
Expired activity
Please go to the PowerPak
homepage and select a course.
Perioperative Pharmacy Care—What Every Pharmacist Should Know
INTRODUCTION
Pharmacists' roles in surgery and anesthesiology are growing. Pharmacists have expanded their
involvement in monitoring surgical stock-distribution systems, enforcing controlled-substance
procedures, supplying drug information, and providing pharmaceutical care to surgical patients.
One reason pharmacists are more critical in the operative theater is a growing understanding of
the need for rational drug therapy in the perioperative period.
This continuing education module addresses 4 perioperative areas in which pharmacists are key
resource personnel: surgical site infection prevention; recognition and treatment of malignant
hyperthermia (MH); appropriate use of alvimopan (Entereg); and recognition and treatment of
local anesthetic systemic toxicity.
SURGICAL CARE IMPROVEMENT PROJECT AND SURGICAL SITE INFECTION
Surgical procedures possess inherent risk. The dangers of surgery, some of which are readily
apparent, are demonstrated in full by a brief review of the arena in which many surgical
advances developed: war theaters through the centuries. Until World War II, disease and
nonbattle injuries caused an inordinate amount of morbidity and mortality, and much of what
was labeled "war pestilence" was infectious in nature. In the Civil War in the United States
(U.S.) and World War I, for example, for every soldier who died in combat, 7 soldiers died of
disease or postsurgical complications such as surgical site infections (SSIs). After sulfanilamide
and penicillin were discovered, surgeons began to hope that infection-free surgery might be
possible.1
During the 1950s and 1960s, researchers studied perioperative antibiotics arduously. They
produced high-quality laboratory and clinical evidence that supported the idea that appropriate
antibiotic administration decreased SSI rates.1 The Centers for Disease Control and Prevention
(CDC) has been monitoring nosocomial infections via the National Nosocomial Infections
Surveillance system (NNIS, now called the National Health Care Safety Network) since 1970. The
CDC has found little uniformity in how hospitals addressed SSI. Prevention has not been not a
priority; most patients have received organism-appropriate antibiotics, but the timing of doses
has been erratic. In one study, only 56% of surgical candidates received antibiotics at the most
ideal time—within 1 hour of incision.2
Despite medical and surgical advances, the threat posed by SSIs persists. Data collected as
recently as 2011 indicate that approximately 750,000 SSIs occur annually at a cost of more than
$2 billion to the U.S. health care system.3 An SSI that extends a hospital stay by a week or more
can triple the cost of care.4 Numerous other adverse outcomes are possible after surgery
including failure to resume crucial preoperative medications, thromboembolism, or prolonged and unnecessary catheterization. This section focuses on SSI, a continuing serious concern that
is influenced by a constellation of factors as shown in Table 1.
| Table 1. Risk Factors for Surgical Site Infection |
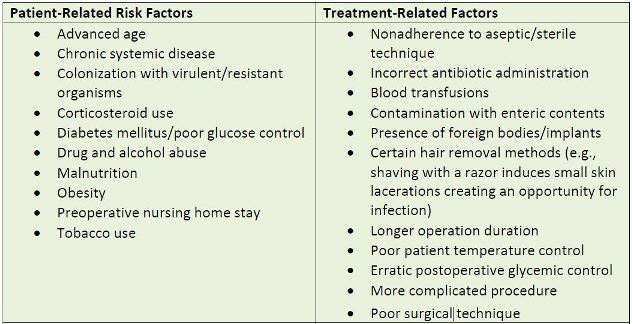 |
| Source: Reference 2 |
THE SURGICAL CARE IMPROVEMENT PROJECT
In 2002, the Centers for Medicare & Medicaid Services (CMS) partnered with more than 30
national organizations—including the American Hospital Association, CDC, Institute for Health
Care Improvement, and The Joint Commission—to standardize surgical quality improvement. In
the National Surgical Infection Prevention Project (SIP), hospitals began collecting data in July
2004. The project was later renamed The Surgical Care Improvement Project (SCIP) and
broadened to include performance measures that would reduce postoperative surgical
complications. The program's goal was to reduce surgical complications by 25% by 2010.2,3
A technical expert panel developed a mechanism through which hospitals could address
publicly reported individual SCIP core measures.5 Core (meaning central or most important)
measures are evidence-based criteria for specific conditions that gauge timeliness and
effectiveness of care. These describe specific actions that, when consistently applied,
contribute to successful outcomes. Publicly reported quality measures have the potential to not
only improve patients' outcomes, but also affect health care providers' business models. Core
measures are costly to collect and report; if a hospital's performance falls short, corrective
actions are equally time-consuming and costly to implement. To a certain extent, cost exerts pressure on hospitals to perform better, but the public nature of these measures is a larger
driver. Poor scores may convince patients to seek health care services elsewhere.3
SCIP now consists of nine measures, all of which address factors that can compromise
outcomes, shown in Table 2. Three core measures pertain to preventing health care-associated
infections (HAIs) and are of specific interest to health-system pharmacists:
• Use of prophylactic antibiotics appropriate for the patient's specific procedure.
• Initiation of prophylactic antibiotics no less than 1 hour before surgical incision (or
within 2 hours in patients receiving vancomycin or fluoroquinolones).
