
Expired activity
Please go to the PowerPak
homepage and select a course.
Addressing Hypoglycemia in Type 2 Diabetes Mellitus: The Pharmacists' Role in Optimizing Patient Outcomes
PART 1 - CLINICAL DIALOGUE
Moderator:
Jerry D. Meece, RPh, CDE, CDM, FACA, FAADE
Faculty:
Philip Raskin, MD, FACP, FACE, CDE
Scott Drab, PharmD, CDE, BC-ADM
Jerry D. Meece, RPh: I'd like to welcome Dr. Philip Raskin and Dr. Scott Drab this morning. I'm going to get started on the subject of hypoglycemia, a topic that seems to trouble and bother so many people with diabetes. How common is hypoglycemia among patients with type 1 diabetes versus type 2 diabetes? How do we even define hypoglycemia?
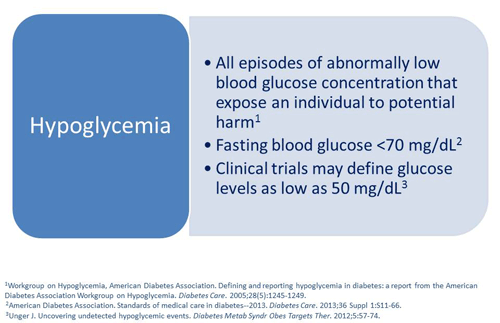
Scott Drab, PharmD: First, I think it's important to stress and understand that patients who suffer from hypoglycemia can really feel bad when they have an event and that's why it's so important. Hypoglycemia is having an episode of a low blood glucose concentration that can expose an individual to potential harm, ranging from falls to cardiovascular incidents.1 The American Diabetes Association defines hypoglycemia as typically having a blood glucose value less than 70 mg/dL,2 and that differs a little bit than what we use in clinical trials where they're measuring true hypoglycemic effects, which might occur at levels around 50 mg/dL.3
Jerry D. Meece, RPh: Dr. Raskin, what's your thoughts on the definition of hypoglycemia?
Philip Raskin, MD: The American Diabetes Association says that hypoglycemia occurs at blood sugar levels less than 70 mg/dL because that's when regulatory hormones fire off. Glucagon and epinephrine are released and that's what they use as the official definition. Now in clinical trials the hypoglycemia levels are predetermined. Generally, hypoglycemia is something that impacts a patient. They feel it and they have some bad outcomes from it.
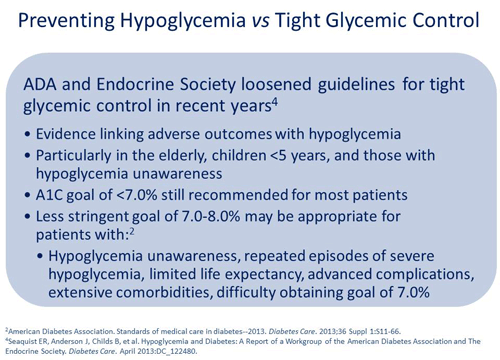
Jerry D. Meece, RPh: So this brings up the next question. After all these years of making sure that we're reaching out to clinicians and urging them to maintain the tightest control, the ADA and the Endocrine Society are both now advising that for some populations, preventing hypoglycemia is more important than tight control.4 So we need to have a balance between tight glycemic control and avoiding hypoglycemia.
How do these recommendations differ in various populations such as the young, old, etc.? How do you decide what goals are for whom?
Scott Drab, PharmD: Jerry, let me talk a little bit about older individuals because of my experience in long-term care. One of the things that we've known for quite some time is the fact that older individuals respond differently to hypoglycemia. We knew this back in the '90s, even probably before that. There was an excellent piece published in Diabetes Care in 1997 that showed that when we induce hypoglycemia in younger individuals, in their 40s, versus individuals in their 60s and 70s, that older individuals had less symptoms of hypoglycemia and it took longer for them to actually realize they were having the symptoms.5 These individuals also lost psychomotor function in this situation much sooner and to a higher degree than did younger individuals. So we know that older individuals respond differently. Now Dr. Raskin, can you explain a little bit about why that happens and what's going on physiologically?
Philip Raskin, MD: The brain only operates on glucose and ketones as a fuel. The brain cannot use free fatty acids as fuel as other tissues can, so when you're hypoglycemic and you haven't had time to make ketones, you're in trouble. The function of the brain depends on the concentration of glucose in the blood, the uptake of glucose from the blood into the brain, and also blood flow.
As we age, there are changes in our cerebral blood vessels which limit flow. So that's probably the reason why patients who are older take longer to recognize if they have hypoglycemia. Although the consequences of hyperglycemia can result in diabetic complications, often that happens over a long period of time after 20 or 25 years. Not everybody gets it. However, if you have a low blood sugar and you wreck your car, you can die right then and there. So in my view, to avoid hypoglycemia is the healthcare provider's first job.
Jerry D. Meece, RPh: I would add that there was a time in which I think we, as clinicians, looked at patients’ levels differently in an effort to avoid hypoglycemia completely. But I think total avoidance of hypoglycemia is something that we can't do. It's going to happen at some point. Fear of hypoglycemia drives up A1C just as much as actual hypoglycemia itself. So total avoidance of hypoglycemia, especially in type 1 diabetes mellitus is just not workable. Even in type 2 diabetes patients on insulin I think you're going to see hypoglycemia if we're getting to tighter goals. It's no one's fault. We didn’t do anything wrong; it is just a consequence of tight control.
We certainly want to avoid severe hypoglycemia. So Dr. Raskin, what is the difference between symptomatic hypoglycemia and severe hypoglycemia?
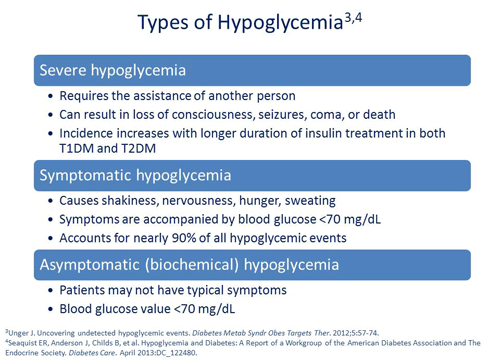
Philip Raskin, MD: Severe hypoglycemia is defined as hypoglycemia that requires the assistance of another person.2 That includes being so shaky that you can't get to the refrigerator to get a glass of orange juice or having to call the paramedics to give intravenous glucose. Symptomatic hypoglycemia causes you to have some symptoms that are compatible with hypoglycemia: shakiness, nervousness, hunger, and sweating.3 Then there's biochemical hypoglycemia, which you read on your blood glucose meter. There's a difference amongst all those things. Clearly, serious hypoglycemia needs to be avoided at all costs because it can end up in death, eg, if you're driving your car and you have an accident.
Scott Drab, PharmD: It's a balancing act. I had a patient who was having 30 hypoglycemic events per month and out of those 30, at least probably 10 of them required the help of somebody else. We were able get that patient down to three hypoglycemic events per month. We didn’t avoid it but we tried to create a balancing act.
Jerry D. Meece, RPh: So to that point, if we're looking at the physiological consequences of hypoglycemia, what do you see?
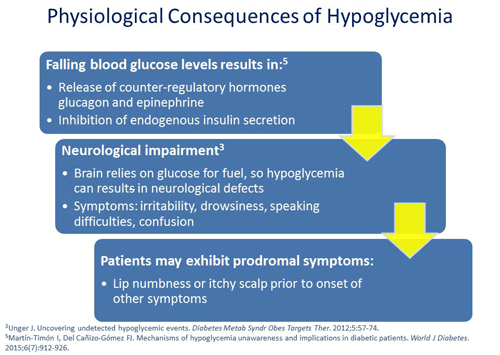
Philip Raskin, MD: If you were to lower the blood sugar to 70 mg/dL you would see first release of glucagon, which is the primary counter-regulatory hormone, and then epinephrine. As the blood sugar continues to fall, there are central nervous system changes. It doesn’t work right. People start to act silly or can look like they’ve had a stroke. All of those symptoms are sort of progressive but there's no real order. They feel the shakiness, the flight or fight feeling that you get when there's a big rush of epinephrine. That comes from the response to falling blood sugar.
Jerry D. Meece, RPh: When I'm interviewing patients I’ll ask them about their first symptoms from their perspective. I’ll say, "Tell me the very first sign of hypoglycemia that you feel." I'm really surprised sometimes at their responses. I've got one patient whose upper right lip gets numb. One of my patient's scalp itches like crazy and feels like it's on fire just preceding the sweating, tremors, and heart racing. I think it just shows the individualization of people with diabetes when we start really listening closely to the way they describe their symptoms.
Scott Drab, PharmD: I agree with that, Jerry, and I have a patient who says that they can't hear. Some individuals will get a sort of suspicion that this is coming on and that's a good indicator. I always try to categorize their symptomatology as a mild incident of hypoglycemia, a moderate incident or a severe incident to understand what they're experiencing. This helps with the treatment, too.
Philip Raskin, MD: It is a good indicator, but some people don't recognize the symptoms because their brain isn't working right. As the blood sugar falls, their cognitive function is reduced. To some extent, this depends on how fast the blood sugar falls and a whole series of other things. You have to educate people to pay attention to those things and to have a glucose source available, either in their pocket or in the glove box of their car, so that they can abort the episode as quickly as possible.
Scott Drab, PharmD: Not everybody goes through this little series of mild, moderate to severe episodes. They may go right to severe very, very quickly.
Philip Raskin, MD: That is correct. The first symptom may be a hypoglycemic seizure.
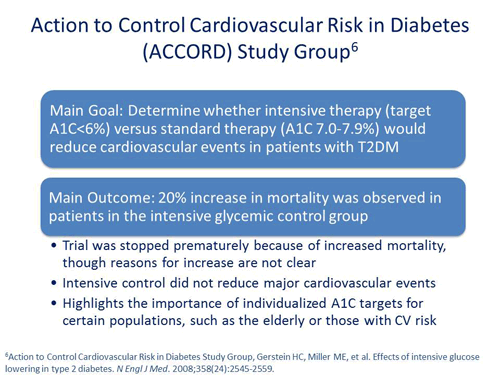
Jerry D. Meece, RPh: Let’s change directions a bit because this topic keeps popping up on the radar when discussing the aggressive treatment of diabetes and trying to drive A1C levels lower. In the ACCORD trial, the Action to Control Cardiovascular Risk in Diabetes study, researchers intensively tried to lower glucose. After some of the results came out, they stopped the trial because of the association with poor cardiovascular outcomes. Dr. Raskin, how did the ACCORD trial change the outlook of what physicians were trying to do? What is your view on it today?
Philip Raskin, MD: In the trial, they evaluated people that had diabetes for a very long time and already had advanced diabetic complications. The target for the intensive group in that trial was a hemoglobin A1C of 6%. It turns out that the trial was stopped because there were increased mortality in that intensive treatment group.6 Now, what caused that mortality? We still don't know. It turns out that the people that had the worst outcomes were the people that had the highest hemoglobin A1Cs to start. I think that it really solidified my long-term bias about the fact that you should not treat elderly and sick people aggressively as you might treat somebody with new diabetes.
Jerry D. Meece, RPh: Dr. Drab, did that change your view and those around you as far as what we're going to do to get A1C levels down lower?
Scott Drab, PharmD: Absolutely. I can remember hearing diabetes educators, endocrinologists, and opinion leaders throughout the country saying we need to treat patients to get their A1C level as low as we can get them. Of course, what we saw is exactly what Dr. Raskin said; there's a U-shaped curve that occurs in certain patients.7 As we lower A1C, we see an increased risk of hypoglycemia and therefore may be placing patients in danger. We're really now starting to identify who those patients are. They are patients that have had diabetes for long periods of time. They are individuals who are perhaps older or who may already have existing cardiovascular disease.
Philip Raskin, MD: The healthcare provider must decide what the targets are. The targets are not the same for every person and they have to be individualized. For some people, you shouldn’t treat aggressively. I think that targets are something that take common sense. You can't get common sense from reading a bunch of guidelines.
Jerry D. Meece, RPh: What I'm hearing from both of you is that it depends on the patient. So we've talked about the physiological consequences of hypoglycemia. Dr. Drab, what do you think are the psychological consequences of hypoglycemia? When a patient is having repeated episodes, what goes through their head?
