
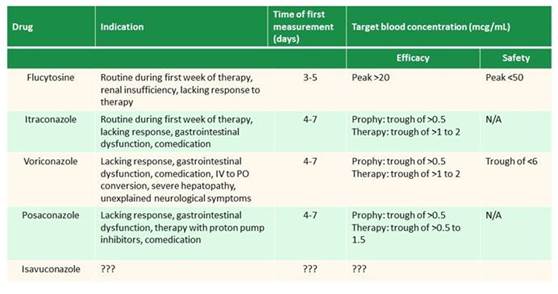
Expired activity
Please go to the PowerPak
homepage and select a course.
Invasive Mycoses: Evaluating the Emerging Paradigms and Their Practical Implications
OVERVIEW
This enduring activity is based on content presented at the 2016 MSGERC symposium at ASM Microbe. With content spanning from updates on diagnostics and pharmacologic considerations for therapies to challenges in the management of invasive aspergillosis, invasive mucormycosis, and breakthrough infections, this activity addresses key clinical issues for optimal invasive fungal disease (IFD) management. The activity also includes commentary on interactive audience questions from the live meeting.
SECTION 1: DIAGNOSTICS: EMERGING DATA
MAIKEN CAVLING ARENDRUP, MD
I've been asked to review what's new in diagnostics, what emerging data we have. I've decided to do that by organism.
Aspergillus diagnostics
We start out with Aspergillus spp. We have several nonculture techniques that you could use for diagnosing invasive Aspergillus infection. These include antigen detection (galactomannan, lateral flow device, and β-D-glucan) and PCR testing. Additionally, some tests are in development (including eNose breath test and PET/MRI). Then, we also have some news in the antifungal susceptibility testing arena. As I'm sure you're all familiar with the galactomannan test and β-D-glucan, I'm going to talk about the lateral flow device, PCR, and antifungal susceptibility testing.
Lateral Flow Device
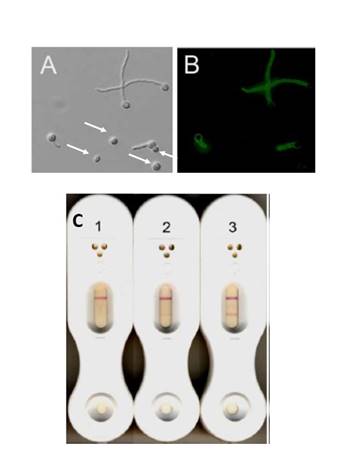
The lateral flow device uses a sort of pregnancy test format, which targets an antigen that is only secreted while Aspergillus is in growth. So this extracellular glycoprotein is secreted, and this test then detects this antigen using antibodies on the test strip that are directed toward it. The idea being that we might be able to discriminate between colonization, where you just have inhaled a few conidia, vs actual fungal growth in the lungs. This is highlighted here in the Section 1, Graphic 1, panel A, when you have conidia shown; those with the arrow have not germinated, in contrast to those up at the top, which have germinated. In panel B, they are stained with these antibodies. You can see that the antibody detects the growing hyphae, but not the conidia that were in panel A, marked with an arrow. [Thornton 2008]
The pros for this antigen test include its rapidity. You can perform the test in less than 20 minutes and you don't need fancy technology. For instance, you don't need an ELISA reader. One con is its lack of objectivity. If you look at the examples in Section 1, Graphic 1, panel C, you have a negative test on the left and a positive test on the right. The upper most band is a control band. The lower band is a test band. And in the center test, there is a weak test band. You can see that this is, of course, not an objective reading. You don't get a quantitative result that you can compare across laboratories or over time for the same patient. [Thornton 2008]
Section 1, Graphic 1. Functional characteristics of the lateral flow assay. From Thornton CR. Clin Vaccine Immunol. 2008;15:1095-1105.
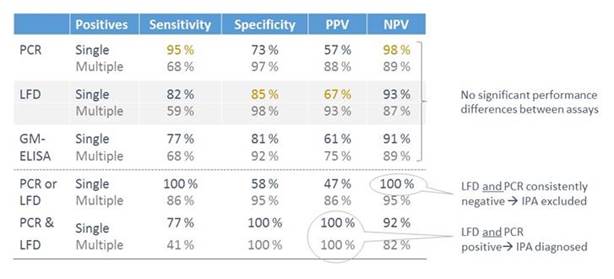
Now this test has been evaluated both for serum and for BAL. In Section 1, Graphic 2, we have data from a study in which the LFD was evaluated in serum and compared to PCR and galactomannan test. You can see the sensitivities, the specificities, the positive and negative predictive values. And you have PCR, the lateral flow device in gray, and the galactomannan test. You see there are small numeric differences, but actually these differences do not reach statistical significance.
You can also see that for each of these lines, you have a line for single vs multiple testing to determine positivity. Of course, the sensitivity goes down for all of these if you require more than one positive test result to determine if it is positive. But the specificity, of course, goes up. You see that in most cases here: you have a sensitivity that is quite nice. The positive and negative predictive values are listed as well. And they're not perfect, but it's an addition to the tests we have already. It is also clear that if you combine testing – so if you, for instance, combine the LFD test with the PCR, then you will have increased sensitivity and an increased negative predictive value. So if both tests are negative, then you could exclude infection. If both are positive, then you have diagnosed your patients and then you can focus your further diagnostics on those that do not fulfill these criteria. [White 2013]
Section 1, Graphic 2. Aspergillus spp. LFD, PCR, and galactomannan in serum/blood. Adapted from White PW et al. J Clin Microbiol.2013;51:1510-156.