• Discontinuation of prophylactic antibiotics within 24 hours after surgery completion
(within 48 hours for cardiothoracic surgery).6-9
These core measures are quite specific and the entire health care teams needs to be aware of
them. In terms of surgery-related HAIs, pharmacists must appreciate four specific components:
antibiotic selection, timing of initial dose, intraoperative re-dosing recommendations, and
antibiotic discontinuation.
| Table 2. 2015 SCIP Measures |
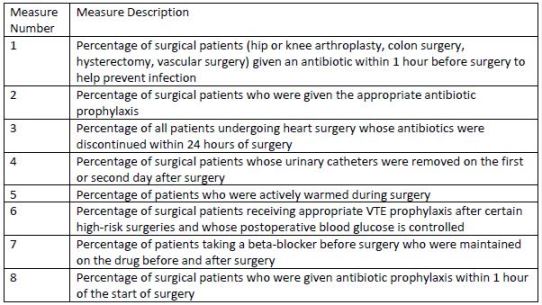
 |
| Source: Adapted from references 3 through 6 |
| Abbreviation used: VTE - venous thromboembolic |
Antibiotic Selection
Antibiotics, when used properly, augment proper aseptic technique, and are an additional
protection against infection. However, prophylactic antibiotics create some risks including
adverse reactions and development of antibiotic-associated diarrhea. Antibiotic overuse can
increase bacterial resistance.8
An appropriate prophylactic antibiotic has five properties9:
1. It is effective against microorganisms anticipated to cause postoperative infection in the
specific wound type and the hospital's geographic location.
2. It will achieve adequate local tissue levels that exceed the minimum inhibitory
concentration needed for the most likely infecting organism, and maintain those levels
from incision through wound closure.
3. It will cause minimal adverse effects.
4. The cost will be reasonable.
5. It will be unlikely to cause or contribute to increasing organism virulence.
Staphylococcus species cause infection in the majority of procedures that do not violate mucosa
or hollow viscera (the small and large intestines, stomach, esophagus, bladder, and uterus). .
Often, a cephalosporin fulfills these criteria and is a preferred agent. The SCIP
recommendations include an extensive, detailed chart that describes preferred antibiotics by
surgical site. Adequate doses are those calculated with consideration of patient weight or body
mass index.8,9
Timing of Initial Dose
SCIP specifies timing of antibiotic administration because presence of the drug at the surgical
site before the surgeon makes the first incision reduces the likelihood of SSI. Studies have
confirmed that when antibiotics are administered between 30 minutes and 2 hours before
incision, infection rates are very low. National guidelines recommend beginning antibiotic
infusion within 60 minutes of incision (or within 2 hours in patients receiving vancomycin or
fluoroquinolones).9
Intraoperative Redosing
Some surgical procedures can be lengthy. When the operative period is longer than the half-life
of the prophylactic antibiotic or there is excessive blood loss (i.e., more than 1,500 mL), re-administration of the antibiotic may be needed to ensure adequate coverage throughout the
procedure. Currently, 2013 clinical practice guidelines suggest administering a second dose of
the same antibiotic at intervals of 2 times the drug's half-life. Pharmacists who work in the
surgical arena should have copies of the guidelines available for ready reference at all times.
Clinicians need to refer to the document's redosing recommendation for the patient's specific
procedure and the antibiotic being used.9
Antibiotic Discontinuation
Studies have also demonstrated that administering antibiotics after the surgeon closes the
incision is unnecessary, so SCIP recommends discontinuing antibiotics within 24 hours. If
adequate tissue levels are achieved during the procedure, the antibiotic should not be needed
after the procedure is complete.9 However, certain surgeries, such as those for perforated
appendix, may require active treatment with antibiotic continuation needed after surgery.9
Additional Notes
Antibiotic prophylaxis is used in all surgical incisions, but patients who have wounds classified
as contaminated (open wounds after injury, viscus spillage, subject to break in sterile
technique, or incision through inflamed tissue) or dirty (traumatic in nature, necrotic or clearly
infected) will need to receive continuing therapeutic antibiotic treatment.4,10,11 More serious
wounds are associated with infection rates of up to 10%.11
Other nonantimicrobial interventions can reduce SSIs and may require the pharmacy team to
mobilize. Controlling the patient's intraoperative temperature, administering supplemental
oxygen, and aggressively hydration have been shown to decrease infection rates, although
more research is needed.12-15 Aggressive perioperative blood glucose control with intravenous
insulin in patients undergoing cardiac operations decreases SSI rates, and avoiding
intraoperative hyperglycemia is critical.11,16
Have these reporting measures actually decreased infection rates? Stulberg et al. found that
none of the individual SCIP measures was significantly associated with a lower probability of
infection. Composite analysis looking at all measures collectively found lower infection rates.6 Another study found that SSI risk varied by patient, procedure, and antibiotic properties but
was not significantly associated with prophylactic antibiotic timing.17
Surgical suite staff cannot pick and choose among these measures. They need to implement all
of them. Attention to the full set of metrics collectively appears to improve care and outcomes.
MALIGNANT HYPERTHERMIA
Rare and life-threatening, MH is associated with an autosomal-dominant calcium channel
mutation. Ordinarily, most MH-susceptible (MHS) individuals are unaware of their condition.