Scott Drab, PharmD: Certainly there's an elevation in anxiety because you're always waiting for the next hypoglycemic reaction to occur. Also I've seen social isolation, where individuals are not engaging in activities.
We also see it in younger individuals, especially in patients with type 1 diabetes. When they eat or drink something to bring blood glucose values up, they become very conscientious about their weight and issues with their weight. So then oftentimes they'll go off of their insulin to try to lose weight and it creates a lot of different problems.
Jerry D. Meece, RPh: Dr. Raskin, is there a difference in the psychological makeup of someone who experienced hypoglycemia in type 1 versus type 2 disease?
Philip Raskin, MD: In patients with type 1 diabetes they're afraid of hypoglycemia, especially if they’ve had a really serious episode. I have patients who get up in the middle of the night every night to check because they're afraid. Some won't take certain doses of insulin at night because they're afraid. It's really a problem for type 1 diabetes.
In patients with type 2 diabetes, I guess it depends on whether or not these people with type 2 diabetes are having serious hypoglycemia.
Scott Drab, PharmD: Oftentimes in individuals with type 1, because they don't want to take insulin and because they are so frightened, this deep fear of hypoglycemia will drive up A1C values.
Jerry D. Meece, RPh: I had a patient that told me that one of her biggest fears was waking up dead. The statistics show that dead-in-bed syndrome occurs in 5% of patients with type 1 diabetes. The cause of death is still a bit of a mystery and it’s unclear whether this syndrome is due to heart arrhythmias or genetics combined with heart arrhythmias.8
So let's move on. What therapeutic strategies come into play when we're aiming for an A1C goal of 7%, while balancing the risks of hypoglycemia?
Scott Drab, PharmD: I make a general assessment of the patient and try to determine if there is an obvious need to be really concerned about hypoglycemia. Is this person operating heavy equipment? Is this person at a higher risk for hypoglycemia? Are they taking other medications that would concern me with regards to hypoglycemia?
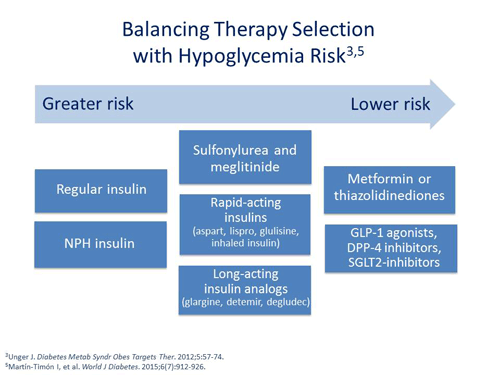
If this is the case, I'm going to try to use those particular therapies that are not associated with hypoglycemia, drugs like metformin, which is an older drug and is recommended as a first-line agent, or GLP-1 receptor agonists, DPP-4 inhibitors and the new SGLT2-inhibitors.9 The older drugs, such as sulfonylurea and meglitinide, cause the secretion of insulin, and are associated with hypoglycemia.10 I may use metformin, sulfonylurea or meglitinide if medication cost is an issue.
When it comes to insulin therapy, I have shied away from the older human preparations, such as Human Regular and NPH insulin. I think there are fundamental problems with them. NPH has a peak effect and that peak effect can lead to hypoglycemia. Regular insulin lasts sometimes in the body 8 to 10 hours. The activity of Regular insulin from one injection to the next injection can overlap and lead to insulin stacking. With insulin stacking comes a greater risk of hypoglycemia. I've tended to use the newer insulin analogs that allow for a little bit greater flexibility and less instances of hypoglycemia.11
Jerry D. Meece, RPh: Dr. Raskin, how do you choose a drug for your patients? How much does hypoglycemia potential play into getting A1C down?
Philip Raskin, MD: I don't like hypoglycemia and any kind of insulin can cause hypoglycemia. I think drugs that are typically not associated with hypoglycemia are what you ought to use in older people or sicker people. If you have somebody with type 1 diabetes, you're obliged to use insulin. Sometimes the hypoglycemia is unavoidable. As a clinician, you try to minimize it by making sure that people take their shots like they're supposed to do and that you do your best to teach them how to balance food with insulin.
Scott Drab, PharmD: Do you see some differences in the insulin products? Do you see certain ones that might be associated with less hypoglycemia that you might be using in your practice?
Philip Raskin, MD: The analogue insulins are great but they're very expensive. I work in a public hospital and the cost of the medication is a really big deal to us. You get no better hemoglobin A1C effects with any insulin. NPH insulin is just as good as glargine 300 or glargine 100 at lowering A1C. The difference is that there's less hypoglycemia with glargine 300 or glargine 100 compared to NPH insulin.
Jerry D. Meece, RPh: The benefit of analogue insulin is you can rely on consistent dosing whether it's basal or bolus. If you compare that to NPH, we see much more variability.11 So the dose-to-dose variability in patients with non-analogue insulin is one thing that we look for very specifically when we're trying to tightly control A1C levels and avoid the major consequences of hypoglycemia.
So with that, we'll move on to another topic. Is there a link between having repeated episodes and a greater risk for cardiovascular issues or possibility of more hypoglycemic episodes to follow?
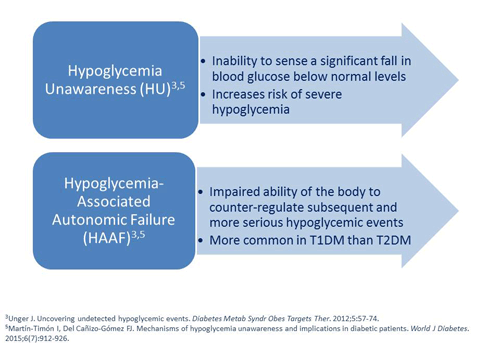
Philip Raskin, MD: You have less counter-regulatory action if you’ve had a recent hypoglycemic episode. So that having one makes you more likely to have another one. Also having repeated hypoglycemic episodes means that the level at which the counter-regulatory hormones are released gets lower - the blood sugar has to be lower and lower before they fire. Then you have a hypoglycemic episode and you have no symptoms. This inability of patients to detect a significant fall in their blood glucose levels is known as hypoglycemia unawareness.12 Sometimes patients think that's great. However, it's a terrible thing because there's no warning and patients can go from being seemingly okay to being in a coma instantly and it's dangerous. Sometimes the only way to identify hypoglycemia is to do a blood glucose test. This is especially true for people with type 1 diabetes.
Jerry D. Meece, RPh: What about hypoglycemia-associated autonomic failure or the body's inability to counter-regulate over time? Dr. Raskin, how much hypoglycemia unawareness do you see? How common is this syndrome?
Philip Raskin, MD: In type 1 diabetes it's very common. The reason is that as you have more and more hypoglycemia, the level at which the counter-regulatory hormones, glucagon and epinephrine, fire, and what you feel when you have hypoglycemia keeps getting lower and lower. If you’ve had hypoglycemia over and over again, then the counter-regulatory system doesn’t work so the blood sugar gets lower. Over time, you don't get the usual flight or fight symptoms or the ability to counter-regulate these events, and that is known as hypoglycemia-associated autonomic failure.3,11
Jerry D. Meece, RPh: Dr. Drab, in your patient population, is there a higher incidence with type 1 diabetes?
Scott Drab, PharmD: Dr. Raskin is right; it is more common with type 1 patients. But I've seen it in our type 2 diabetes patients when they progress and get older because there's less and less beta cell function. These patients are typically on basal bolus insulin. They're also fairly sensitive to insulin.
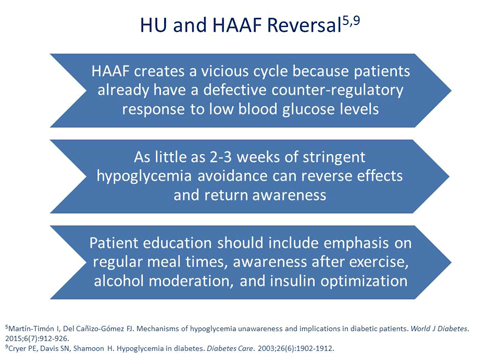
To counteract this problem, we'll artificially allow the patient’s blood glucose values to go up a little bit and prevent the hypoglycemic reactions from occurring. Studies have shown in as little as a week's time of not having repeated episodes of hypoglycemia, we can restore the normal firing mechanisms that Dr. Raskin is talking about. Two or three weeks might even be better but in as little as a week, we can restore that effect.13
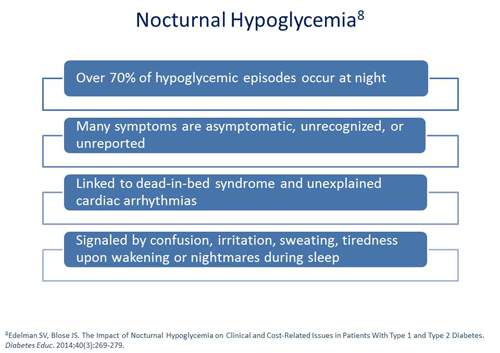
Jerry D. Meece, RPh: We’ve talked about dead-in-bed syndrome and we know that nocturnal hypoglycemia occurs, but how do we control it? What steps do you take?

Scott Drab, PharmD: As Dr. Raskin already alluded, patients need to monitor. When you place patients on continuous glucose sensors, it's interesting to see what happens overnight. In many patients who have stated that they’ve never experienced nocturnal hypoglycemia in fact actually did. Although many type 2 patients are not using continuous sensors on their own, we should take note of those patients that wake up with obvious symptoms of nocturnal hypoglycemia, such as not sleeping well, having nightmares, or soaking the bed. By using a continuous glucose sensor for several days in these patients, we may be able to confirm that indeed they are suffering from nocturnal hypoglycemia.
Philip Raskin, MD: Continuous glucose monitoring is good and it also has its downside. It goes off all the time in the middle of the night and wakes people up. Most of my patients that have used it don't like it. Most of them only use it periodically because it's annoying, especially if you have the hyperglycemia alarm on. I think measuring the blood sugar the old-fashioned way with the finger stick works well. Patients with type 1 diabetes are often so fearful of hypoglycemia at night that you don't really have to worry about it because they take care of it themselves.
Scott Drab, PharmD: Let me give you one example. I have a patient in her 50s with type 1 diabetes. Every night her husband would wake her up several times a night concerned about her having hypoglycemia. She never had a good night's sleep. We put her on a continuous sensor with the alarm. And she said, "I'm actually sleeping better. I'm sleeping the whole night through now," because her husband waits to hear the alarm. And she said, "It's made an amazing difference," not only just in her overall morale and attitude but also in her A1C values now.
Jerry D. Meece, RPh: Sometimes, we never even realize night time issues unless we ask specifically about hypoglycemia and how it's affecting patients. A few things that we suggest for patients is setting a specific goal for night time blood glucose levels and monitoring their levels if they exercise hard after supper. I think that little bit of advice goes a long way.
Philip Raskin, MD: I try to advise my patients not to go to bed with a blood sugar under a certain level, the same as if you were advising them to check their levels before driving a car.
Jerry D. Meece, RPh: How you go about choosing a therapy in order to reduce the occurrence of nocturnal hypoglycemia? Are you more likely to change medications or are you more likely to make a dose adjustment?
Scott Drab, PharmD: I already mentioned the use of insulin analogs. One of the problems we run into with the NPH insulin is the fact that it has a peak effect. When NPH is given in the evening, it would peak at 1:00 or 2:00 in the morning causing nocturnal hypoglycemia unless the person had a snack. A lot of it comes down to using better products that alleviate some of the problems we've seen with the older ones.
Philip Raskin, MD: Not all hypoglycemia occurs because of insulin. The sulfonylureas and the meglitinides tend to cause hypoglycemia. If you have a person that's older, frail, and more susceptible to hypoglycemia, you might choose drugs that don't usually cause hypoglycemia, such as metformin, DPP-4 inhibitors, or GLP-1 receptor agonists. Your choice of drugs for people with type 2 diabetes can be influenced by your concern of preventing hypoglycemia.
Scott Drab, PharmD: We're both saying that the use of specific products that tend to cause either less hypoglycemia or are not really associated with hypoglycemia may be important for certain people.
Philip Raskin, MD: Absolutely. That's exactly what we're saying.