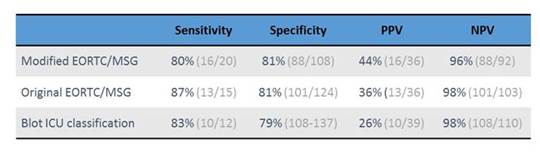
This test has also been evaluated in BAL fluid. A multicenter study from Austria looked at 4 centers that had 133 patients with 149 BALs. Sixteen of these patients—so that means a little bit more than 10%—were either proven or probable invasive Aspergillosis cases, using modified EORTC/MSG criteria, where an ICU stay for more than 4 days was included as a host factor. Most of the patients were characterized as pulmonary disease patients (29.5%), followed by heart disease (16.8%), hematologic malignancy (16.8%), GI disease (8.7%), neurologic disease (9.4%), or trauma (8.1%). [Eigl 2015a] These are normal risk groups for invasive aspergillosis. Independently, as shown in Section 1, Graphic 3, if you use the modified EORTC criteria—the original ones, or another set of classification criteria that has been designed specifically for the ICU Department—you see sensitivities here in the mid-80s; and specificities around 80 for the LFD. [Eigl 2015a]
Section 1, Graphic 3. Evaluation of LFD in BAL for diagnosis of IPA in ICU patients. Adapted from Eigl S et al. Crit Care. 2015;19:178.
This gives you a positive predictive value of around 40%. Maybe that doesn't seem too impressive. But the negative predictive value is quite high. You can use this test to sort of exclude IPA, but not really to diagnose it because the positive predictive value is too low.
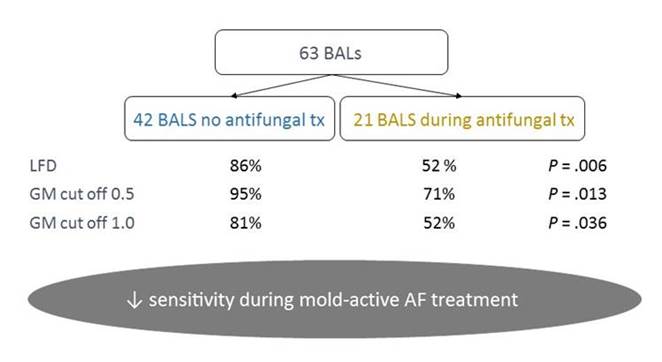
As in any other diagnostic test, the sensitivity depends on if your patient is already on antifungal treatment or not. That is clearly true for culture, it's true for antigen detection, and basically, it's true for everything. Because as soon as you give mold-active therapy, then you decrease your fungal load and the chance to diagnose. This is shown in Section 1, Graphic 4 for the LFD device in a study, also by Eigl and colleagues, where they had 63 BALs from 60 cases of proven or probable IPA cases. Here you have the sensitivity where BAL was performed in patients with no antifungal vs those with antifungal treatment. You can see that the sensitivity goes down from 86% to 52% with antifungal treatment. In addition, for galactomannan, the sensitivity goes down as soon as your patient is on anti-mold prophylaxis. That is quite logical. So it is, of course, important that you take the tests that are necessary before you put your patient on therapy. [Eigl 2015b]
Section 1, Graphic 4. Aspergillus BAL and LFD test sensitivity and the impact of mold-active antifungal therapy. Adapted from Eigl S et al. Int J Antimicrob Agents. 2015;46:401-405.
PCR
Let's move on to PCR. There's no commercial PCR out there for Aspergillusspp., but there's been a lot work going on over the past years to standardize the methodologies that are used for PCR testing for Aspergillus. The major initiative here is the European Aspergillosis PCR Initiative. The work that has been done by this group has been focused on what sample type should be used, how that sample type should be prepared for DNA extraction, and how the PCR should be performed.