However, exposure to volatile anesthetics or depolarizing muscle relaxants such as
succinylcholine (other drugs have also been implicated as seen in Table 3) disrupts intracellular
calcium homeostasis, allowing uncontrolled calcium release from the sarcoplasmic reticulum. A
skeletal muscle metabolic crisis rapidly develops as calcium builds in this tissue and may
progress to a fatal hypermetabolic state called MH crisis.18,19 Until the 1970s, MH was
associated with a mortality rate of 70% to 80%.20,21
| Table 3. Potential Triggers of Malignant Hyperthermia |
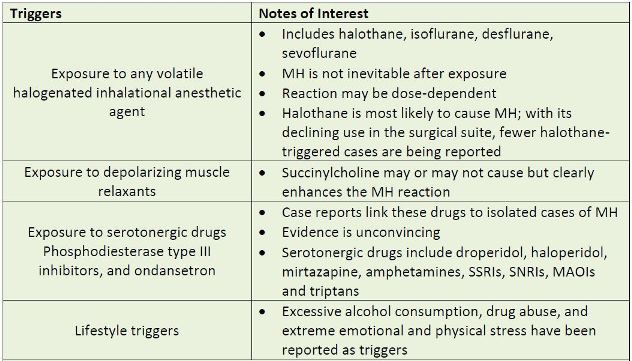 |
| Source: Reference 2 |
| Abbreviations used: MH – malignant hyperthermia; SSRIs – selective serotonin reuptake inhibitors; SNRIs – serotonin–norepinephrine reuptake inhibitors; MAOIs – monoamine oxidase inhibitors |
The caffeine-halothane contracture test (CHCT) remains the gold standard for diagnosing MHS)
individuals and requires a muscle biopsy. It is based on the observation that MH muscle
samples were more sensitive to the contracture-inducing properties of caffeine, caffeine with
halothane, or halothane alone, than normal muscle.19 Very few centers are capable of
performing the test (only four in the U.S. and 30 in the entire world). Since 2001, building on
research that identified MH-associated mutations in the ryanodine receptor gene, the
European Malignant Hyperthermia Group (EMHG) has maintained a guideline for genetic testing to identify patients with MH susceptibility. In July 2015, EMHG updated the guideline for
the diagnostic pathway for patients with potential increased risk for developing malignant
hyperthermia.19
For obvious reasons, most MH occurs in the surgical suite when patients are challenged with
anesthesia and muscle relaxants. Due to its tendency to progress rapidly, MH is considered an
emergency requiring immediate treatment.19
Epidemiology and Presentation
MH affects children and young adults of all races, but males are affected 2.5 to 4.5 times more
often than females.20 The true genetic prevalence of MH is probably higher than the estimated
incidence of 1 case for every 2,000 individuals for several reasons:
• Many susceptible people never receive an MH- triggering drug and never develop MH.
• Some susceptible people are exposed to MH-triggering agents but experience only mild
reactions that resolve quickly and spontaneously; their healthcare providers either do
not see the symptoms or interpret them as something else.
• MH follows an autosomal-dominant inheritance pattern in humans, so its prevalence is
increasing.22
MH's symptoms range from abortive courses (episodes that start insidiously, but for some
reason have lighter symptoms or end quickly) to fulminant MH crises.22
In the surgical suite, signs of impending MH begin with excessive carbon dioxide (CO2)
production. End tidal CO2 concentrations increases or hyperventilation develops while the
patient is breathing spontaneously. Between 50% and 80% of patients develop tachycardia,
supraventricular or ventricular arrhythmia, and isolated masseter (jaw) spasm if succinylcholine
has been used or generalized muscular rigidity with other agents.22 Additional signs include
profuse sweating and mottled (spotted or blotchy) skin.
As the crisis progresses, patients develop hallmark cyanosis due to oxygen consumption that is
3-fold greater than normal. Although the condition is called hyperthermia, temperature spikes
to 38.8°C (102oF) or more often occur relatively late or are prevented altogether if the
anesthesiologist recognizes the problem and starts treatment quickly. Untreated, MH
progresses to rhabdomyolysis and its cascade of hyperkalemia, elevated creatine
phosphokinase, and myoglobinemia. Acute renal failure becomes a strong possibility as the
kidneys strain to eliminate myoglobin, and at end stage, multiorgan failure and circulatory
collapse occurs.20,22
MH is managed as an emergency, and requires specific monitoring and treatment in the
postacute phase.
Medical Emergency
MH treatment follows the same protocol employed for any drug reaction, with the situation
being complicated if the patient is presently undergoing surgery and is unconscious. Surgical
suite staff need to be prepared to act quickly, modifying their approach based on the patient's
clinical presentation and unique needs. Staff should take the following actions22:
• Withdraw the offending agent as quickly as possible.
• Call for help if necessary, often making the call to the pharmacy response team.
• Give dantrolene 2.5 mg/kg (1 mg/lb) and repeat every 5 minutes until the patient's
respiratory symptoms are stable. Large doses (more than the recommended 10 mg/kg)
may be required for patients with persistent contractures or rigidity. Note that 10 mg/kg
is not a ceiling dose; however, do not exceed 30 mg/kg.
• Assess the need for continuing anesthesia, and if surgery must continue, start
intravenous opioids, sedatives, and if necessary, nondepolarizing muscle relaxants. End
the surgery as quickly as possible.
• Concurrently, the anesthesiologist should remove the vaporizer used to administer the
volatile anesthetic, replacing it with 100% oxygen at maximum fresh gas flow to restore
end tidal pCO2 to within normal limits.
The hydantoin derivative dantrolene, a specific ryanodine receptor antagonist, decreases
calcium loss from skeletal muscle's sarcoplasmic reticulum and restores normal metabolism.
Injectable dantrolene is available in 3 forms. Dantrium 20 mg vials that must be diluted in 60 mL
of sterile water before administration (do not use diluents that contain bacteriostatic agents).
Revonto 20 mg and Ryanodex 250 mg are packaged with their own solutions for reconstitution.
