett DH, et al. Multicenter clinical evaluation of the (1→3) β-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41:654-659.
- Ostrosky-Zeichner L, Pappas PG, Shoham S, et al. Improvement of a clinical prediction rule for clinical trials on prophylaxis for invasive candidiasis in the intensive care unit. Mycoses 2011; 54:46–51.
- Ostrosky-Zeichner L, Shoham S, Vazquez J, et al. MSG-01: a randomized, double-blind, placebo-controlled trial of caspofungin prophylaxis followed by preemptive therapy for invasive candidiasis in high-risk adults in the critical care setting. Clin Infect Dis. 2014;58:1219-1226.
- Pfaller MA, Rhomberg PR, Messer SA, Jones RN, Castanheira M. Isavuconazole, micafungin, and 8 comparator antifungal agents' susceptibility profiles for common and uncommon opportunistic fungi collected in 2013: temporal analysis of antifungal drug resistance using CLSI species-specific clinical breakpoints and proposed epidemiological cutoff values. Diagn Microbiol Infect Dis. 2015;82:303-313.
- PR Newswire. Astellas provides update on phase 3 study evaluation isavuconazole in patients with candidemia and other invasive Candida infections. July 30,2015. http://www.prnewswire.com/news-releases/astellas-provides-update-on-phase-3-study-evaluating-isavuconazole-in-patients-with-candidemia-and-other-invasive-candida-infections-300121072.html. Accessed December 17, 2015.
- Redding SW, Marr KA, Kirkpatrick WR, Cooc BJ, Patterson TF. Candida glabrata sepsis secondary to oral colonization in bone marrow transplantation. Medical Mycology. 2004;42:479-481.
- Thornton CR. Development of an immunochromatographic lateral-flow device for rapid serodiagnosis of invasive aspergillosis. Clin Vaccine Immunol. 2008;15:1095-1105.
- Townsend R, Desai A, Azie N, Jones M, Englehardt M, Schmitt AH. Drug interaction profiles of isavuconazole, voriconazole and posaconazole with immunosuppressants metabolized by CYP4503A4 (CYP3A4). Poster presented at 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID); 25–28 April, 2015; Copenhagen, Denmark; P0216.
- Trifilio S, Pennick G, Pi J, et al. Monitoring plasma voriconazole levels may be necessary to avoid subtherapeutic levels in hematopoietic stem cell transplant recipients. Cancer. 2007;109:1532-1535.
- Viscoli C, Machetti M, Cappellano P, et al. False-positive galactomannan Platelia Aspergillus test results for patients receiving piperacillin-tazobactam. Clin Infect Dis. 2004;38:913-916.
- White PL, Parr C, Thornton C, Barnes RA. Evaluation of real-time PCR, galactomannan enzyme-linked immunosorbent assay (ELISA), and a novel lateral-flow device for diagnosis of invasive aspergillosis. J Clin Microbiol. 2013;51:1510-1516.
- White PL, Wingard JR, Bretagne S, et al. Aspergillus polymerase chain reaction: systematic review of evidence for clinical use in comparison with antigen testing. Clin Infect Dis. 2015;61:1293-1303.
- Wiederhold NP, Pennick GJ, Dorsey SA, et al. A reference laboratory experience of clinically achievable voriconazole, posaconazole, and itraconazole concentrations within the bloodstream and cerebral spinal fluid. Antimicrob Agents Chemother. 2014;58:424-431.
- Wingard JR, Carter SL, Walsh TJ, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116:5111-5118.
- Winston DJ, Hathorn JW, Schuster MG, Schiller GJ, Territo MC. A multicenter, randomized trial of fluconazole versus amphotericin B for empiric antifungal therapy of febrile neutropenic patients with cancer. Am J Med. 2000;108:282-28.
Back to Top