I have just selected 3 papers out of this initiative to illustrate where we are currently (Section 1, Graphic 5). You can see summarized in each of the 3 studies—serum comes out the worst. It's not as good as plasma in study 1, it's not as good as plasma in the second study, and also whole blood pellet seems not so good here, but this study made use of frozen samples (where part of the pellet content may be lost during processing)—that may explain it. And in the third study as well, PCR in serum is not so good. Therefore, serum is not the preferred sample type. [White 2015; Springer 2016; White 2016]
Section 1, Graphic 5. Summary of key Aspergillus PCR studies from the EAPCRI PCR initiative. [White 2015; Springer 2016; White 2016]
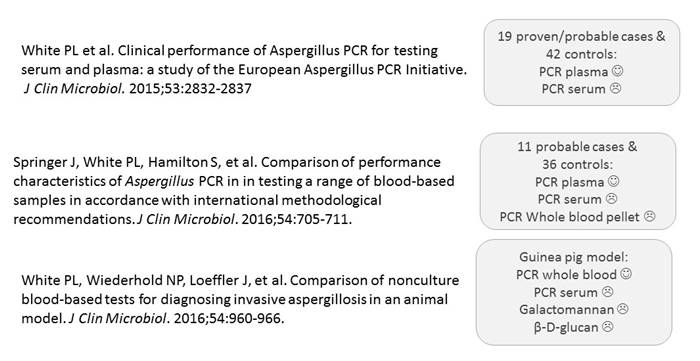
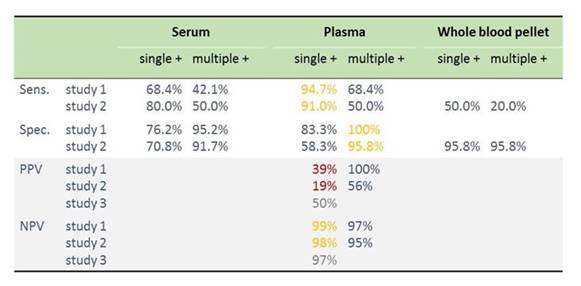
We can also look at the performance characteristics of the PCR tests in these studies. I've summarized that into 1 table here, as shown in Section 1, Graphic 6. You have the 3 studies up here—one using selective cases, one conducted in the hematological setting, and a third one comparing these methodologies in a guinea pig model. Overall, you can see that if you compare sensitivity between serum and plasma, plasma is better. If you look at specificity, it's again better in plasma than it is in serum. You can also see that you have the higher sensitivity, of course, if you just require 1 positive sample, and you have the highest specificity if you require more samples to determine positivity. Now, in these settings, the invasive aspergillosis infection rate was quite high. It was above 30%. That's not typical for the clinical setting. Typically, we have in most settings around 10% prevalence.
I've calculated the positive predictive values (bottom of the table) in the 3 studies based on prevalence rate of 10%. Again you see that the positive predictive value is not too good for PCR—it's below 50%. But the negative predictive value is very high. You can use the test to exclude disease. You can use the test to determine which patients do not have Aspergillus spp. infection and then focus your further diagnostics on those that were positive.
I'd like to talk a bit more about the guinea-pig model (study 3, Section 1, Graphic 5). Any animal model is nice because you really standardize your model, you're sure you're taking samples from the same situation, the same infected animal. In this model, the guinea pigs were inoculated with nebulized fumigatus conidia and samples were drawn at 1 hour, 5 days, 7 days and 9 days after inoculation. They found, in the guinea-pig model, that whole blood was better than serum. The performance characteristics of serum was poor, once again. That specificity was greater for β-D-glucan than it was for the other ones. And actually quite a low sensitivity for β-D-glucan here, which is quite surprising compared with the other studies that have been out there. But again, just as before, if you combine more than one test, then you can improve your performance of your diagnostics. So if you combine whole blood testing for galactomannan and β-D-glucan with whole blood PCR, then you get a sensitivity of 100%. Or if you combine all 3 tests—which of course, would be expensive—you improve your diagnostic performance. If all 3 are positive, you can diagnose infection. And if all 3 are negative, then you can exclude it. And otherwise, you can narrow your patient group where you have to use more intensive diagnostic approaches.
Section 1, Graphic 6. Performance data from selecting key Aspergillus PCR studies from the EAPCRI PCR initiative. [White 2015; Springer 2016; White 2016]
Aspergillus: Testing for Susceptibility and Resistance
Let's move on to susceptibility and to resistance. Section 1, Graphic 7 summarizes the reports that have reported resistance due to environmental resistance mechanisms in Aspergillus fumigatus.
We have seen 3 environmental mechanisms in Aspergillus fumigatus causing azole resistance. They are shown here, with the dark blue here for the TR34/L98H mechanism that confers pan-azole resistance. The light blue dot is for the mechanism that confers voriconazole and isavuconazole resistance. The green dot at the bottom is for a new mechanism that also confers azole resistance.
And you can see that you have a lot of dots here in Europe, where I come from. All those that I encircled, they come from publications that came out from around March last year until today. There are lots of circles here showing that—or highlighting that azole resistance is really becoming a global problem. Even if, in some regions, the proportion of isolates of resistance is not very high, more and more countries find these resistant isolates, and typically resistance doesn't go away so easily. I think resistance is becoming a larger issue. [Mellado 2007; Denning 2011; Snelders 2007; Mortensen 2010; Kuipers 2011; Lockhart 2011; Rath 2012; Stensvold 2012; Bader 2013; van der Linden 2013; van der Linden 2015; Prigitano 2014; Chowdhary 2012; Chowdhary 2013; Badali 2013; Seyedmousavi 2013; Steinmann 2014; Kurzyk 2015; Kidd 2015; Lavergne 2015; Wu 2015; Chen 2015; Pelaez 2015; Hagiwara 2016; Wiederhold 2016; Le Pape 2016; Castanheira 2016]
Section 1, Graphic 7. Fungicide use and azole resistant A fumigatus reports. Graphic courtesy of Professor Maiken Cavling Arendrup.