The difference between the products is significant. Ryanodex reconstitution yields a more
concentrated solution with 250 mg dantrolene suspended in 5 mL of sterile water for injection
in less than 30 seconds; it can be administered in less than 1 minute. An initial treatment dose
of dantrolene of 2.5 mg/kg for an 80 kg patient requires only 4 mL (200 mg). For Dantrium and
Revonto, reconstitution produces 60 mL, a considerably larger volume that cannot be
administered as quickly.23-26
Pharmacists need to remember that calcium channel blockers, when given with dantrolene,
may precipitate severe hyperkalemia and should be avoided. Although dantrolene has few, rare
adverse effects, pharmacists should be aware of them. They include prolonged breathing
problems, tissue necrosis after extravasation, nausea, vomiting, headache, and dizziness.24
If the patient continues to display symptoms following the cumulative administration of 10
doses of dantrolene, MH is an unlikely diagnosis.25,26 Table 4 describes additional supportive
care.
| Table 4. Additional Interventions for Acute MH |
 |
| Source: References 22, 24-26 |
| Abbreviations used: MH – malignant hyperthermia. |
Postacute MH
Patients who experience an episode of MH should be relocated to the intensive care unit for at
least 24 hours. During that time, dantrolene 1 mg/kg every 4 to 6 hours or 0.25 mg/kg/h should
be administered continuously for at least 24 hours and longer if they remain symptomatic.
Clinicians must monitor for damage to the kidney. Table 4 describes standard interventions
during this time.22-26
Patients should be referred for counseling and assessment to ensure they do not experience
MH again.
Treating an MH reaction early usually results in complete recovery, but a small number of
patients with MH die from organ failure annually. Every surgical suite should have a poster
posted in a prominent location to guide staff through the appropriate treatment steps should a
patient develop MH.
Additional Notes
The Malignant Hyperthermia Association of the United States (MHAUS, www.mhaus.org) educates the public and health care workers about MH and supports relevant research and education. The organization also provides a 24-hour hotline [(800) MH-HYPER or (800) 644-9737] to answer questions about MH and assist treating clinicians with acute cases. The group also encourages reports to the North American registry of acute MH cases, a database that researchers can use to further advance understanding of the condition.27
MH management has improved, as reflected by mortality declining from 80% to less than 5%. Improved treatment and better resources have made the difference.28
USING ALVIMOPAN SAFELY
Alvimopan (Entereg) is a peripherally acting μ-opioid receptor antagonist indicated to accelerate the time to upper and lower gastrointestinal (GI) recovery following surgeries that include partial bowel resection with primary anastomosis (connection of the healthy sections of the colon or rectum after a cancerous or otherwise diseased portion has been surgically removed). Clinical trials of alvimopan identified a potential for patients to develop myocardial infarction with long-term use, a potential problem that the U.S. Food and Drug Administration (FDA) addressed by requiring a risk evaluation and mitigation strategy (REMS) program.29,30 As a result, alvimopan is available only to hospitals that perform surgeries that include a bowel resection and dispensed by pharmacies that are enrolled in the E.A.S.E. ENTEREG® REMS Program.
Hospital pharmacies may receive requests to use alvimopam for off-label uses. These are usually based on small studies using alvimopan in cystectomy.31-34 Pharmacists should follow their hospitals’ procedures for approval of off-label drug requests, but cannot exceed the dosing parameters for alvimopan approved by the FDA.
REMS Programs
The concept of risk evaluation and mitigation is rooted in the Food and Drug Administration Amendments Act of 2007, which expanded the FDA’s authorities and responsibilities. The authority to require a REMS was designed to improve drug safety. The FDA can direct manufacturers to design and implement a REMS to ensure that a drug or biological product’s benefits outweigh its risks. The FDA can require a REMS as a condition for approval of a new product or for an approved product if new safety concerns are identified. REMS are designed to manage a known or potential serious risk associated with drugs or biologics.35
Every REMS is tailored to the drug or biologic it addresses. Depending on the agent and its
adverse event profile and monitoring requirements, the FDA may require any or all of the items
listed in Table 5.35
| Table 5. Possible Components of FDA-Mandated REMS Plans |
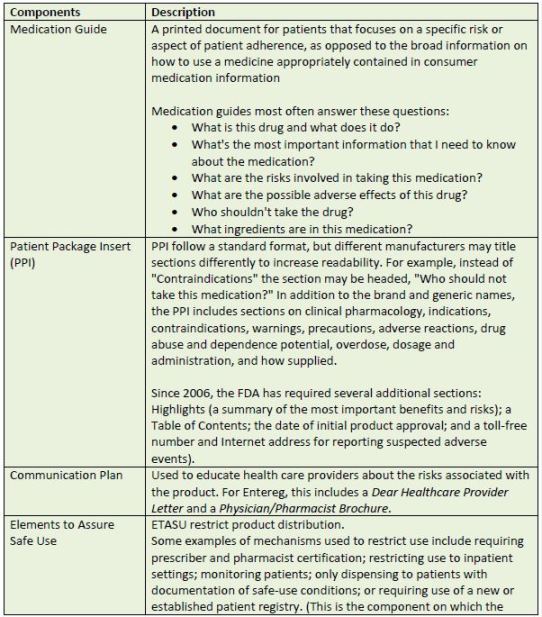
 |
| Source: References 35 |
| Abbreviations used:ETASU –elements to assure safe use; FDA – U.S. Food and Drug Administration; PPI – patient package insert; REMS, Risk Evaluation and Mitigation Strategy |
The Entereg EASE Program
The Entereg Access Support & Education (EASE) program (http://www.enteregrems.com/) is a
product-specific REMS designed to ensure that prescribers and patients use alvimopan in
accordance with the FDA-approved label.29,30 This program is intended to reduce the potential
risk for myocardial infarction (MI) with long-term use of this drug. Its 3 main components
include the following:
• The manufacturer must ensure the hospital receives the EASE REMS program kit and all
health care practitioners responsible for ordering, dispensing, or administering
alvimopan have been educated on alvimopan's benefits and risks.
• The certified hospital pharmacy has pharmacy systems, order sets, protocols, and/or
other measures in place to limit the use of alvimopan to inpatient settings and a
maximum of 15 doses.
• The certified hospital pharmacy will not dispense alvimopan to outpatients and will not
transfer alvimopan to any hospital pharmacy not enrolled with the EASE REMS Program.