Jerry D. Meece, RPh: One thing I think that is easy to sneak up on a clinician is the introduction of a new drug. When we're adding new drugs, we need to remember that drugs that may not have caused hypoglycemia in the beginning can now cause it. We may need to reduce the dose of that first drug going forward.
Scott Drab, PharmD: I agree with that, Jerry. I would also add that I think it's very important that clinicians not only treat A1C values but actually look at the blood glucose values. That's where frequent blood glucose monitoring is important. Certain medications work on fasting blood glucose, certain medications work on postprandial glucose and still others work on both. That’s why it is important to know which glucose value is elevated. It’s targeting drug therapy to the specific abnormality.
Jerry D. Meece, RPh: It's a reminder to all of us that A1C is the average over the period of three months, being weighted in the last month. But it's an average.

So let’s discuss motivational interviewing techniques. How do you approach patients about overall hypoglycemia and address how they approach it?
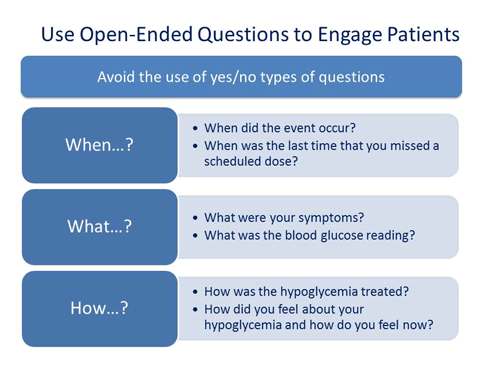
Scott Drab, PharmD: You constantly have to engage patients so they will deliver answers to you. In other words, not using yes/no types of questions and instead asking "How did you," "Why did you," "What did you."
Jerry D. Meece, RPh: The whole idea of motivational interviewing techniques and skills is to get a story from the patient. When I ask a patient, "How did you feel about your hypoglycemia and how do you feel now?” It's amazing what the power of silence really does. Ask those questions in the right way and allow the silence to fill the room. The patient is not going to have a quick response. We mistake that sometimes for them not knowing the answer but what they're really doing is processing. And I think because we're in a very rapid-fire world, clinician visits are short, we're moving patients in and out, and we don't allow that 30 seconds of total silence to occur while the patient is trying to figure out how they really feel about something like hypoglycemia. So our answers sometimes come by not talking more but by listening more.
For example, did a patient use the 15/15 rule? Did they check and 15 minutes later recheck after eating 15 grams of carbohydrate? More often than not, I don't see it in our patients.
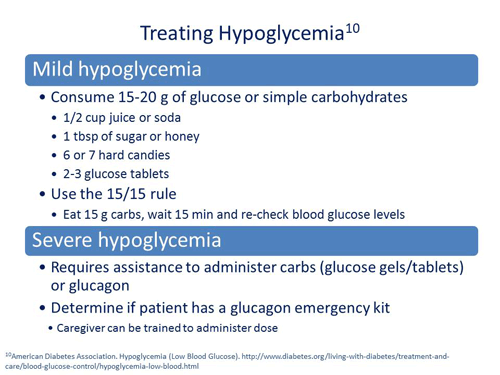
Scott Drab, PharmD: Right. That provides the pharmacist with a great opportunity to now enter into a discussion how you treat hypoglycemia. When are glucose tablets or glucose gels appropriate? When do we need glucagon? This gives the pharmacist a wonderful opportunity to discuss how to treat and how to manage hypoglycemia
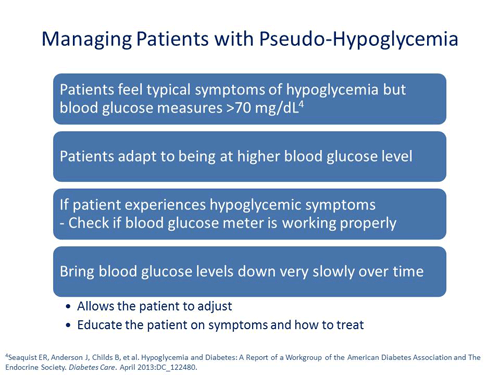
Jerry D. Meece, RPh: How do you address a patient when they say they feel like they’ve had a hypoglycemic event but there’s no reading of hypoglycemia anywhere on their meter?
Scott Drab, PharmD: The first thing I recommend is to check the meter to make sure it is functioning properly. We've seen that many times being a problem.
In addition, there are that subset of patients whose bodies are used to being at higher glucose values. If their glucose level is lowered too rapidly, they may start to feel signs and symptoms of hypoglycemia at normal glucose values. What is the solution? Some people tough it out but I don't think that's for everybody. What I like to do is bring glucose levels down very, very slowly in small increments which allow patients to adjust and adapt.
Jerry D. Meece, RPh: Dr. Raskin, how would you approach a patient in this situation?
Philip Raskin, MD: In the same way that people can become hypoglycemic and don't feel it, people can have blood sugar that's higher and have symptoms. It's just sort of an adaptation. If you persistently bring the blood sugars down in that patient, then they won't feel that hypoglycemia at say 110 or 120 mg/dL.
Jerry D. Meece, RPh: Taking the time to explain to patients is so key. From the standpoint of motivational interviewing techniques, we've learned that if a question is generalized and we've taken the guilt out of it then questions are more likely to help. We nudge patients. They get yelled at enough and I think the gentle nudges make the difference.
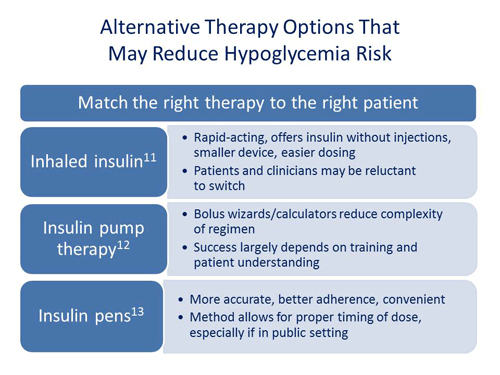
As far as regimen selection and timing, what effects do you think that inhaled insulin will have on hypoglycemia? Will it be something that we'll see used more?
Philip Raskin, MD: Years ago, there was an inhaled insulin on the market. Clinicians and patients tried it. However, there were some challenges with the delivery device and for many patients it wasn’t the best option. And as a result, there wasn’t a large acceptance. In contrast, the newly available inhaled insulin provides a better and simplified delivery device. Although there is a place for inhaled insulin, I gravitate towards other insulin therapies and I just don't see inhaled insulin overtaking the use of injectable insulin.
Scott Drab, PharmD: I agree with Dr. Raskin. I don't think that inhaled insulin will ever overtake injectable but I do have a number of patients that use inhaled insulin. Many patients were taking multiple injections of insulin and they were experiencing pain and bruising. These patients embraced the opportunity to switch to inhaled insulin. In addition, inhaled insulin appears to be more rapid-acting in the sense that it has a time course profile that more closely mimics our body’s own physiological insulin secretion. It appears to be more rapid acting than injectable insulin analogs and has a shorter duration of action, which may help reduce hypoglycemia. I think for certain patients it is and will be beneficial. It's another tool in the armamentarium that we have and it’s certainly easier to use than the old inhaled insulin.
Jerry D. Meece, RPh: I think time will tell. We take things, we work with them, and we try small changes. I think it's going to be for people who just want a boost of insulin and for those patients it might be a good choice.
Moving from that, do you feel that we've made big strides in using insulin pump therapy as far as reducing hypoglycemia?
Philip Raskin, MD: It’s important to realize that patients can get hypoglycemic on a pump. If you get continuous insulin overnight, it's the same problem. I think pumps are a great way to give insulin for the right kind of person but I don't think that it necessarily results in less hypoglycemia.
Scott Drab, PharmD: I agree with Dr. Raskin that you can get hypoglycemia on a pump. However, the data shows that we tend to use less insulin when we have patients on insulin pumps and they typically have better A1C levels.4 But, in motivated patients we do see less hypoglycemia. One of the big factors is the technology that's involved in bolus wizards and calculators. Life is not the same from day to day and mealtimes can vary. A bolus wizard will help to calculate the dose of bolus insulin that you need for say lunchtime, taking into account that you still have active insulin onboard from the morning bolus. Many people don't do that when they're calculating their dose of insulin.
Jerry D. Meece, RPh: I train patients on two different pumps. And the one thing that I've noticed is that it depends so much on the trainer. I agree with Dr. Raskin. A pump is just an insulin delivery device. With the right instruction and with the right training in the right patient, you can do incredible things with it. The pump in itself doesn't do a miracle job; it's the training and the patient understanding.
Do insulin pens make any difference versus the insulin vials? Do you see that pens help to lower hypoglycemia to any extent?
Philip Raskin, MD: I think insulin pens are great. They're convenient. People don't have to carry a syringe and a vial in their pocket. They're much more accurate than drawing up and eyeballing it. Of course depending on the kind of syringe you have and the vision of your patient. For some people it might make a difference but for most people it's just a more convenient and more expensive way of giving insulin.
Scott Drab, PharmD: From the patient standpoint, many individuals using a vial and syringe are very self-conscious about injecting mealtime insulin. For example, they do not want to take vials and syringes in to restaurants because they're concerned about people thinking they are IV drug users. Also, they don't want to go into a bathroom with a syringe. So, for many individuals they will inject their insulin at home. This creates a real problem because the insulin is acting while they are driving to the restaurant and essentially have no food on the stomach. This scenario can and often does lead to hypoglycemia.
A pen makes it so much more convenient. Individuals feel more comfortable injecting in public places. So, I think from that standpoint you will see correct use and better adherence, which could ultimately lead to less hypoglycemia.
Jerry D. Meece, RPh: It's also about timing. If I have the pen right there at the time that I need to use it, then I may be more likely to give myself the dose especially in a public setting.

As we wrap up here, Dr. Raskin, in your opinion, how can pharmacists make better use of their training and knowledge as they work with physicians? What tools can they bring? What can they offer? How can they help implement change and lower A1C as well as lower hyperglycemic effects?
Philip Raskin, MD: Pharmacists in our institution see patients. They are able to change insulin doses and interview patients and instruct them on various issues. They are another form of healthcare provider. It's less work for the physician if somebody else is doing some of those other things. Patients feel much more comfortable talking to people other than their doctor. Oftentimes the non-physician healthcare provider gets a better sense of what’s really going on because the patient is much more honest with them than they would ever be with the physician.
Jerry D. Meece, RPh: Dr. Drab, from your perspective and the collaborative practice agreements you have, how do you feel that pharmacists can make a big difference in going forward?
Scott Drab, PharmD: I will agree with Dr. Raskin. In collaborative care you have the ability to change doses, meet one-on-one with the patients, and serve as an extension to the physician. It does not mean replacing the physician but rather to serve as an integral member of the healthcare team.
However, I'm going to take a little different approach because many pharmacists may not have the opportunity to practice in a collaborative care environment. So what about the pharmacist who is dispensing medications? Where can these individuals be most helpful?
First and foremost, understanding the patients. Ask your patients about the instances of hypoglycemia using open-ended questions. Get more of the story. It doesn’t have to be a 30-minute discussion; it could be a 1-minute discussion that you have with a patient. They may say something that signals to you that they are having an issue with hypoglycemia and then you can decide whether you can address the issue or decide if they need to discuss this with their physician.
In regards to dispending products, having a greater understanding of what products can cause hypoglycemia and which ones do not is critical.
Jerry D. Meece, RPh: I think that's where our role comes in. Pharmacists need to build better, stronger relationships with physicians, by bringing that knowledge forward.

Finally, I’d like to ask each of you to provide clinical pearls or key points that you would leave pharmacists with when addressing hypoglycemia in patients with diabetes.
Scott Drab, PharmD: First, identify those patients at risk of hypoglycemia. Who is on medications that may cause hypoglycemia? Make sure that you ask about hypoglycemia at every encounter. Make sure that you use open-ended questions so that you can get a sense of what's going on and then offer solutions to help solve potential problems.
Philip Raskin, MD: I think hypoglycemia is really bad and ought to be avoided as much possible. From a patient's perspective, there are lots of things that you can address such as medication choices and doses. But the most important thing from the patient's perspective is the willingness to monitor blood glucose levels. And that often means sticking their finger many times during the day. This is a sure way to avoid hypoglycemia.