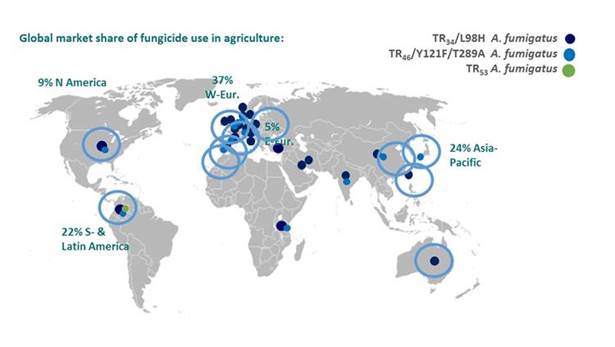
The reason why we have more in Europe is probably due to fact that we use more azole pesticides in our agriculture because we need to optimize the yield per hectare, since Europe is a more densely populated area. But because of this resistance, it is important that we can actually detect these isolates, and which infections, are caused by isolates with resistance. Doing mold susceptibility testing is not very easy in a routine laboratory for clinical microbiology. But actually there is a screening test, currently undergoing EUCAST validation, that was originally designed by Paul Verweij in the Netherlands. It uses a 4-well agar plate, where the agar contains azole antifungals in 3 of the wells: itraconazole (4 mg/L) in one, voriconazole (1 mg/L) in a second, posaconazole (0.5 mg/L) in the third, and no antifungal in a final one. You simply touch your colonies with a moist swab. You prepare a suspension of your conidia to a MacFarland 0.5-2. You then put 25 µL in each of the wells, then read the plate after 2 days and see if there is sufficient growth in the control well. And if these wells are clear, then the likelihood of resistance is really low. [van der Linden 2015] That is a very easy screening test that you could adopt in routine laboratories. I think that is an important consideration for invasive infections due to the rising frequency of azole resistance.
With that, we also just wanted to mention the Etest®. That is a commercial approach. In the upcoming European Committee on Antimicrobial Susceptibility Testing (EUCAST) Guidelines, or ESCMID Guidelines, we only give a C recommendation for this test because there has been quite a lot of variation from laboratory to laboratory in the MIC values that you get using this test. Therefore, the reference method gets a high score, and high strength of recommendation. EUCAST is best for clinical testing because we have breakpoints for Aspergillus. The Clinical and Laboratory Standards Institute (CLSI) has not established set breakpoints yet. But importantly, we also give a high score for use of this screening test for MIC testing. And we also recommend that you test on more than 1 colony, because when you breathe (and most of us do), then you don't inhale just a single genotype of Aspergillus. You inhale a lot of different Aspergillus conidia. So then you could have different genotypes in your lungs and you could easily have both susceptible and resistant strains. If you only test 1 colony on 1 occasion, then you really risk missing a resistant organism that is also in the lungs. [Astvad 2014]
Candida Testing
Now with that, we move to diagnostics for Candida. If we look at the nonculture techniques, the mannan antigen/antibody and the β-D-glucan test are well known. But there are some new data on the T2 Assay, PCR, and antifungal susceptibility testing, which I would like to focus on.
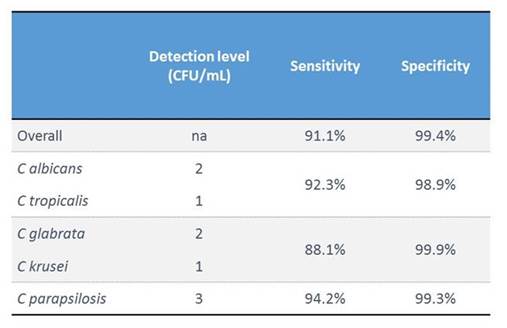
We start with the commercial T2Candida® test, which was launched last year and which detects the 5 most prevalent Candida species. It was tested in a big clinical study, which enrolled many patients but had a low prevalence of invasive candidiasis. Therefore, for the performance assessment, they also used spiked samples for calculating sensitivity and specificity. Section 1, Graphic 8 shows the table from the publication, the sensitivity and specificity rate. They found a sensitivity above 90%, and a high specificity overall. [Mylonakis 2015]
Section 1, Graphic 8. Performance characteristics of T2Candida® assay. Adapted from Mylonakis 2015.
In Denmark, we have conducted a 2-center evaluation, which is still ongoing, that compares the T2Candida® test, the mannan antigen and antibody test, and also blood culture. It is being conducted in the ICU setting and includes high-risk patients.
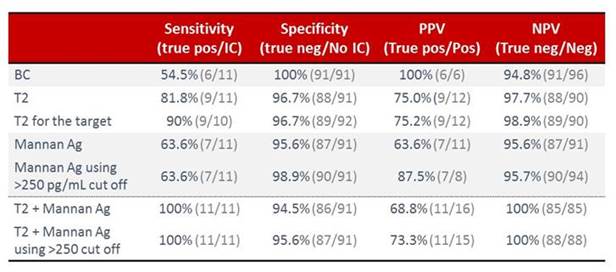
We now have included 100 cases—with 11 cases that are either proven or likely invasive candidiasis test cases. An external patient case review is pending. But still the proportion of positive cases was exactly as we expected, around 10%. If we look at the performance rates as shown in Section 1, Graphic 9, then you see that the T2Candida® test apparently comes out a bit better than the blood culture and also better than mannan antigen, even if we use a more stringent cutoff. And importantly, if we combine testing, that is if you combine T2 with mannan testing, then we can come up with 100% sensitivity and 100% negative predictive value. If you use a stringent mannan antigen cutoff, then the specificity is really also pretty nice. [Arendrup 2016]
Section 1, Graphic 9. Performance characteristics of the T2Candida® test compared with the mannan antigen/antibody test and blood culture. Adapted from Arendrup 2016.
In conclusion, I think that there is no such thing as a perfect test. However, by having a broad armamentarium and by combining the tests, we can increase our detection rate.