Dear Health Care Provider Letter
The key point in alvimopan's Dear Health Care Provider Letter is that this drug is indicated for
short-term use only. The letter describes a 12-month study (the length of study that the FDA
considers the basis of long-term evaluation) in which 805 patients treated with opioids for
chronic pain received either 0.5 mg alvimopan (n = 538) or placebo (n = 267) twice daily.
Alvimopan-treated patients were more likely to experience myocardial infarction than placebo-treated patients, with most events occurring between 1 and 4 months after treatment
initiation. The manufacturer indicates that this imbalance has not been observed in other
alvimopan studies and no causal relationship has been established definitively.29,36
In keeping with current FDA requirements, the Dear Health Care Provider Letter also provides
information to remind health professionals to report adverse events. To report suspected
adverse reactions contact Cubist Pharmaceuticals at 1-877-CUBIST-6 (1-877-282-4786) or contact the FDA at 1-800-FDA-1088 (1-800-332-1088) or at
www.fda.gov/medwatch/default.htm .
Prescriber and Pharmacist Brochure
Cubist Pharmaceuticals also provides a 3-page Prescriber and Pharmacist Information Brochure (located here). Table 6 describes the brochure's main messages.30
| Table 6. Prescriber and Pharmacist Brochure Summary |
 |
| Source: References 30 |
| Abbreviations used:EASE – Entereg Access Support & Education Program; REMS – Risk Evaluation and Mitigation Strategy. |
The brochure highlights several important sections of the complete prescribing information.
First, it reminds health care providers that patients who receive more than 3 doses of an opioid
within the week before surgery may be more sensitive to alvimopan. They may experience
unwanted abdominal pain, nausea and vomiting, and diarrhea.
Next, the brochure addresses organ impairment and serious contraindications. Alvimopan is not
recommended for use in patients with severe hepatic impairment, end-stage renal disease,
complete gastrointestinal obstruction, pancreatic or gastric anastomosis, or in patients who
have had surgery for correction of complete bowel obstruction.
The brochure reports that alvimopan's most common adverse reaction—dyspepsia—occurs at
an incidence of 1.5% or more.
Additional Notes
Pharmaceutical manufacturers evaluate their products' safety throughout their lifecycles.
During clinical trials and product development, manufacturers often anticipate safety concerns
and the possibility that the FDA will require a REMS. When serious potential risks are identified,
manufacturers develop effective risk management strategies that are as flexible as possible.35
In the surgical space, pharmacists need to be aware of the EASE program. Its efforts are
directed at health professionals to ensure that appropriate patients receive the medication in
the hospital, 15 doses or fewer are administered, and that MI risk is minimized.
LOCAL ANESTHETIC TOXICITY
Unlike general anesthetics that act systemically, local anesthetics (LAs) block sensations
(especially pain) only in the affected area. Anesthesiologists and physicians often target specific
nerve pathways or induce paralysis when they perform specific procedures. They may employ
LAs for topical or surface anesthesia, infiltration, plexus block, epidural block, or subarachnoid
block.37-39
Structurally related to cocaine, synthetic LAs are rarely abused and produce no hypertension
and very little vasoconstriction. Often, LAs are administered with another drug to decrease
bleeding (e.g., lidocaine) or to decrease the anesthetic's acidity and quicken its onset of action
(e.g., sodium bicarbonate). Use of LAs has increased in the last decade as minimally invasive
and ultrasound-guided techniques have grown in popularity. These techniques improve
postoperative analgesia and lead to better perioperative outcomes.37-39
A large number of LAs are now available and are classified by chemical structure either as
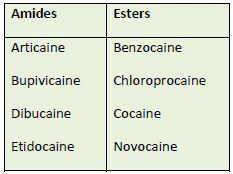
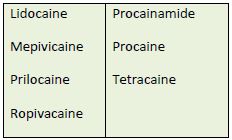
amides or esters.37-39 Table 7 lists some of the currently used LAs in these two categories. Many
health professionals remember an agent's classification in this way: amides generally have 2 i's
and esters have 1. Procainamide—an ester with 2 i's—is the sole exception. Usually, patients
who have an allergy or hypersensitivity to an amide LA do not cross-react to ester LAs.40
| Table 7. Local Anesthetics |
 |
Local anesthetic systemic toxicity (LAST) usually results from elevated LA plasma
concentrations.37-39 In fewer than 1% of patients who receive LA, systemic toxicity can be life-threatening.39
Most experts agree that LAs have the greatest potential to cause adverse reactions when
prescribers use inappropriate doses or routes or use a faulty injection technique. However,
unintended reactions to local anesthetics are possible even when dose and route are
appropriate. Patients with renal or hepatic impairment, respiratory acidosis, preexisting cardiac
block, hypoxia, or ischemic heart disease are at elevated risk for reaction. Patients who are
pregnant, very young, or very old also are more likely to have adverse reactions to LA.37-39
The most common cause of LAST is inadvertent intravascular injection, which can occur even if
the anesthetic was administered within the recommended dose range.37-39
Clinical Presentation of LAST
LAST's signs, symptoms, and timing are capricious, and a high level of vigilance is crucial since
early detection is associated with successful outcomes. One can think of LAST as a progressive
biphasic reaction that starts in the central nervous system (CNS) and progresses to the
cardiovascular system, although presentations vary. Both systems are very sensitive to changes
in tissue electrophysiology. Symptoms most often occur within 50 seconds of LA injection but
can be delayed for an hour or more after injection. Red flags in any patient who has received an
LA include altered mental status and cardiovascular instability.38
CNS symptoms are usually the first to develop. They may be subtle, or even absent altogether.