Jerry D. Meece, RPh: My nugget of advice is to always tell your patients that you can never get into trouble by checking too often. So when in doubt, check, and learn from that blood glucose check
References
- Workgroup on Hypoglycemia, American Diabetes Association. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28(5):1245-1249.
- American Diabetes Association. Standards of medical care in diabetes-2013. Diabetes Care. 2013;36 Suppl 1:S11-66.
- Unger J. Uncovering undetected hypoglycemic events. Diabetes Metab Syndr Obes Targets Ther. 2012;5:57-74.
- Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and Diabetes: A Report of a Workgroup of the American Diabetes Association and The Endocrine Society. Diabetes Care. April 2013:DC_122480.
- Matyka K, Evans M, Lomas J, Cranston I, Macdonald I, Amiel SA. Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Diabetes Care. 1997;20(2):135-141.
- Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
- Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA1c in people with type 2 diabetes: a retrospective cohort study. The Lancet. 2010;375(9713):481-489.
- Unger J, Parkin C. Hypoglycemia in insulin-treated diabetes: a case for increased vigilance. Postgrad Med. 2011;123(4):81-91.
- Campbell RK. Clarifying the role of incretin-based therapies in the treatment of type 2 diabetes mellitus. Clin Ther. 2011;33(5):511-527.
- Fisher M. Hypoglycaemia in patients with type 2 diabetes: minimising the risk. Br J Diabetes Vasc Dis. 2010;10(1):35-41.
- Martín-Timón I, Del Cañizo-Gómez FJ. Mechanisms of hypoglycemia unawareness and implications in diabetic patients. World J Diabetes. 2015;6(7):912-926.
- Moghissi E, Ismail-Beigi F, Devine RC. Hypoglycemia: minimizing its impact in type 2 diabetes. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2013;19(3):526-535.
- Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26(6):1902-1912.
PART 2 - eCase Challenge #1
The role of the pharmacist in the care and management of patients with diabetes is well established. Pharmacists practicing in community settings have shown that they can accurately identify patients with poor adherence; educate patients about their diabetes, medications, and self-monitoring; and provide support for adherence, resolve complications, and improve quality of life.1,2 Patients often interact with pharmacists more frequently than their healthcare provider;1 as such, pharmacists can act as an important intermediary support as part of the patient’s multi-disciplinary care team.
Motivational interviewing is a technique with the aim of eliciting a positive behavioral change using collaborative and nonconfrontational conversation with the patient. It should promote patient motivation and self-confidence regarding a specific question or concern.3 Through the use of open-ended questions, pharmacists may identify barriers to adequate glycemic control or detect symptoms of common treatment-related adverse events, such as hypoglycemia.4
Pharmacists should recognize that every patient treated with an anti-hyperglycemic therapy is at some risk of iatrogenic hypoglycemia.5 Given that hypoglycemia places some patients, especially the elderly, children and those with hypoglycemia unawareness, at additional risk of adverse events,6 pharmacists should be vigilant in using motivational interviewing techniques to detect potential cases of hypoglycemia and provide patients with education on its symptoms, risk factors, and potential alternative treatment strategies.
CASE PRESENTATION
Allen is a 76-year-old retired Caucasian male who was recently diagnosed with type 2 diabetes (T2DM). He has a documented 12-year history of hypertension and 7-year history of dyslipidemia. Three years ago, he had a myocardial infarction.
Allen presented to his primary care physician 4 months ago with blurry vision, frequent urination and increased thirst, and unintentional weight loss of 15 pounds. A random glucose level at that time was 247 mg/dL and his HbA1C level was 10.2%. His physician began him on a regimen of premixed 75/25 neutral protamine lispro (NPL)/insulin lispro twice daily. After 3 months, his HbA1C dropped to 7.8% and his physician added 500 mg metformin once daily in order to reach a jointly decided A1C goal of 7.5%.
When Allen arrives at the pharmacy to refill his metformin prescription, he greets everyone by name including you, his pharmacist, whom he has known for years. You refill Allen’s prescription and, as per your usual routine, ask Allen if he has any questions about his medication or if he has experienced any recent changes in symptoms. Allen indicates that since he starting taking the metformin last month, he has experienced periodic episodes of shakiness accompanied by sweating and rapid heart rate. During this time, he has also occasionally experienced blurred vision and headaches. He expresses that he has reservations about calling his doctor about his symptoms and notes that his next appointment is not scheduled for another 3 weeks. You mentally recall the symptoms of hypoglycemia and recognize that Allen may be experiencing some of them. Concerned, you begin to ask Allen a serious of open-ended questions that address whether hypoglycemia may be the root cause of these symptoms and whether Allen is monitoring his blood glucose levels properly.
Biometrics:
- Height: 5 feet 10 inches
- Weight: 207 lbs
- Current BMI: 31.4 kg/m2
Vital Signs:
- Pulse: 72 bpm
- BP: 121/73 mmHg
- Respirations: 16/minute
Past Medical History:
- Hypertension for 12 years
- Dyslipidemia for 7 years
- Myocardial infarction 3 years ago
Family History:
- Father with T2DM managed with insulin therapy
- Sister and brother with history of T2DM, managed with oral combination therapy; both also have history of hypertension
Social History:
- Non-smoker
- Alcohol use: social/on occasion (2-3 beers on weekends)
- Occupation: factory worker, now retired
- Spouse: married, three children ages 47, 49 and 52
Current Medications:
- Premixed 75/25 [neutral protamine lispro (NPL)/insulin lispro] 30 units in the morning and 20 units in the evening
- Metformin 500 mg once daily
- Atorvastatin 40 mg daily
- Lisinopril 20 mg daily
Known Allergies:
Recent Laboratory Findings:
- 4 months ago, A1C – 10.2%
- 1 month ago, A1C – 7.4%
Question #1:
What is your initial approach with Allen?
- Detail the progressive nature of T2DM
- Use motivational interviewing to explain the symptoms of hypoglycemia
- Indicate a need to repeat laboratory testing
- Suggest that alternative therapies should be explored
- Approach the situation with a “hands-off” mentality
Motivational interviewing is a patient-centered, directive method for enhancing motivation to change and instilling confidence using specific, open-ended questioning. In this scenario, the recognition that Allen may be experiencing hypoglycemia should prompt you to begin a series of open-ended questions to determine whether hypoglycemia is causing these symptoms. Because Allen feels comfortable with you, his pharmacist, he has already expressed that he has had notable symptoms changes since his most recent medication change. If Allen had not been forthright with his symptoms, you would likely want to probe the patient with questions such as, “Can you tell me some signs of low blood sugar? How do you feel when you have low blood sugar?” and “What are the reasons for low blood sugar?” However, because Allen is newly diagnosed with T2DM, he does not seem to be aware that his symptoms may be caused by low blood sugar. Therefore, the correct answer is B.
In this scenario, one of the first starting points of patient education on hypoglycemia should begin with an explanation of hypoglycemia and some of the usual reasons for low blood sugar. Explain to Allen that his symptoms may be caused by low blood sugar, defined as a condition characterized by abnormally low blood glucose levels, usually less than 70 mg/dL. Explain, that some of the most common causes of hypoglycemia are excessive or incorrect insulin administration, delayed or missed meals, excess alcohol consumption, weight loss, and exercise.7 Follow-up questions to Allen might be, “When and how often are you experiencing these symptoms? Under what circumstance did they occur (ie, missed meal, following exercise, etc.). Sometimes, when our lives get busy we don’t get all of our medication doses in or get them injected or taken on time. How many times in a week do you think that you missed getting your medicines in on time or taken/injected as prescribed? How many times were you unsure of whether or not you have taken your insulin?
Allen reports that he takes his medications regularly and rarely misses a dose, thanks to his wife who keeps a list of his medications and when they should be taken. He states that his symptoms have appeared in mid-to-late morning. When you ask Allen how often he skips breakfast, he says that he is not much of a breakfast person and prefers to have black coffee only. Sometimes, his wife will make him a few scrambled eggs and a piece of toast.
At this point, it may be helpful to address the importance of maintaining regular mealtimes as part of managing T2DM and preventing hypoglycemia. In one international study, insufficient food consumption was the most common cause of hypoglycemia in patients with type 1 diabetes mellitus (T1DM) or T2DM.8 The American Diabetes Association (ADA) indicates that one of the easiest ways to treat hypoglycemia is to consume 15-20 grams of glucose or simple carbohydrates, such as 2 tablespoons of dried fruit, ½ cup of juice or soda, 1 tablespoon of sugar or honey, or 6 or 7 hard candies.9 Patients may also wish to use glucose tablets or gel (following package instructions), which provide a standardized, easy-to-measure dose of carbohydrates and may be less tempting to snack on than candy. You suggest implementing the 15/15 rule, which indicates that a patient eat 15 grams of carbohydrates and wait 15 minutes for the sugar to get into the blood before checking blood sugar levels again. If hypoglycemia persists and blood glucose remains <70mg/dL, the treatment should be repeated.10
Question #2:
What is your next best point to address while interacting with Allen?
- Reprimand Allen for not following regular mealtimes
- Suggest consultation with a dietician
- Assess when and how often he is checking his blood sugar levels
- Indicate that his insulin regimen may need to be optimized
Careful blood glucose monitoring is one way in which detection and prevention of hypoglycemia can dramatically be improved. This rationale supports why the correct answer is C. After asking Allen when and how often he is monitoring his blood glucose levels, he reveals that he checks his levels just once in the morning and once later in the evenings. He reports that his fasting blood glucose levels in the morning are usually between 70 and 80 mg/dL. Though these values are not hypoglycemic, they are in the low range according to ADA guidelines for preprandial glucose.10 The combination of his recent treatment intensification with his lack of consistent carbohydrate intake may also be causing Allen’s hypoglycemic symptoms that he describes. You might also want to inquire about any recent addition of activity or exercise in the evenings.
Allen would benefit and likely reduce the number of potential hypoglycemic events if he checked his blood sugar levels more frequently. Frequent self-monitoring using a blood glucose meter will allow Allen to observe his glycemic patterns throughout the day. Testing ideally should be performed before and 2 hours after each meal, in addition to periodic checks during the night for a period of time to determine when low blood glucose levels are present.5 In Allen’s case, more frequent testing is needed because of his recent treatment intensification, especially in the mornings and after eating breakfast to see if his blood sugar levels have risen. Part of the successful self-management of diabetes relies on a detailed blood glucose log, which should provide entries for the timing and dosage of treatment, carbohydrate intake, physical activity, and any circumstances surrounding recent episodes of hypoglycemia. Such a log, accompanied with a detailed food log for a four day period, with a patient’s personal perspective of the hypoglycemic events, may help to predict times when Allen is more likely to experience an event or help to detect early signs and symptoms.11
When evaluating a patient’s hypoglycemic events, an important aspect to consider is how low blood sugar levels influence choices about driving. Hypoglycemic events are associated with significantly increased risk of accidents, including accidental falls and motor vehicle accidents.12 When speaking with Allen, you should ask if he tests his blood glucose before driving and his thoughts on how low his blood glucose would need to go before he thinks he should not drive.
Driving during episodes of hypoglycemia poses a significant risk not only for the patient but also for others on the road or around the vehicle. One useful technique is a “vectoring” procedure, which recommends that patients check their blood glucose one hour before they plan to drive and again before getting behind the wheel. Such a technique may help alert the patient to changing blood glucose levels (are BG levels rising, staying the same or decreasing?) and any problems that could occur while driving. Longer trips may warrant more frequent checking to ensure safety.11
Question #3:
Which of the following is an appropriate treatment for a severe hypoglycemic event that occurs at home?
- Glucose- or carbohydrate-rich food
- Insulin
- Hard Candy
- Glucagon
Severe hypoglycemia is defined as a hypoglycemic event that requires the assistance of another person to administer carbohydrates or glucagon due to confusion or unconsciousness.10 Though Allen appears to have been experiencing mild, symptomatic hypoglycemic events, he may be at greater risk of a future severe hypoglycemic event because of his history of mild events, current treatment regimen with insulin, and age. Elderly patients, especially those with cognitive impairment or who experience cognitive decline have significantly greater risk of experiencing a subsequent severe event.13 As part of your assessment of Allen’s hypoglycemic events, an inquiry into his and friends or family member’s understanding of the use of glucagon emergency kits should be employed. You might ask, “If you take insulin, do you have a glucagon emergency kit? When would glucagon be used to treat hypoglycemia? Who in your family knows how to administer glucagon?”