Resistance and Susceptibility Testing in Candida spp.
With that, we move to resistance and susceptibility testing for Candidaspp. I'm sure that most of you are aware that echinocandin resistance is emerging, particularly in Candida glabrata. Section 1, Graphic 10 shows data from the CDC Surveillance Program, where you see the rate of echinocandin resistance going up over the past 6 years, from 2008 to 2014. Now, in this study, the authors also looked at risk factors for having a resistant isolate. It may not be too surprising that, for instance, prior echinocandin use is—in multivariate analysis—associated with having a resistant Candida spp. isolate. [Vallabhaneni 2015] But it was somewhat surprising that fluconazole resistance is, as well. They also found that previous candidemia and recent hospitalization were also predisposing factors. But what was really surprising—to me, at least—was that almost 60% of the patients were echinocandin naive, at least in that time frame, when they were in the hospital. I haven't seen that magnitude of nonexposure previously in echinocandin resistance. The first impression when I read the paper was that maybe they had a very poor susceptibility test. But actually if you look at only the isolates for which they had the FKS sequence, they actually had a molecular mechanism behind the Candida resistance, and 44% of the cases with a hot-spot mutation were echinocandin-naive patients. [Vallabhaneni 2015]
This could mean that resistant isolates are transferred in the hospital from one patient to the other. It has been previously reported that you can have small colonial outbreaks and that not only parapsilosis or bacteria could be transferred from one patient to another. Of note, the CDC adopted a central venous catheter bundle policy in 2011. [CDC 2011] And, after that, the candidemia rate went down. That was particularly true in Atlanta/Baltimore for the patients that had a CVC. These findings strongly suggest that there was some kind of pathogen transfer in the hospital. [Vallabhaneni 2015] It also really underscores that susceptibility testing is important.
Section 1, Graphic 10. Echinocandin resistance in C glabratain the CDC surveillance program. From Vallabhaneni 2015. Ga = Atlanta metropolitan area; MD = Baltimore city and county, Maryland; OR = Tri-county region of Portland, Oregon, and TN = Knoxville, Tennessee.

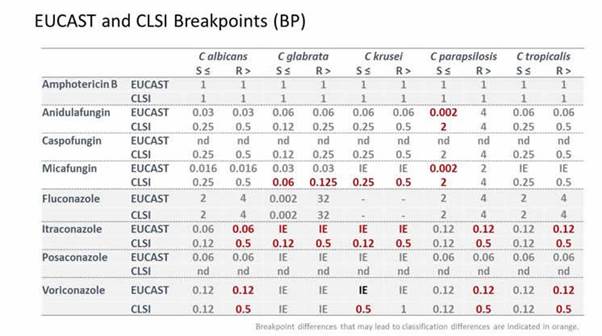
I think that, in that respect, it's good to see that both CLSI and EUCAST now have breakpoints for most drug combinations when it comes to Candidaspp. And also that these breakpoints for most of the compounds and organisms have been harmonized (Section 1, Graphic 11). Note that for us to make the comparison more direct, we adopted the EUCAST resistance designation of > rather than the CLSI designation of ≥ for the resistance breakpoints (and hence the breakpoint values are one 2-fold dilution lower than the exact number one would find in the CLSI tables). Section 1, Graphic 11 also highlights those where there might be minor differences. For instance, for parapsilosis, we would classify a wild-type organism's susceptibility as intermediate in Europe whereas it would be classified as susceptible by the CLSI. But these are really minor differences. We've come a long way in harmonizing breakpoints for the Candida. [EUCAST 2015; CLSI 2012]
Section 1, Graphic 11. EUCAST AND CLSI breakpoints for various Candida spp. Minor differences are indicated in maroon. Adapted from EUCAST 2015; CLSI 2012]
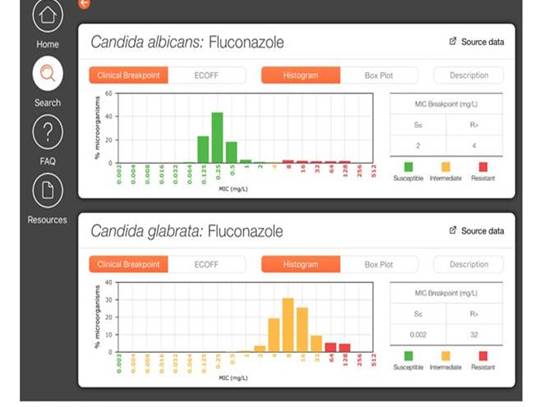
We have tried to make life a little easier for you by developing an app that you can download for iPhones or iPads or Android devices where you can see the MICs. If you download this app (FungiMICs) to your device, you can go in and you can choose any sort of drug combinations. Here I've selected Candida albicans and fluconazole, and glabrataand fluconazole. The breakpoints are shown in Section 1, Graphic 12, and all the MICs from our collated MIC distributions show up in green if it's a susceptible area; yellow if it's intermediate; and red if it's resistant. If you have an MIC, you can look into this and see if that MIC places you in the susceptible or the resistant area. For glabratain the lower panel, where the entire wild-type population is intermediate, you see there is only yellow and red. [ISHAM/EUCAST FungiMIC 2016]
Section 1, Graphic 12. Output of the FungiMAP app for Candidaspp. Developed by EUCAST/ISHAM. Available in Appstore and PlayMarket for iPhones/iPad tablets.