CNS excitation causes agitation, confusion, twitching, and seizures. CNS depression may also
occur and can cause drowsiness, obtundation, coma, or apnea. In addition, patients may
develop nonspecific neurologic symptoms including metallic taste, circumoral paresthesias
(burning, prickling, or formication around the mouth), diplopia, tinnitus, and dizziness.41
In the heart, LAST is manifested through conduction and contractility abnormalities. Both
tachyarrhythmia and bradycardia are possible. Hypotension or bradycardia may be a symptom
of severe local anesthetic toxicity. In severe cases, progressive hypotension and bradycardia can cause asystole. Ventricular ectopy, multiform ventricular tachycardia, and ventricular fibrillation
are common problems.
One critical point to remember is that LAST, especially when caused by potent and long-acting
LAs, is mechanistically different from cardiac arrest due to ischemia or a re-entrant arrhythmia.
This makes LAST somewhat resistant to traditional resuscitation methods.38,39
Mechanisms of Toxicity
The actual cause of LAST is not fully understood at this time, though it may be related to the
specific LA's mechanism of action: A flux of sodium ions across plasma membranes initiates and
propagates axonal action potentials. LAs bind to any of nine specific voltage-gated sodium
(NaV) channels and reduce sodium ion flux during depolarization in cardiac, nerve, and skeletal
muscle tissues. LAs appear to cause toxicity mainly when they block cardiac sodium channels.
Other proposed mechanisms include interference with ionotropic and metabotropic signaling
systems, and effects on mitochondrial metabolism and oxidative phosphorylation that prevent
electron transport and the ability to use fatty acids as fuel in cardiac myocytes.39
In the CNS, LAs block inhibitory pathways in the cerebral cortex, causing excitation. Now
unfettered, facilitatory neurons begin to work or function unopposed, increasing excitatory
activity further and leading to convulsions. As the LA dose increases, both inhibitory and
facilitatory circuits are inhibited, resulting in generalized CNS depression.39
As LA toxicity develops, hypercarbia and/or acidosis also decrease LA plasma protein binding.
Elevated carbon dioxide levels and falling pH also increase the available free drug to diffuse into
the brain. Conversely, acidosis increases the cationic form of the LA, which decreases its
diffusion rate through lipophilic cell membranes, a situation called ion trapping. The anesthetic
gets trapped in the intracellular space, and cannot be eliminated. Clinically, comorbid
hypercapnia and acidosis create a precarious situation. Seizures and CNS depression can lead to
hypoventilation and respiratory acidosis; decreased cardiac output leads to metabolic acidosis,
which further exacerbates the CNS and cardiac toxicity.37-39
Anesthetics have different affinities for NaV channel isoforms, and various cardiac and nerve
isoforms probably contribute to differences among LAST syndromes. LAs with short clinical
durations of action and lower potencies (e.g., lidocaine and mepivacaine) tend to depress
cardiac contractility without producing cardiac arrhythmias. Those with longer durations of
action and greater potencies (e.g., bupivacaine, levobupivacaine, and ropivacaine) seem more
likely to produce conduction defects with cardiac arrhythmias, with or without evidence of
reduced contractile function.39
Prevention of LAST
Since LAs have the greatest potential to cause LAST if inappropriate doses or routes are used or
the health professional's injection technique is faulty, prevention of most (but not all) reactions
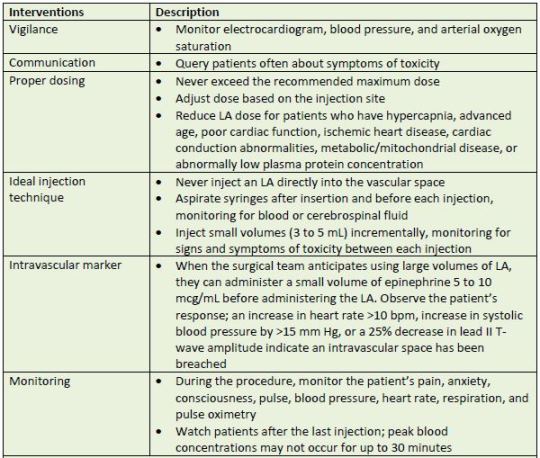
is possible. Table 8 describes reasonable interventions that may reduce the likelihood of LAST
occurring.
| Table 8. Steps for Preventing LAST |
 |
| Source: References 38, 42, 43 |
| Abbreviations used:BPM, beats per minute; LA, local anesthetic |
Prompt Treatment: Assisted Ventilation and Circulatory Support
At the first sign of LAST, the surgical team should not inject any more LA, secure the patient’s airway, ventilate with 100% oxygen, and call for help. If the patient does not have intravenous (IV) access established, a team member should insert an IV line. Team members should have a defibrillator ready and retrieve the crash cart or emergency box. Next, someone should secure
an LA toxicity kit (20% fat emulsion, off-label use). This is often a role for the pharmacy
response team.41,42,44
The LA toxicity kit consists of four 250-mL bags of 20% fat emulsion. The kit was designed with
consideration of the potential for recurrence of cardiovascular instability after initial successful
reversal of MH. Clinicians may need access to a total dose of 1000 mL. The kit also includes a 3-way lock, two 50-mL Luer-lock syringes, intravenous access and venoclysis equipment, and the
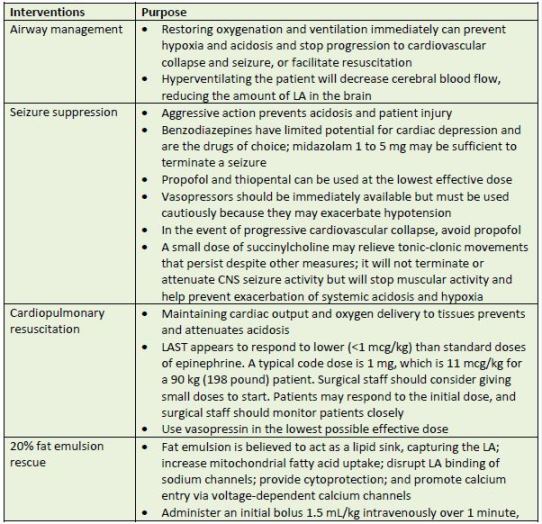
protocol sheet.45 Table 9 describes various interventions and their purposes.