Glucagon, the main counter-regulatory hormone of insulin, should be utilized as the first-line treatment for severe hypoglycemia in patients treated with insulin-based regimens who lose consciousness and are unable to chew or swallow. In contrast to dextrose, which must be administered intravenously, a trained caregiver can administer glucagon by subcutaneous or intramuscular injection. Use of glucagon emergency kit can expedite treatment that would otherwise be delayed by waiting for the arrival of paramedics in an emergency situation. Although some risks, such as nausea and vomiting, can result from higher doses of glucagon, these events are rare and much less severe than some potential outcomes of a severe hypoglycemic event, such as coma, seizure, or death.7 The correct answer choice is D, ‘Glucagon’.
Multiple glucagon emergency kits are available. They typically are in a brightly colored case to locate easily during an emergency situation and contain glucagon powder, a syringe filled with solvent and text and graphic instructions for use. For adults and children over 25 kg in weight, 1 mg of glucagon reconstituted in 1 mL of sterile water is recommended. The glucagon can be injected into the patient’s buttock, arm or thigh.7 After providing the glucagon injection, patients should be moved to their side in order to prevent aspiration pneumonia in the event of vomiting with treatment. Caregivers may also need to follow-up with a supplementary carbohydrate immediately after the episode.
Caregivers of patients with diabetes should receive continuous education of the use of glucagon kits. They should always have knowledge of the kit location, proper storage, and awareness of the expiration date. Proper training of caregivers may include the practice of injecting with saline in order to provide confidence if an emergency occurs. Caregivers should also be advised on the importance of not delaying treatment so that blood sugar levels can be elevated as quickly as possible.14 It is also important that caregivers recognize that they should not try to provide juice or other food to a patient who cannot swallow properly. In Allen’s situation, his wife would be the most likely person to administer glucagon in the event of severe hypoglycemia, and she should receive training on how to use an emergency glucagon kit.
Question #4:
All of the following are likely to result from fear of hypoglycemia except?
- Tighter glycemic control
- Elevated anxiety
- Failure to comply with therapies
- Limited exercise or physical activity
- Avoidance of being alone
Fear of hypoglycemia is a significant factor that influences how a patient manages glycemic levels and is a major limiting factor in successful clinical management of the disease. In fact, fear of hypoglycemia is almost as much of a barrier to glycemic control as hypoglycemia itself.15 Understandably, fear can drive up A1C values because patients want to avoid experiencing the unpleasant effects of hypoglycemia,11 which can sometimes leave patients debilitated for a day or more. Hypoglycemia can result in failure to comply with therapeutic regimens, high levels of anxiety, and low levels of happiness and satisfaction with their disease management. Patients may begin to exhibit behaviors to avoid hypoglycemia, the most likely of which is maintaining higher blood glucose levels than recommended.5 As such, the correct answer, A, reflects that patients are less likely, rather than more likely, to exhibit tighter glycemic control when they are fearful of hypoglycemia.
From the pharmacist’s perspective, you can use your motivational interviewing skills to determine what a hypoglycemic event meant to the patient. You might inquire about how the event(s) affected them and if they loosened their glucose control because of it. Allen expresses that he has been feeling pretty anxious about these episodes and is fearful of leaving the house without his wife. He is wondering whether the episodes are being brought on by something that he is doing or whether a change in medication might be necessary, though his wife is adamant about sticking to his current prescribed regimen. You should remind Allen that recognizing his specific symptoms of hypoglycemia may help to prevent future episodes and lessen the likelihood of a severe event. Through motivational interviewing, you may be able to identify unique symptoms, such as changes in taste or smell or numbness, that precede an event and are different from the classic adrenergic signs of shaking, racing heart rate, and tingling or the neuroglycopenic signs of headache, hunger, blurry vision, or drowsiness.11
You can provide Allen with positive reinforcement that understanding his signs and symptoms of hypoglycemia can help him to make decisions that provide better glycemic control and hopefully reduce the incidence of his hypoglycemia. Let him know that maintaining a scheduled mealtime regimen, especially in the mornings when he is prone to symptomatic events, testing his blood glucose levels more frequently, and keeping a detailed log of his levels should reduce the odds of subsequent events. In addition, you may also review with Allen a self-titration schedule, which provides recommendations on reducing doses, should his blood glucose levels become too low.16
Question #5:
Which one of the following alternative therapies would NOT be appropriate for you to suggest to Allen’s physician to address Allen’s concerns about hypoglycemia?
- GLP-1 receptor agonist
- DPP-4 inhibitor
- SGLT2-inhibitor
- Metformin
- Sulfonylurea
All of the above therapies are associated with a low risk of hypoglycemia except the secretagogue, sulfonylurea, which increases insulin output from the pancreas and is a major contributor to hypoglycemia in early T2DM. Therefore, the correct answer is E. The risk of hypoglycemia with each sulfonylurea depends on its pharmacokinetic properties; the greatest risk is highest among long-acting sulfonylureas (chlorpropamide and glyburide) and much lower in short-acting agents, such as glipizide.17
GLP-1 (glucagon-like peptide-1) agonists function as incretin mimetics. They increase insulin secretion and suppress glucagon secretion in a glucose dependent manner, and they slow gastric emptying. In addition, they increase satiation and have been associated with weight loss.18 Though reductions in A1C levels were comparable, the incidence of hypoglycemia was lower with the addition of a GLP-1 agonist to metformin or sulfonylurea compared with the addition of insulin glargine.19
The enzyme, DPP-4 (dipeptidyl peptidase-4), degrades endogenous GLP-1 and glucose-dependent insulinotropic peptide (GIP). DPP-4 inhibitors, also called gliptins, prolong the action of endogenous incretin hormones. In patients inadequately controlled with metformin, DPP-4 inhibitors have shown similar glycemic control to sulfonylureas but significantly lower incidence of hypoglycemia (<5%).17
SGLT2 (sodium glucose co-transporter 2)-inhibitors are oral agents that lower glucose by blocking its reabsorption in the renal tubules and subsequently increasing glucose urinary excretion. In addition to lowering glucose levels, SGLT2-inhibitors reduce body weight, blood pressure, and cholesterol. Hypoglycemia has occurred infrequently (<5%) in clinical trials.20
Metformin is an oral biguanide that is the recommended first-line therapy for T2DM according to ADA guidelines.10 Metformin decreases gluconeogenesis and glycogenolysis in the liver and increases glucose disposal in the musculature. Because metformin does not directly stimulate insulin secretion, it causes less hypoglycemia than sulfonylureas and glinides when used alone.21 However, combination therapy, such as with a sulfonylurea or insulin, is associated with a greater risk of hypoglycemia than with sulfonylurea monotherapy.17,22
Until Allen begins frequent and consistent blood glucose monitoring, it is not clear whether his hypoglycemia is linked to his lack of carbohydrate intake in the mornings, or to the addition of metformin to his premixed insulin treatment regimen. Since the initiation of Allen’s hypoglycemia seemed to occur after he began metformin therapy, a re-evaluation of his treatment strategy may be warranted. You should remember that hypoglycemia risk may increase with metformin when used in combination with other therapies, though it is often viewed as a “non-hypoglycemic” agent. When consulting with Allen’s physician, you might suggest decreasing Allen’s insulin regimen to 20 units BID and titrating his metformin to 500 mg BID or one of the alternative strategies discussed above provided that drug cost is not an issue.
Question #6:
Less stringent A1C goals (such as <8%) may be appropriate for all of the following patients except?
- Those with a history of severe hypoglycemia
- Those with limited life expectancy
- Those with no significant cardiovascular disease
- Those with extensive comorbid conditions
- Those with difficulty attaining a 7.0% A1C target despite intensive self-management education, repeated counseling, and effective doses of glucose-lowering agents
For many years, clinicians were advised to pursue aggressive glucose management for diabetes treatment because of the reduced risks of microvascular complications such as nephropathy, neuropathy, and retinopathy. The traditional goal was to achieve and maintain hemoglobin A1C levels below 7.0%. However, clinical trials in recent years have suggested that setting and achieving such goals may place certain patients with diabetes at a greater risk for cardiovascular complications and mortality.
In 2012, the ADA and European Association for the Study of Diabetes (EASD) released a consensus report outlining the serious consequences of hypoglycemia and the importance of setting individualized treatment goals.23 The report indicates that less stringent goals, between 7.0-8.0%, may be appropriate for patients with the characteristics noted above, whereas a more stringent goal of <6.5% might be appropriate in select patients with short disease duration, long life expectancy, and no significant cardiovascular disease if the goal can be achieved without significant hypoglycemia or other adverse effects from treatment. Indeed, current ADA guidelines also reflect a more individualized approach based on patient needs, preferences, and tolerances.10
In conjunction with the 2012 report, the ADA and Endocrine society published a consensus report in 2013 advising clinicians that preventing hypoglycemia is more important than tight glycemic control in some patients.24 The report outlines evidence that connects hypoglycemia with adverse outcomes, especially among the elderly, children younger than 5 years, and those with hypoglycemia unawareness and provides guidance on setting an appropriate target to reduce hypoglycemia.
Part of the rationale for providing individualized, multiple A1C targets stemmed from the results of the large clinical study, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. In the study, which was terminated prematurely, patients who received intensive therapy with an A1C goal of 6.0% or lower were 22% more likely to experience a non-fatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes compared with patients who received standard therapy with an A1C goal of 7.0-7.9%.25 Though the study did not definitively demonstrate that hypoglycemia was the cause of increase mortality, there seemed to be a negative association between targeting aggressive glycemic control in patients with or at risk of significant cardiovascular disease.
Furthermore, there appears to be a U-shaped curve in regards to A1C levels and increased risk for mortality and cardiac events. The lowest all-cause mortality in diabetic patients is found with an A1C level of 7.5%, while patients with lower or higher A1C levels have an elevated risk.26 All of this data above supports why answer C is correct.
Given that Allen has cardiovascular risk factors (hypertension and dyslipidemia) and a history of cardiovascular disease (myocardial infarction 3 years ago), pursing a stringent A1C target of <7.0% may not be the optimal therapeutic strategy for his particular case. When discussing Allen’s potential hypoglycemia and alternative therapeutic strategies with his physician, you may want to address the risk for mortality from hypoglycemia. As a clinician, a fine balance must be found between glycemic control and risk of therapeutic complications. The hypoglycemic risks must be weighed against whether the patient will live long enough to get complications from diabetes.
FOLLOW UP
Since Allen’s last visit to the pharmacy, he has started eating breakfast consistently and checking his blood glucose levels more frequently, especially in the mornings. He reports that after breakfast, his blood glucose levels typically rise 50-75 mg/dL, taking his postprandial glucose levels to 125-150 mg/dL. His hypoglycemic symptoms have dissipated and his physician did not modify his treatment regimen though his last A1C level was 7.3%. After discussing the risks and benefits of tighter glycemic control, both Allen and his physician decided to not pursue more aggressive treatment until his diabetes progresses and his A1C levels rise significantly. Allen is now aware of the symptoms and risks of hypoglycemia and more consistently checks his glucose levels before driving, especially on trips more than 30 minutes away. Allen obtained an emergency glucagon kit, and he and his wife received training on how to use the kit should an emergency hypoglycemic event occur. Allen reports feeling comfortable managing his diabetes and frequently checks in with his pharmacist when he picks up his refills.
CONCLUSION
Pharmacists can be influential partners in the diabetes management team. They can proactively work with patients to identify potential signs and symptoms of hypoglycemia and work with individuals, and their physicians, to come up with solutions. Motivational interviewing is an extremely useful tool in identifying issues with diabetic complications, not only for hypoglycemia, but also for therapy selection and medication adherence, and should be a collaborative process that requires active patient involvement. By using open-ended questioning, pharmacists can help patients to identify and solve issues related to their condition by providing structured guidance.
Pharmacists should be familiar with hypoglycemia, its risk factors, methods of prevention, and complications that may result. They should promote patient awareness of hypoglycemia, frequent blood glucose monitoring, maintenance of regular mealtimes, dosing and tracking logs, and consider recommending options for alternative therapies if warranted. Pharmacists should also be cognizant that individualized A1C goals are often best and that less stringent goals may be appropriate for certain populations, such as the elderly and those with cardiovascular risks.