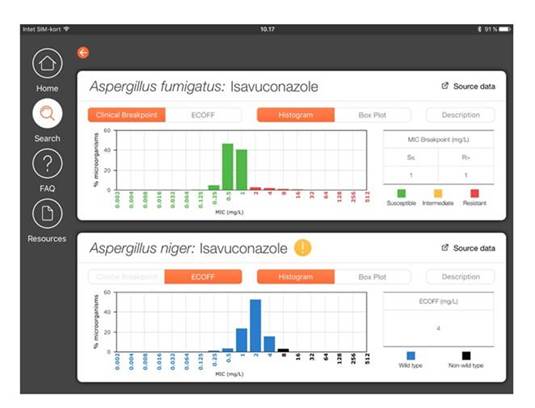
We can also generate similar figures for management of Aspergillus spp, as shown in Section 1, Graphic 13 with Aspergillus fumigatusand isavuconazole in the upper diagram and A niger and isavuconazole in the bottom diagram. For A niger, you see there is no clinical breakpoint tab. That's because we haven't set breakpoints for this organism yet. But you can still see the wild-type distribution because we've shown the MICs. And we've shown in blue those that are wild-type; and in black those that are not wild-type. [ISHAM/EUCAST FungiMIC 2016]
Section 1, Graphic 13. Output of the FungiMAP app for Aspergillus spp. Developed by EUCAST/ISHAM. Available in Appstore and PlayMarket for iPhones/iPad tablets.
Peter asked me specifically to specify what the differences are among epidemiologic cut-off values (ECOFFs), wild-type/nonwild-type, and breakpoint classifications into susceptible, intermediate, resistant.
I've tried to explain this in a little bit more detail in Section 1, Graphic 14. Here is a graphic developed from more than 800 isolates from 12 different sources from the EUCAST for C glabrata for fluconazole MICs. They give this typical 5-dilution-step-MIC distribution (the blue bars), documenting that the MIC variation that you see within this distribution can be explained by methodologic variation. But if you move out to the right of that distribution, then you have an isolate with a resistance mechanism. That isolate may or may not respond to treatment, depending on if the dosing overcomes this elevated MIC. So therefore, the epidemiological cutoff value, the ECOFF—as we call it, or ECV, as abbreviated here—describes the upper limit of what is normal for that organism. And when it's not normal, then you can't rely on your clinical experience with that drug-drug combination. That is also what you can see in the app. It's nice for you to note if an organism is wild-type or not.
Section 1, Graphic 14. Epidemiologic cut-off values (abbreviated ECOFF or ECV) and resistance in fungal pathogens. Adapted from EUCAST 2016.
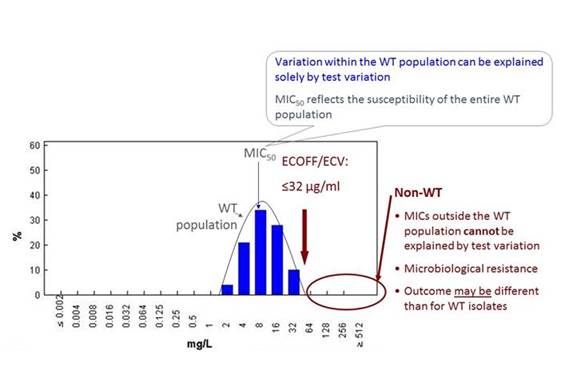
We can also generate such diagrams for fluconazole for Candida albicans, glabrata, and krusei. All have Gaussian distributions, and all have an ECOFF that describes the upper limit or ECV—depending on how you abbreviate it—that describes the upper limits. It's 1 for albicans, but it's 32 for glabrata. And it's 128 for krusei. But not all organisms have a clinical breakpoint because we know that krusei is not a good target for fluconazole, so we haven't set breakpoints for that organism. And for Candida albicans, for instance, the breakpoint is actually 1 step higher than the ECOFF because we have clinical data showing that you can treat that sort of organism well. That's the difference between ECOFFs and wild-type/nonwild-type and breakpoints.
Diagnostics for Other Fungal Infections
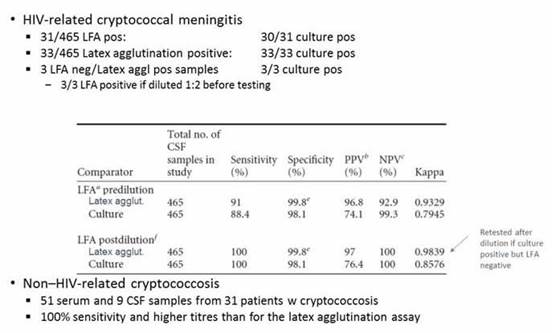
We now move to cryptococcal antigen, which for a long time period was usually detected by using a latex agglutination test. But a lateral flow test has been developed to test for cryptococcal infections, just as for Aspergillusspp. As shown in Section 1, Graphic 15, that test performs if you are looking at HIV-related Cryptococcus, as well as nonrelated HIV-related cryptococcal infection in the serum or CSF. [Lourens 2014; Jitmuang 2016] That's also an easy to use, almost bedside test for detection of the antigen.