| Table 9. Management of LAST |
 |
| Source: References 38, 41, 42, 44, 46, 47 and 48 |
| Abbreviations used:CNS – central nervous system; LA – local anesthetic; LAST – local anesthetic system toxicity |
In treatment-resistant cases of LAST in which there is inadequate response to epinephrine and
other standard therapies, cardiopulmonary bypass should be considered as a bridging therapy
until tissue levels of LAs have cleared.37-39
Additional Notes
LAST is a growing concern. Anesthesiologists are trained to watch carefully for LAST. Other
health professionals—those who work in offices, outpatient facilities, and small surgical
centers—need education so that misdiagnosis and underreporting can be reduced. Knowledge
about LAs and management of complications is important. Prevention strategies can reduce the
rate of LAST, but will not eliminate it. Pharmacists can provide comprehensive education and
ensure that the crash cart is augmented with a LAST rescue kit.
SUMMARY
Pharmacists' can expand their roles in the perioperative setting by having an antibiotic
surveillance program that addresses SCIP measures. All pharmacists who work with the surgical
suite should be prepared to treat malignant hyperthermia and have dantrolene available.
Alvimopan should be available preoperatively, and pharmacists need to be familiar with the
FDA's approved REMS, limiting dosing to 15 postoperative doses during hospitalization. In the
event that a patient develops LAST, rapid response with all the necessary components is critical.
References
1. Murray CK, Hinkle MK, Yun HC. History of infections associated with combat-related injuries. J Trauma. 2008;64(3)(suppl):S221-S231.
2. Munday GS, Deveaux P, Roberts H, Fry DE, Polk HC. Impact of implementation of the Surgical
Care Improvement Project and future strategies for improving quality in surgery. Am J Surg. 2014;208(5):835-840.
3. Cataife G, Weinberg DA, Wong HH, Kahn KL. The effect of Surgical Care Improvement Project
(SCIP) compliance on surgical site infections (SSI). Med Care. 2014;52(2)(suppl 1):S66-S73.
4. Tillman M, Wehbe-Janek H, Hodges B, Smythe WR, Papaconstantinou HT. Surgical care
improvement project and surgical site infections: can integration in the surgical safety checklist
improve quality performance and clinical outcomes? J Surg Res. 2013;184(1):150-156.
5. The Joint Commission. Surgical Care Improvement Project Available at:
http://www.jointcommission.org/surgical_care_improvement_project/. Accessed March 8,
2016.
6. Stulberg JJ, Delaney CP, Neuhauser DV, et al. Adherence to surgical care improvement
project measures and the association with postoperative infections. JAMA. 2010;303(24):2479-
2485.
7. Salkind AR, Kavitha CR. Antibiotic prophylaxis to prevent surgical site infections. Am Fam
Physician. 2011;83(5):585-590.
8. Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup.
Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical
Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
9. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial
prophylaxis in surgery. Am J Health-Syst Pharm. 2013;70:195-283.
10. Classification of surgical wounds. Master of Medicine.com http://masterofmedicine.com/classification-of-surgical-wounds/. Accessed July 22, 2015.
11. Furnary AP, Kerr KJ, Grunkemeier GL, et al. Continuous intravenous insulin infusion reduces
the incidence of deep sternal wound infection in diabetic patients after cardiac surgical
procedures. Ann Thorac Surg. 1999;67(2):352-360.
12. Kurz A, Sessler DI, Lenhardt RA. Perioperative normothermia to reduce the incidence of
surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334(19):1209-1215.
13. Grief R, Akca O, Horn E-P, et al. Supplemental perioperative oxygen to reduce the incidence
of surgical wound infection. N Engl J Med. 2000;342(3):161-167.
14. Melling AC, Ali B, Scott EM, et al. Effects of preoperative warming on the incidence of
wound infection after clean surgery: a randomized controlled trial. Lancet. 2001;358:876-880.
15. Sessler DI, Akca O. Nonpharmacologic prevention of surgical wound infections. Clin Infect
Dis. 2002;35(11):1397-1404.
16. Zerr KJ, Furnary AP, Grunkemeier GL, et al. Glucose control lowers the risk of wound
infection in diabetics after open heart operations. Ann Thorac Surg. 1997;63(2):356-361.
17. Hawn MT, Richman JS, Vick CC, et al. Timing of surgical antibiotic prophylaxis and the risk of
surgical site infection. JAMA Surg. 2013 Jul;148(7):649-57.
18. Bandschapp O, Girard T. Malignant hyperthermia. Swiss Med Wkly. July 31, 2012;142:w13652.
19. Hopkins PM, Rüffert H, Snoeck MM, et al; European Malignant Hyperthermia Group.
European Malignant Hyperthermia Group guidelines for investigation of malignant
hyperthermia susceptibility. [published online ahead of print July 18, 2015]. Br J Anaesth. doi: 10.1093/bja/aev225 .
20. Rosenberg H, Davis M, James D, Pollock N, Stowell K. Malignant hyperthermia. Orphanet J
Rare Dis. Published April 24, 2007. doi:10.1186/1750-1172-2-21.