REFERENCES
- Al Hamarneh YN, Charrois T, Lewanczuk R, Tsuyuki RT. Pharmacist intervention for glycaemic control in the community (the RxING study). BMJ Open. 2013;3(9).
- Wubben DP, Vivian EM. Effects of pharmacist outpatient interventions on adults with diabetes mellitus: a systematic review. Pharmacotherapy. 2008;28(4):421-436.
- Pladevall M, Divine G, Wells KE, Resnicow K, Williams LK. A Randomized Controlled Trial to Provide Adherence Information and Motivational Interviewing to Improve Diabetes and Lipid Control. Diabetes Educ. 2015;41(1):136-146.
- Unger J. Insulin initiation and intensification in patients with T2DM for the primary care physician. Diabetes Metab Syndr Obes Targets Ther. 2011;4:253-261.
- Unger J. Uncovering undetected hypoglycemic events. Diabetes Metab Syndr Obes Targets Ther. 2012;5:57-74.
- Slomski A. Avoiding hypoglycemia at all costs is crucial for some with diabetes. JAMA. 2013;309(24):2536-2537.
- Kedia N. Treatment of severe diabetic hypoglycemia with glucagon: an underutilized therapeutic approach. Diabetes Metab Syndr Obes Targets Ther. 2011;4:337-346.
- Lammert M, Hammer M, Frier BM. Management of severe hypoglycaemia: cultural similarities, differences and resource consumption in three European countries. J Med Econ. 2009;12(4):269-280.
- American Diabetes Association. Hypoglycemia (Low Blood Glucose). Am Diabetes Assoc. 2015.
- American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36 Suppl 1:S11-66.
- Meece J. Hypoglycemia Awareness Training. AADE Pract. 2014;2(3):48-51.
- Signorovitch JE, Macaulay D, Diener M, et al. Hypoglycaemia and accident risk in people with type 2 diabetes mellitus treated with non-insulin antidiabetes drugs. Diabetes Obes Metab. 2013;15(4):335-341.
- Punthakee Z, Miller ME, Launer LJ, et al. Poor Cognitive Function and Risk of Severe Hypoglycemia in Type 2 Diabetes Post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care. 2012;35(4):787-793.
- Harrism G, Diment A, Sulway M, Wilkinson M. Glucagon administration - underevaluated and undertaught. Pract Diabetes Int. 2001;18(1):22-25.
- Wild D, von Maltzahn R, Brohan E, Christensen T, Clauson P, Gonder-Frederick L. A critical review of the literature on fear of hypoglycemia in diabetes: Implications for diabetes management and patient education. Patient Educ Couns. 2007;68(1):10-15.
- Khunti K, Davies MJ, Kalra S. Self-titration of insulin in the management of people with type 2 diabetes: a practical solution to improve management in primary care. Diabetes Obes Metab. 2013;15(8):690-700.
- Fisher M. Hypoglycaemia in patients with type 2 diabetes: minimising the risk. Br J Diabetes Vasc Dis. 2010;10(1):35-41.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet Lond Engl. 2006;368(9548):1696-1705.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther. 2007;29(11):2333-2348.
- Rosenwasser RF, Sultan S, Sutton D, Choksi R, Epstein BJ. SGLT-2 inhibitors and their potential in the treatment of diabetes. Diabetes Metab Syndr Obes Targets Ther. 2013;6:453-467.
- Bodmer M, Meier C, Krähenbühl S, Jick SS, Meier CR. Metformin, Sulfonylureas, or Other Antidiabetes Drugs and the Risk of Lactic Acidosis or Hypoglycemia. Diabetes Care. 2008;31(11):2086-2091.
- Lewin A, Lipetz R, Wu J, Schwartz S. Comparison of extended-release metformin in combination with a sulfonylurea (glyburide) to sulfonylurea monotherapy in adult patients with type 2 diabetes: a multicenter, double-blind, randomized, controlled, phase III study. Clin Ther. 2007;29(5):844-855.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of Hyperglycemia in Type 2 Diabetes: A Patient-Centered Approach Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364-1379.
- Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and Diabetes: A Report of a Workgroup of the American Diabetes Association and The Endocrine Society. Diabetes Care. April 2013:DC_122480.
- Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
- Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA1c in people with type 2 diabetes: a retrospective cohort study. The Lancet. 2010;375(9713):481-489.
PART 3 - eCase Challenge #2
Type 2 diabetes mellitus (T2DM) is a progressive and heterogeneous disease characterized by β-cell dysfunction, insulin resistance, and impaired hepatic glucose production. Insulin resistance can be present for years prior to diagnosis, as initially β-cells are able to compensate for elevated glucose levels by increasing insulin production. However, over time and with disease progression, β-cells become refractory to glucose despite hyperinsulinemia, insulin deficiency develops, and hyperglycemia worsens. This initial progression from impaired glucose tolerance to early T2DM will later be followed by further β-cell dysfunction marked by complete loss of insulin secretory capacity and an absolute insulin deficiency.1,2 Because of this progressive nature of T2DM, patients who initially respond to oral antidiabetic drugs, such as metformin or thiazolidinediones, and to sulfonylurea therapy, which increases insulin output from the pancreas, will often require exogenous insulin for proper glycemic control much in the same way that a patient with type 1 diabetes mellitus (T1DM) has an insulin requirement.3 Thus, patients with advanced T2DM, who are near the insulin-deficient end of the spectrum, face many of the same challenges in the clinical management of glycemic control as patients with T1DM.
One important aspect in the therapeutic management of T2DM is the risk of iatrogenic hypoglycemia. Though the overall prevalence of iatrogenic hypoglycemia is considerably lower in T2DM than in T1DM, patients with advanced T2DM who require therapeutic intervention with insulin will have a higher risk of developing hypoglycemia than those with earlier staged disease.4 Clinicians may underestimate the prevalence of hypoglycemia in patients with T2DM; observational studies have shown similar rates of hypoglycemia in patients with T1DM and T2DM when on insulin therapy.5 Furthermore, the loss of β-cell function associated with progressive T2DM parallels a loss of glucagon counter-regulation and epinephrine release with hypoglycemia similar to that seen in T1DM. As a result, falling blood glucose levels often do not trigger hypoglycemic symptoms6 or are shifted to a lower glycemic threshold for producing an autonomic or symptomatic response.7
Though the American Diabetes Association (ADA) has recognized that less aggressive glycemic goals may require therapy adjustments to decrease the risk of iatrogenic hypoglycemia,8 the incidence of hypoglycemia, especially at night, is difficult to identify because many events are asymptomatic, unrecognized, or unreported.9 Nocturnal hypoglycemic episodes may occur in as much as 70% of cases.10 As such, many patients are simply unaware of symptoms making diagnosis even more complex.4 This is further compounded by the fact that many patients are not periodically monitoring their blood glucose levels during sleeping hours.11
Understanding and recognizing the symptoms of nocturnal hypoglycemia, and its complications such as hypoglycemia unawareness (HU) and hypoglycemia associated autonomic failure (HAAF), is challenging for both patients and clinicians. However, pharmacists are uniquely positioned to provide an interface for patients to express any concerns about their symptomology and current treatment regimens. A greater understanding of HU and HAAF may help to eliminate some of the detrimental effects of hypoglycemia on patient quality of life and raise awareness of alternative treatment strategies that may be appropriate.
CASE PRESENTATION
Janet is a 62-year-old African American woman with type 2 diabetes mellitus who arrives at the pharmacy to pick up her prescription for insulin aspart protamine/insulin aspart. She has a documented 8-year history of dyslipidemia and 3-year history of depression.
Janet was diagnosed with T2DM 22 years ago and initially was treated with 500 mg metformin twice daily. Along with diet and exercise, this regimen was sufficient to control her A1C levels for approximately 6 years, but after her A1C levels rose to 8.6% her physician increased dosing to 1000 mg twice daily. This dosage controlled her A1C for an additional 4 years. After that time, her A1C level increased again to above 8.5%, which prompted her physician to add in a regimen of 2.5 mg glyburide twice daily. This regimen maintained Janet’s A1C level to below 7.0% for another 5 years. However, as Janet’s disease progressed, her glyburide dosage was increased incrementally because of increasing A1C levels until it was clear that the metformin-glyburide regimen was no longer sufficient for adequate glycemic control. Seven years ago, her physician switched her therapy to a 70/30 premix of insulin aspart protamine/insulin aspart of 25 units before breakfast and dinner (0.6 units/kg/day). At a visit with her primary care physician last month, her random blood glucose level was 206 mg/dL and her A1C level was 7.6%. Her physician increased her treatment regimen to 30 units before breakfast and 35 units before dinner (0.8 units/kg/day).
When you refill Janet’s prescription, you ask Janet if she has any questions about her medication or if she has experienced any recent changes in symptoms. She indicates that since the increase in her dosage last month, she has felt confused and irritated when she wakes up, doesn’t feel rested and just last week experienced two violent nightmares during sleep. Feeling concerned, you begin to ask a series of open-ended questions to determine what may be causing these symptoms and whether Janet has been monitoring her blood glucose levels properly. You learn that Janet has been checking her blood glucose levels just three times throughout the day and that she has not been checking periodically during the night.
Biometrics:
- Height: 5 feet 2 inches
- Weight: 186 lbs
- Current BMI: 33.2 kg/m2
Vital Signs:
- Pulse: 68 b/m
- BP: 130/78 mmHg
- Respirations: 16/minute
Past Medical History:
- T2DM for 22 years
- Dyslipidemia for 8 years
- Depression for 3 years
- No history of CV risk factors or CV disease
Family History:
- Both parents deceased (father, myocardial infarction, age 67; mother T2DM, breast cancer, age 71)
- 2 siblings – both with dyslipidemia and hypertension; brother with T2DM and sister with gestational diabetes during both pregnancies
Social History:
- Non-smoker
- Occupation: real estate agent
- Social alcohol use (~2-3 drinks/week)
- Divorced, 2 children ages 33 and 35
Current Medications:
- Premixed 70/30 insulin aspart protamine/insulin aspart 30 units morning, 35 units evening
- Atorvastatin 40 mg/day x 8 years
- Paroxetine 30 mg/day x 2.5 years
Known Allergies:
Recent Laboratory Findings:
Question #1:
What is your initial approach with Janet right now?
- Suggest that alternative therapies be explored
- Determine whether Janet is experiencing nocturnal hypoglycemia
- Indicate that she should discuss these symptoms with her physician
- Outline the importance of diet and exercise in T2DM management
- Review her current medications to see if there are any contraindications
Hypoglycemia is defined as a blood glucose level <70 mg/dL (3.9 mmol/L).12 Reports indicate that over 70% of all hypoglycemic events occur during the night, when the patient is usually asleep and unaware of symptoms.4 With a normal physiological response to hypoglycemia, the body exerts strong counter-regulatory mechanisms to quickly increase blood glucose levels to protect itself from the negative consequences that might occur. These regulatory responses include the inhibition of endogenous insulin secretion, as well as the stimulation of glucagon, norepinephrine, epinephrine, and cortisol secretion. Together, these mechanisms stimulate hepatic glucose production and reduce glucose utilization in peripheral tissue, ultimately resulting in an increase in blood glucose levels.13
Hypoglycemia unawareness (HU) is the inability to sense a significant fall in blood glucose below normal levels.14 Patients with HU are unable to detect falling levels of blood glucose before the onset of symptoms such as palpitations, sweating, anxiety, or tremors.13 Though Janet has not indicated that she is experiencing some of the more classic symptoms of hypoglycemia, she has indicated that she awakens with confusion, irritation, and tiredness and reports having nightmares during sleep.3 You recognize that these are common symptoms of nocturnal hypoglycemia and that she may be experiencing HU. Based on this rationale, the correct answer is B.