Section 1, Graphic 15. Performance of the lateral flow assay in cryptococcal meningitis. From Jitmuang 2016 and Lourens A, Jarvis JN, Meintjes G, Samuel CM. Rapid diagnosis of cryptococcal meningitis by use of lateral flow assay on cerebrospinal fluid samples: influence of the high-dose "hook" effect. J Clin Microbiol. 2014;52(12):4172-4175. © American Society for Microbiology. With permission
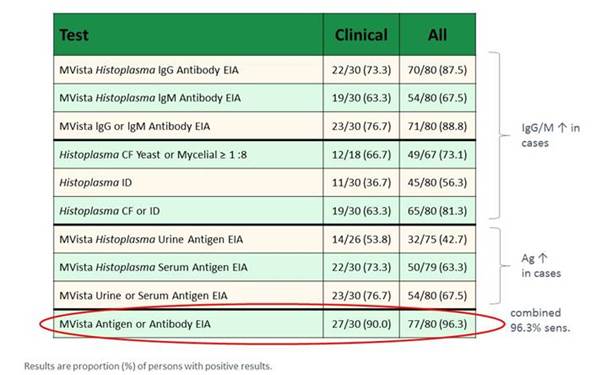
Finally, there is also news for Histoplasma detection. A very recent publication this year in Clinical Infectious Diseases looked at the performance for antibody detection, antigen detection, or both. As shown in Section 1, Graphic 16, the authors again found that you have a high sensitivity if you combine antigen and antibody detection. In the natural history, first you have antigen, then you have IgM, then you have IgG. If you combine these tests, you have a higher sensitivity. [Richer 2016]
Section 1, Graphic 16. HistoplasmaAg, IgM, and IgG Ab detection, positive results. Adapted from Richer 2016.
Commentary on Traditional Methods
These were all the things that have come up over the past years. But I just also want to highlight that we always criticize the classical techniques, the microscopy and the culture. But part of that low sensitivity that we see here may be due to how we performed classical diagnostics. In other words, we may have options for improving sensitivity.
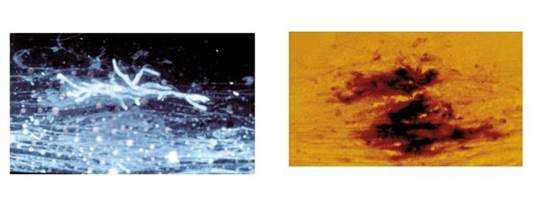
One possibility is to use a fluorescent brightener for detection when you do microscopy. [Rüchel 1999] As shown in Section 1, Graphic 17, left panel, with use of such a brightener, you can clearly see hyphae. And if you have it in real life, you can even see septae. You can see the dichotomous branching here, typical for Aspergillus. That is exactly the same slide on the right, where it's just a wet mount. The Aspergillus could easily be overlooked.
Section 1, Graphic 17: Differences between fungal preparations examined with fluorescent brightener (left side) and a plain wet mount (right side). Reproduced with permission from the Fungal Infection Trust.
http://www.aspergillus.org.uk/content/fumigatus-stained-blankophor%C2%AE;http://www.aspergillus.org.uk/content/fluorescence-microscopy-fumigatus
Also, you can optimize your culture by culturing out a suitable amount of your sample. If you increase the volume, you increase the sensitivity. That's been shown for BALs. [Fraczek 2014] In a study comparing the British normal standard culture vs a higher-volume-undiluted-culture method from respiratory specimens suspected of having Aspergillus fumigatus, 20% of samples were positive with the higher-volume method vs 10% for the standard method. [Fraczek 2014] If you increase your volume, you'll increase your chance to see it on your plate compared with the normal microbiology test, which was originally designed for detecting bacteria.
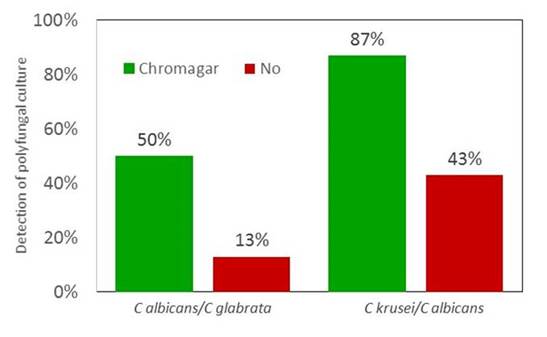
Finally, you can enhance your culture by including selective agar. We tested that in an Exterma; Quality Assessment Program (EQA) involving 6 laboratories in the Nordic countries. We sent them artificial samples, or simulated samples, if you will. And in 2 different samples, there were mixed cultures of 2 different Candida species (Section 1, Graphic 18). The laboratories that used chromogenic agars are in green. They actually detected both organisms in a much higher proportion if they used a chromogenic agar that is selective and that led to different colors for the different Candida species as compared with that if a selective agar was not used. [Arendrup 2007; Mortensen 2007]
Section 1, Graphic 18. Use of chromogenic selective agar vs normal media for the detection of polyfungal Candida spp. cultures. Adapted from Arendrup 2007; Mortensen 2007.
You can optimize how you do your classical techniques and thereby optimize your sensitivity. I think that's important. Even though we have fancy techniques, culture is the only method that gives you an isolate on which you can perform susceptibility testing.