21. Britt BA, Kalow W. Malignant hyperthermia: a statistical review. Can Anaesth Soc J. 1970;17(4):293-315.
22. Schneiderbanger D, Johannsen S, Roewer N, Schuster F. Management of malignant
hyperthermia: diagnosis and treatment. Ther Clin Risk Manag. 2014;10:355-362.
23. Wappler F. Malignant hyperthermia. Eur J Anaesthesiol. 2001;18(10):632-652.
24. Schuster F, Muller-Reible CR. Malignant hyperthermia-diagnostics, treatment and
anaesthetic management [in German]. [published online ahead of print Nov 16, 2009]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2009;44(11-12):758-763. doi: 10.1055/s-0029-1242125.
25. Glahn KP, Ellis FR, Halsall PJ, et al. Recognizing and managing a malignant hyperthermia
crisis: guidelines from the European Malignant Hyperthermia Group. Br J Anaesth.
2010;105(4):417-420.
26. Podranski T, Bouillon T, Schumacher PM, Taguchi A, Sessler DI, Kurz A. Compartmental
pharmacokinetics of dantrolene in adults: do malignant hyperthermia association dosing
guidelines work? Anesth Analg. 2005;101(6):1695-1699.
27. Malignant Hyperthermia Association of the United States. http://www.mhaus.org. Accessed
July 2, 2015.
28. Rosero EB, Adesanya AO, Timaran CH, Joshi GP. Trends and outcome of malignant
hyperthermia on the United States, 2000 to 2005. Anesthesiology. 2009;110(1):89-94.
29. Entereg [complete prescribing information]. Lexington, MA: Cubist Pharmaceuticals;
October 2014. http://www.merck.com/product/usa/pi_circulars/e/entereg/entereg_pi.pdf.
Accessed July 21, 2015.
30. Entereg Access Support & Education Program. [prescriber and pharmacist brochure]. http://www.enteregrems.com/pdf/ENTEREG%20REMS-Prescriber%20and%20Pharmacist%20Information%20Brochure.pdf. Accessed July 21, 2015.
31. S44#. Lee CT, Chang SS, Kamat AM, et al. Alvimopan accelerates gastrointestinal recovery
after radical cystectomy: a multicenter randomized placebo-controlled trial. Eur Urol. 2014;66:265-272.
32. Kauf TL, Svatek RS, Amiel G, et all. Alvimopan, a peripherally acting μ-opioid receptor
antagonist, is associated with reduced costs after radical cystectomy: economic analysis of a
phase 4 randomized, controlled trial. J Urol. 2014;191:1721-17277.
33. Tobis S, Heinlen JE, Ruel N,et al. Effect of alvimopan on return of bowel function after robot-assisted radical cystectomy. J Laparoendosc Adv Surg Tech A. 2014;24(10):693-697.
34. Manger JP, Nelson M, Blanchard S, Helo S, Conaway M, Krupski TL. Alvimopan: A cost-effective tool to decrease cystectomy length of stay. Cent European J Urol. 2014;67:335-341.
35. Dieck GS, Sharrar RG. Preparing for safety issues following drug approval: pre-approval risk
management considerations. Ther Adv Drug Saf. 2013;4(5):220-228.
36. Entereg Access Support & Education Program. [dear health care provider letter]. http://www.enteregrems.com/pdf/ENTEREG%20REMS-Dear%20Healthcare%20Provider%20Letter.pdf. Accessed July 21, 2015.
37. Cummings DR, Yamashita DD, McAndrews JP. Complications of local anesthesia used in oral
and maxillofacial surgery. Oral Maxillofac Surg Clin North Am. 2011;23(3):369-377.
38. Ciechanowicz SJ, Patil VK; Association of Anaesthetists of Great Britain and Ireland.
Intravenous lipid emulsion-rescued at LAST. Br Dent J. 2012;212(5):237-241.
39. Wolfe JW, Butterworth JF. Local anesthetic systemic toxicity: update on mechanisms and
treatment. Curr Opin Anaesthesiol. 2011;24(5):561-566.
40. González-Delgado P, Antón R, Soriano V, Zapater P, Niveiro E. Cross-reactivity among amide-type local anesthetics in a case of allergy to mepivacaine. J Investig Allergol Clin Immunol. 2006;16(5):311-313.
41. Di Gregorio G, Neal JM, Rosenquist RW, Weinberg GL. Clinical presentation of local
anesthetic systemic toxicity: a review of published cases, 1979 to 2009. Reg Anesth Pain Med. 2010;35(2):181-187.
42. Guinard JP, Mulroy MF, Carpenter RL. Aging reduces the reliability of epidural epinephrine
test doses. Reg Anesth. 1995;20(3):193-198.
43. Kahn RL, Quinn TJ. Blood pressure, not heart rate, as a marker of intravascular injection of
epinephrine in an epidural test dose. Reg Anesth. 1991;16(5):292-295.
44. Fettiplace MR, Weinberg G. Past, present, and future of lipid resuscitation therapy. [published online ahead of print July 17, 2015]. JPEN J Parenter Enteral Nutr. doi:10.1177/0148607115595979.
45. Marwick PC, Levin AI, Coetzee AR. Recurrence of cardiotoxicity after lipid rescue from
bupivacaine-induced cardiac arrest. Anesth Analg. 2009;108:1344-6.
46. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice guideline on local anesthetic
systemic toxicity. Reg Anesth Pain Manag. 2010;35(2):152-161.
47. Weinberg, GL. Treatment of local anesthetic systemic toxicity (LAST). Reg Anesth Pain
Manag. 2010; 35(2):188-193.
48. Neal JM, Weinberg GL. A checklist for treating local anesthetic systemic toxicity. APSF
newsletter. Available at www.apsf.org/newsletters/html/2012/spring/06_checklist.htm.
Accessed September 3, 2015.
Back to Top