Based on your knowledge of risk factors and Janet’s medical history, you should apply motivational interviewing techniques using open-ended questions to try to assess whether Janet is experiencing nocturnal hypoglycemia and hypoglycemia unawareness. HU can occur in patients with type 1 or type 2 diabetes mellitus, and over 80% of patients with T2DM have experienced a hypoglycemic event with over 40% of those events being asymptomatic.15 Risk factors for HU include a long duration of diabetes, a history of recent hypoglycemic events, intensive glycemic therapy, and advanced age.4 Though Janet is not elderly, she is at greater risk because of her long history of disease and recent treatment intensification with insulin. Patients with T2DM have a three-fold increased risk of hypoglycemia when treated with insulin therapy for more than 5 years versus treatment for less than 2 years.16
After asking Janet a few questions, such as how often she wakes feeling unrested and confused, you learn that she wakes feeling this way a few times a week. This is concerning because HU places patients with T2DM at a 17-fold greater risk for a severe hypoglycemic event17 and also for the development of hypoglycemia-associated autonomic failure (HAAF), or the impaired ability of the body to counter-regulate subsequent and more serious hypoglycemic events.3 Over time, repeated episodes of mild hypoglycemia cause the normal threshold for initiating physiological response in future events to shift towards lower blood glucose concentrations, resulting in impairment of the natural defense mechanisms for prevention and reversal. HAAF is most often caused by recent or recurrent hypoglycemia.3
You should explain to Janet that her symptoms probably indicate that she is experiencing nocturnal hypoglycemia with hypoglycemia unawareness and address that nocturnal hypoglycemia may be linked to serious complications, such as dead-in-bed syndrome or other arrhythmias that may lead to a sudden, unforeseen death.18 You should stress to Janet the ongoing importance of blood glucose monitoring and determine if she has a glucagon emergency kit available in the event of a severe hypoglycemic event. Both she and a caregiver should be trained on how to properly use an emergency kit.
Question #2:
Which of the following interventions is best suited for Janet to determine whether she is experiencing nocturnal hypoglycemia?
- Continuous glucose monitoring
- Self-monitoring of blood glucose
- Titration of insulin therapy
- Discontinuation of her current treatment regimen
A true episode of hypoglycemia may be difficult to detect, especially for nocturnal hypoglycemia and its association with HU and HAAF. Continuous glucose monitoring (CGM) has been shown to identify unrecognized hypoglycemia in nearly 60% of patients.19 Furthermore, CGM reduces the incidence of severe hypoglycemic events in patients with HU.20 Given the serious risks of nocturnal hypoglycemia, including the increased risk of severe hypoglycemic events, Janet may benefit from the use of CGM, indicating that the correct answer is A. Because CGM devices can display real-time blood glucose values and also sound an alarm if any extreme changes are detected, an automated system may benefit Janet the most especially if her normal physiologic counter-regulatory mechanisms against hypoglycemia are not working properly and cannot be self-corrected.
After asking Janet how often she checks her blood glucose levels during the night, you learn that in recent months she has become very relaxed in her monitoring and had not been checking her nightly levels at all. In addition, she mentions that she has recently added in a few evening aerobic classes per week and has not checked her glucose levels before or after exercising. Self-monitoring of blood glucose levels periodically during the night and after exercising would have likely detected any severe drops in glucose, especially after a treatment change. The ADA indicates that CGM should be used as a supplemental tool to self-monitoring in patients with HU or frequent hypoglycemic episodes. Not only can CGM lower the incidence of severe hypoglycemia, it can help the patient control A1C levels as well.20 Additional interviewing of Janet reveals that when she does check her morning blood glucose levels, they are sometimes as low as 40-50 mg/dL. Other than generally feeling unrested in the morning, she reports that she otherwise “feels fine.” It is not uncommon for patients with advanced T2DM or T1DM to report that they feel fine even with blood glucose levels as low as 40 or 50 mg/dL. Janet is likely exhibiting the loss of her physiological responses to hypoglycemia and possibly HAAF. You should emphasize that the inability to detect hypoglycemic symptoms puts her at greater risk for a severe event both at night and during the day and is deleterious to her overall health.
The problem of hypoglycemia unawareness can be counterbalanced by artificially allowing blood glucose values to increase temporarily to prevent hypoglycemic reactions from occurring. In as little as two to three weeks of structured avoidance of hypoglycemia, the normal physiological responses and awareness to hypoglycemia can be restored.21 The ADA supports that temporary relaxation of glycemic targets should be utilized in patients with HU or severe hypoglycemia.12 You should indicate to Janet that she should consider discussing with her physician the temporary relaxation of her current treatment regimen in order to restore hypoglycemia awareness. CGM would allow Janet to observe her glycemic patterns throughout the day and night and would likely reduce the number of hypoglycemic events, even if Janet is unable to feel symptoms coming on. Additionally, you should stress to Janet the importance of checking her blood glucose levels before and after exercise and prior to bedtime. She may need to have a snack at any of these times if low blood glucose levels are anticipated and set a slightly higher goal for her bedtime blood glucose checks.
As Janet’s pharmacist, you can provide Janet with positive reinforcement that frequent monitoring of blood glucose levels, especially at night when she may be prone to hypoglycemic events, should reduce her risk of severe hypoglycemia in the future.
Question #3:
All of the following are potential advantages of basal-prandial-correction therapy (using a rapid-acting analog at each meal with a basal insulin) over premixed insulin except?
- Improved patient literacy on correct carbohydrate counting and insulin-to-carbohydrate ratio
- Opportunity for flexible prandial insulin titration
- Elimination of a consistent-carbohydrate requirement
- Reduction in hypoglycemia with proper insulin-to-carbohydrate ratio
- Elimination of multiple dosing injections
After further conversation with Janet, you learn that she feels limited by her current treatment regimen of premixed 70/30 insulin aspart protamine/insulin aspart because she is consistently having to try to match her carbohydrate requirements with her fixed dosage. She admits that her work schedule often prevents her from having a consistent nightly dinner as she frequently has to meet with clients in the evening. She often finds herself eating on the go or skipping meals with her busy lifestyle.
At this point in your discussion with Janet, it may be appropriate to initiate a discussion of the importance of maintaining regular mealtimes and a healthy diet as part of managing T2DM, as well as alternative therapy choices may help to reduce hypoglycemia. Janet’s susceptibility to HU and HAAF may be compounded by the fact that she is not eating regular, balanced meals. Even though Janet has had T2DM for many years, you may view this situation as an opportunity to revisit her understanding of correct carbohydrate counting and proper insulin-to-carbohydrate ratios.
Though the twice daily regimen of premixed insulin allows for greater ease of use with simple administration, it may not offer the flexibility of titration and dose timing of basal-prandial-insulin therapies.22 Using a regimen of peakless basal insulin with a rapid-acting analog works well for many patients with T2DM because it allows for flexible prandial insulin titration, eliminates the need for consistent carbohydrate requirements, and in Janet’s case may reduce her hypoglycemic events during the night with adjustment of either her basal insulin or rapid-acting insulin based on her specific carbohydrate intake. However, unlike premixed insulins, basal-prandial regimens may require more frequent prandial injections based on mealtimes. As such, the correct answer is E.
It is also important to note that regardless of therapy selection, Janet should maintain a detailed blood glucose log, with entries for the timing and dosage of treatment, carbohydrate intake, physical activity, and any incidence of hypoglycemia.23 Should Janet and her clinical care team choose to switch to a basal-bolus regimen, she will need to be sure to adjust her prandial insulin based on her meal selections.
Part of your role as Janet’s pharmacist and part of the clinical care team is to raise awareness of new or alternative therapies that may be better suited for her optimal disease management when the current regimen is associated with adverse effects. The first generation insulin analogs, glargine and detemir, are long-acting basal insulins that are typically associated with lower incidence of hypoglycemia. Detemir is available as a once-daily or twice-daily dosing with a duration of action up to 24 hours, depending on dose. Glargine is considered a peakless insulin with a duration of action of up to 24 hours. Both clinical trials and meta analyses have shown that glargine and detemir are associated with a 30% reduction in the risk of nocturnal hypoglycemia as well as a reduction in severe hypoglycemia compared with NPH.24–26 Glargine-300 was licensed by the FDA for use in the U.S. in February 2015. Compared with glargine-100, glargine-300 demonstrated comparable glycemic control in patients with T2DM. Importantly, treatment with glargine-300 reduced the number of confirmed or severe hypoglycemic events that occurred at any time of the day (14% difference), as well as during the night (31% difference), compared with glargine-100. Additionally, glargine-300 was associated with slightly less weight gain.27
The second generation, ultra-long acting basal insulin, degludec, has a duration of action beyond 42 hours and was just licensed for use in the U.S. in September 2015. Treatment with degludec has shown reduced rates of nocturnal hypoglycemic episodes compared with glargine in patients with T1DM or T2DM.28 The premixed formulation of 70/30 degludec and insulin aspart received FDA licensure concomitantly with the single degludec formulation. Twice daily degludec-aspart therapy and basal-bolus therapy with degludec plus mealtime aspart have comparable glycemic control and severe hypoglycemia rates. However, degludec-aspart caused less nocturnal hypoglycemia than biphasic insulin aspart in patients with T2DM.29
While premixed insulins and basal-bolus insulin therapies have provided alternative treatment options for patients that require tighter glycemic control, both approaches have limitations. These include incomplete 24-hour basal coverage with premixed insulins, potential titration complexity with basal-bolus regimens, and the burden of multiple injections.22 Thus, patients and their clinical care teams must carefully evaluate the potential advantages and limitations of each strategy in order to maintain glycemic control and reduce the incidence of hypoglycemia.
Question #4:
Which of the following situations describe patients who would be eligible for continuous subcutaneous insulin infusion (CSII)?
- Elevated A1C levels with multiple daily injections
- Marked same-day or between-day glucose level fluctuations
- Variability of insulin requirements (ie, dawn phenomenon)
- Recurrent hypoglycemia
- All of the above
CSII is the implantation of an infusion system into the body surface for the delivery of insulin using a pump mechanism. Ideally, any insulin replacement regimen should mimic the endogenous physiologic mechanism whereby basal amounts of insulin are provided throughout the day and additional bolus insulin is provided to cover the prandial needs in response to carbohydrate intake. Patients who have elevated A1C levels despite following their daily treatment levels, have significant glucose fluctuations, exhibit endogenous variability of insulin requirements (such as with the dawn phenomenon), or who have recurrent hypoglycemia may all be candidates for CSII therapy.30 As such the correct answer is E, all of the above. It is important to note that an ideal candidate should also be highly motivated to improve their glycemic control and feel comfortable with insulin pharmacodynamics and carbohydrate counting.
While discussing potential alternative therapeutic strategy options with Janet, you may also want to address some of the potential advantages of CSII therapy, which may be appropriate for dealing with her HU and is often paired with CGM technology. Compared with multiple daily injections, CSII has shown similar A1C reductions but greater treatment satisfaction among patients with T2DM in randomized controlled trials.31,32 Newer systems, using sensor-augmented pump therapy allows for an automated insulin suspension or low glucose suspension function that allows for insulin delivery to be suspended for up to 2 hours when glucose falls before a preset threshold. It is a technology that may reduce the duration and frequency of hypoglycemia. In one study of patients with HU, selected specifically because they have a significantly greater risk for a severe event, sensor-augmented pump therapy reduced the rate of moderate and severe hypoglycemia compared with pump-only therapy, the most commonly used treatment.33 Sensor-augmented pump therapy also reduces nocturnal hypoglycemia as well.34 Such therapy with suspended insulin therapy may benefit Janet given her current issues with nocturnal hypoglycemia, though the potential health improvements of pump therapy should be weighed against the cost effectiveness of multiple daily injection therapy.35
An important consideration in the use of insulin pump therapy is the integration of bolus calculators. Because calculating proper insulin bolus dosing multiple times per day is challenging and many adults with diabetes exhibit poor numeracy skills, bolus calculators may represent a useful tool that may improve glycemic control by helping to determine the proper dosing as well as account for any carry over from previous injections.36 Though the use of bolus calculators and pump therapy may reduce hypoglycemia, it is important to note that these technologies do not completely eliminate hypoglycemia. Like manual multiple dose injections, much of the success of the therapy is dependent on the patient’s commitment to this type of therapy and a thorough understanding of carbohydrate counting and proper insulin-to-carbohydrate ratios. Just as important is the skill set of the trainer, who must provide the right training and education for the patient. When discussing the option of CSII therapy with Janet, it is critical to stress that she must receive proper training in order for this treatment strategy to be successful.
Question #5:
All of the following agents may influence hypoglycemia control except?
- Beta-adrenergic antagonists/beta-blockers
- Alcohol
- Statins
- Serotonin reuptake inhibitors (SSRIs)
Successful clinical management of T2DM is complicated and requires a fine balance between therapy selection and lifestyle choices of the patient. As many patients with advanced T2DM also present with significant comorbidities, it is imperative that pharmacists remain cognizant of other therapeutic regimens, or agents such as alcohol, that may influence the incidence of hypoglycemia in patients with T2DM.
Beta-adrenergic antagonists or beta-blockers alter the effects of physiologic epinephrine and may affect the body’s ability to counter-regulate the effects of hypoglycemia and the return of glycemic levels to normal values after a hypoglycemic episode. This may be particularly true for cardioselective agents rather than noncardioselective agents. As such, these agents should be used with caution in patients with T2DM.13
The ADA recommends that adults with diabetes limit their alcohol consumption to one drink per day for women and two drinks per day for men, with extra precautions taken to avoid hypoglycemia.12 Alcohol consumption inhibits gluconeogenesis in the liver, potentially leading to severe hypoglycemia and delays in glucose counter-regulation after excess drinking.37,38 Though moderation is best, up to 20% of hypoglycemia resulting in hospitalizations has been attributed to excess alcohol in patients treated with insulin. In a small study, alcohol consumption was associated with a delayed hypoglycemic effect, resulting in an increased risk of hypoglycemia the following day.39 Keeping these factors in mind, you may want to address Janet’s alcohol consumption during your conversation and discuss the implications of excess alcohol consumption on her risk of hypoglycemia. You might suggest combining her moderate alcohol consumption with carbohydrates in order to help decrease her risk for hypoglycemia.
Statins are a mainstay therapy for the management of dyslipidemia in patients with diabetes. The ADA recommends that statin therapy be used in conjunction with lifestyle modifications, such as reducing saturated and trans fats and increasing fiber and plant sterols, to improve the lipid profile of patients.12 Therefore, the correct answer is C, statins. Janet’s current therapy of atorvastatin should not influence her risk of hypoglycemia.
Selective serotonin reuptake inhibitors (SSRI), such as sertraline, fluoxetine, and paroxetine, are agents commonly prescribed for depression in patients with diabetes. Clinical case studies have reported that in diabetic patients, SSRI therapy exacerbated hypoglycemia that was otherwise unexplained.40 Though the mechanism through which SSRI might be exhibiting this effect is unknown, some hypothesize that SSRIs alter the perception of hypoglycemia or lead to autonomic dysfunction.13 Conversely, other in vivo and clinical data support that SSRIs may enhance the counter-regulatory response to hypoglycemia through increases in the epinephrine and sympathetic nervous system responses.41,42 Though Janet is currently on a daily treatment regimen of 30 mg paroxetine, it is likely best to not alter her current therapy given the conflicting supporting evidence. If her hypoglycemia does not improve with some of the alternative therapeutic options and with careful monitoring of mealtime carbohydrate intake discussed above, then revisiting the impact of her SSRI therapy may be warranted.
FOLLOW UP
Since Janet’s last visit to the pharmacy, she has reassessed all of her treatment options with her physician and the next best steps to take to determine whether she is truly experiencing nocturnal HU and HAAF. They jointly decided that CGM was the best option to observe her daily and nightly glycemic patterns. After a week of using CGM, her readings clearly showed that she was experiencing nocturnal hypoglycemia with readings dipping to 40-50 mg/dL in the middle of the night. Janet began to eat a light snack before exercising in the evenings and also was much more cautious about her carbohydrate intake in the mornings, making adjustments based on her premixed regimen. These actions improved her blood glucose readings to 70-80 mg/dL overnight and in the morning. Following these adjustments, Janet’s physician decided it would be best to temporarily relax her glycemic control in order to restore awareness of hypoglycemic events. Janet still felt that a traditional basal-bolus regimen might give her more flexibility with mealtime options. Upon further consultation, her physician agreed to make a change to the long-acting insulin glargine with the rapid-acting insulin, aspart. However, the change was predicated upon Janet keeping a daily detailed blood glucose log, which she committed to maintaining. With the changes, Janet reports feeling more satisfied with her treatment regimen and since starting the log has been more cautious about her food choices. Since the change in her treatment regimen, Janet’s A1C reading was 7.4% and her physician is continuing to make incremental adjustments to her dosing regimen to bring that level down further while continuing to monitor for hypoglycemia. She reported that she has been waking feeling better rested since the change in medication and continues to use CGM to alert her to any hypoglycemic events that might occur at night.
CONCLUSION
Hypoglycemia avoidance in patients with advanced T2DM is extremely difficult. However, pharmacists are intermediaries to the physician and the rest of the clinical care team that can help pull out knowledge from the patient and should recognize any issues with medication selection or blood glucose monitoring. As influential partners of the diabetes management team, pharmacists can interact with patients to identify potentially hidden issues in patients, such as hypoglycemia unawareness or HAAF, which can have very serious and detrimental consequences if left untreated. Using motivational interviewing, pharmacists can determine if such problems exist during each visit with the patient.
Pharmacists should familiarize themselves with the prevalence of nocturnal hypoglycemia in patients with T1DM and advanced T2DM and its association with HU and HAAF. They should promote patient awareness of symptoms, frequent blood glucose monitoring either with CGM or self-monitoring, the maintenance of regular and healthy meals, dosing and tracking logs, and alternative therapies if warranted. Pharmacists should also be aware that patient satisfaction with therapy selection is important for adherence and glycemic control.
REFERENCES
- Fonseca VA. Defining and Characterizing the Progression of Type 2 Diabetes. Diabetes Care. 2009;32(suppl 2):S151-S156.
- Gastaldelli A. Role of beta-cell dysfunction, ectopic fat accumulation and insulin resistance in the pathogenesis of type 2 diabetes mellitus. Diabetes Res Clin Pract. 2011;93, Supplement 1:S60-S65.
- Unger J. Uncovering undetected hypoglycemic events. Diabetes Metab Syndr Obes Targets Ther. 2012;5:57-74.
- Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57(12):3169-3176.
- Leese GP, Wang J, Broomhall J, et al. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;26(4):1176-1180.
- Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013;369(4):362-372.
- Segel SA, Paramore DS, Cryer PE. Hypoglycemia-Associated Autonomic Failure in Advanced Type 2 Diabetes. Diabetes. 2002;51(3):724-733.
- Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and Diabetes: A Report of a Workgroup of the American Diabetes Association and The Endocrine Society. Diabetes Care. April 2013:DC_122480.
- Gabriely I, Shamoon H. Hypoglycemia in diabetes: common, often unrecognized. Cleve Clin J Med. 2004;71(4):335-342.
- Chico A, Vidal-Ríos P, Subirà M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care. 2003;26(4):1153-1157.
- Edelman SV, Blose JS. The Impact of Nocturnal Hypoglycemia on Clinical and Cost-Related Issues in Patients With Type 1 and Type 2 Diabetes. Diabetes Educ. 2014;40(3):269-279.
- American Diabetes Association. Standards of medical care in diabetes-2013. Diabetes Care. 2013;36 Suppl 1:S11-66.
- Martín-Timón I, Del Cañizo-Gómez FJ. Mechanisms of hypoglycemia unawareness and implications in diabetic patients. World J Diabetes. 2015;6(7):912-926.
- Moghissi E, Ismail-Beigi F, Devine RC. Hypoglycemia: minimizing its impact in type 2 diabetes. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2013;19(3):526-535.
- Wendel CS, Fotieo GG, Shah JH, Felicetta J, Curtis BH, Murata GH. Incidence of non-severe hypoglycaemia and intensity of treatment among veterans with type 2 diabetes in the U.S.A.: a prospective observational study. Diabet Med J Br Diabet Assoc. 2014;31(12):1524-1531.
- UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50(6):1140-1147.
- Schopman JE, Geddes J, Frier BM. Prevalence of impaired awareness of hypoglycaemia and frequency of hypoglycaemia in insulin-treated type 2 diabetes. Diabetes Res Clin Pract. 2010;87(1):64-68.
- Unger J, Parkin C. Hypoglycemia in insulin-treated diabetes: a case for increased vigilance. Postgrad Med. 2011;123(4):81-91.
- Unger J, Parkin C. Recognition, prevention, and proactive management of hypoglycemia in patients with type 1 diabetes mellitus. Postgrad Med. 2011;123(4):71-80.
- Choudhary P, Ramasamy S, Green L, et al. Real-time continuous glucose monitoring significantly reduces severe hypoglycemia in hypoglycemia-unaware patients with type 1 diabetes. Diabetes Care. 2013;36(12):4160-4162.
- Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26(6):1902-1912.
- Atkin S, Javed Z, Fulcher G. Insulin degludec and insulin aspart: novel insulins for the management of diabetes mellitus. Ther Adv Chronic Dis. 2015;6(6):375-388.
- Meece J. Hypoglycemia Awareness Training. AADE Pract. 2014;2(3):48-51.
- Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues vs. NPH human insulin in type 1 diabetes. A meta-analysis. Diabetes Obes Metab. 2009;11(4):372-378.
- Ratner RE, Hirsch IB, Neifing JL, Garg SK, Mecca TE, Wilson CA. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. U.S. Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care. 2000;23(5):639-643.
- Kølendorf K, Ross GP, Pavlic-Renar I, et al. Insulin detemir lowers the risk of hypoglycaemia and provides more consistent plasma glucose levels compared with NPH insulin in Type 1 diabetes. Diabet Med. 2006;23(7):729-735.
- Ritzel R, Roussel R, Bolli GB, et al. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab. 2015;17(9):859-867.
- Ratner RE, Gough SCL, Mathieu C, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15(2):175-184.
- Fulcher GR, Christiansen JS, Bantwal G, et al. Comparison of insulin degludec/insulin aspart and biphasic insulin aspart 30 in uncontrolled, insulin-treated type 2 diabetes: a phase 3a, randomized, treat-to-target trial. Diabetes Care. 2014;37(8):2084-2090.
- Valla V. Therapeutics of diabetes mellitus: focus on insulin analogues and insulin pumps. Exp Diabetes Res. 2010;2010:178372.
- Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care. 2005;28(7):1568-1573.
- Raskin P, Bode BW, Marks JB, et al. Continuous subcutaneous insulin infusion and multiple daily injection therapy are equally effective in type 2 diabetes: a randomized, parallel-group, 24-week study. Diabetes Care. 2003;26(9):2598-2603.
- Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA. 2013;310(12):1240-1247.
- Choudhary P, Shin J, Wang Y, et al. Insulin pump therapy with automated insulin suspension in response to hypoglycemia: reduction in nocturnal hypoglycemia in those at greatest risk. Diabetes Care. 2011;34(9):2023-2025.
- Bode BW. Insulin Pump Use in Type 2 Diabetes. Diabetes Technol Ther. 2010;12(Suppl 1):S-17-S-21.
- Schmidt S, Nørgaard K. Bolus Calculators. J Diabetes Sci Technol. 2014;8(5):1035-1041.
- Lee YA. Diabetes care for emerging adults: transition from pediatric to adult diabetes care systems. Ann Pediatr Endocrinol Metab. 2013;18(3):106-110.
- Ahrén B. Avoiding hypoglycemia: a key to success for glucose-lowering therapy in type 2 diabetes. Vasc Health Risk Manag. 2013;9:155-163.
- Richardson T, Weiss M, Thomas P, Kerr D. Day After the Night Before Influence of evening alcohol on risk of hypoglycemia in patients with type 1 diabetes. Diabetes Care. 2005;28(7):1801-1802.
- Sawka AM, Burgart V, Zimmerman D. Loss of awareness of hypoglycemia temporally associated with selective serotonin reuptake inhibitors. Diabetes Care. 2001;24(10):1845-1846.
- Sanders NM, Wilkinson CW, Taborsky GJ, et al. The selective serotonin reuptake inhibitor sertraline enhances counterregulatory responses to hypoglycemia. Am J Physiol Endocrinol Metab. 2008;294(5):E853-860.
- Briscoe VJ, Ertl AC, Tate DB, Davis SN. Effects of the selective serotonin reuptake inhibitor fluoxetine on counterregulatory responses to hypoglycemia in individuals with type 1 diabetes. Diabetes. 2008;57(12):3315-3322.
Back to Top