Summary
In summary, there are emerging data that show you now can use the lateral flow devices for Aspergillus and Cryptococcus spp. There is progress for the PCR. There are new data on combined testing of antigen, IgM, and IgG for Histoplasmaspp. There are also new data showing that a combination of tests increases your diagnostic performance. For susceptibility testing, you have the azole agar test for Aspergillus, which is really user friendly. EUCAST and CLSI are today more similar than they are different. But don't forget the classical techniques, because they are still relevant. So with that, thank you for your attention.
SECTION 2. THERAPEUTICS: EMERGING DATA AND PHARMACOLOGIC IMPLICATIONS
ELIZABETH DODDS ASHLEY, PHARMD
Introduction
Thank you very much. This is an exciting time for antifungal therapies. We have more available treatment options than we've ever had before. But with choice and greater opportunities for our patients to have customized therapy, we need to be wiser about how we deploy these agents. And in this time where there is an increased focus on resistance of both bacterial pathogens and fungal pathogens, we have to be wise about how we use our antifungal agents to be sure that we steward these drugs to the best of our ability. Because even though we have more treatment options than we've ever had, we still have a limited number of systemic antifungal agents available to treat our patients.
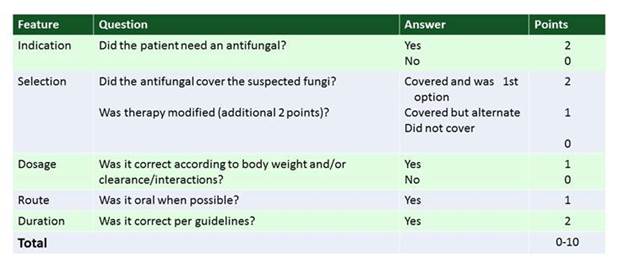
Dr. Munoz's group in Spain has done a lot with antifungal stewardship. One of the nicest studies they put out recently has to do with appropriateness of various antifungal drugs. As shown in Section 2, Graphic 1, they developed this score that I think is a fabulous tool, and I encourage you all to use this when you try to assess your antifungal treatments in your facilities. It goes through a simple set of questions to see if the antifungal therapy that was administered was most appropriate for the patient. It addresses whether an antifungal agent was needed at all. Did it cover the suspected or documented pathogen? Was therapy modified when you had culture results back? Was therapy streamlined, as appropriate? [Valerio 2014]
You can see there are lots of different scenarios encountered when individual treatment courses are reviewed such as the pathogen may have been covered, but a narrower agent would have been more appropriate was certainly one of the options observed. Then they also addressed the appropriateness of other key factors of administering antifungal drugs, including the dosing. Was it given by the right route? And did the duration match the guidelines? Together, you can come up with a composite score that ranges from 0 to 10 points.
Section 2, Graphic 1. Score for evaluating antifungal adequacy. Adapted from Valerio 2014.
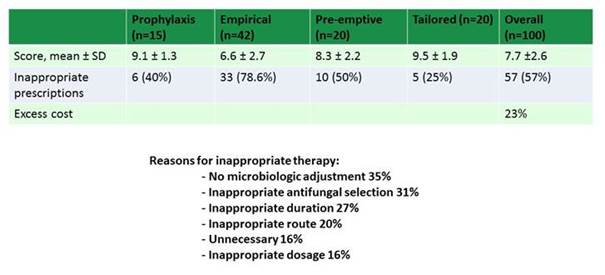
As shown in Section 2, Graphic 2, the investigators applied this to antifungal use at a large facility and found that in 100 cases reviewed, there was a lot of opportunity for improvement. For every type of therapy that was administered from prophylaxis all the way to tailored treatment in patients with confirmed cultures, there were a wide array of scores. The lowest score was for empiric therapy, where there was the least likelihood of having a completely adequate treatment.
When you look through the reasons for inappropriate therapy among the patients treated, a number of factors emerge. There was no dose adjustment or streamlining when that could have been done appropriately in over a third of patients. But also disturbing was the finding that unnecessary antifungal use was observed in 16% of the patients that they reviewed. And then another 16% had inappropriate dosing. So what this tells us is that, across the board, we can do better with our antifungal drug use. And these are data from a facility with a very active antifungal stewardship program and antifungal experts in place. I can only imagine what use might look like at other facilities where there's not quite so much attention paid.
Section 2, Graphic 2. Antifungal use score for different stages of therapy. Adapted from Valerio 2014.
So what I hope to do over the next few minutes is go through some practical advice for how we deploy these new agents to best optimize the use of this precious resource.
Drug-Specific Recommendations
Polyenes
Let's start first with amphotericin B. I remember all too well the days where we used to fight about whether or not we should be using lipid amphotericin agents, and among the lipid amphotericin agents, which ones we should be using. Those discussions have somewhat reemerged. And although lipid amphotericin B has certainly become the standard amphotericin B product administered at most facilities, we now have some new agents emerging internationally that I think are worth mentioning.
These other lipid-based formulations are coming on almost as generic versions of amphotericin B in a lipid carrier. But the lipid carriers are not consistent. I think it is very important that we pause and recognize that not all lipids are the same. They're not all liposomes. They're different lipid carriers. They all have different kinetic properties and as such, they may release the drug differently.
For those of you who remember the arguments about the commercial products available in the United States, being Abelcet and AmBisome—the lipid product, Abelcet, had a much different amphotericin B-release property than the true liposome, which is AmBisome, that may or may not have been related to differences seen with side effects. So when we look at these newer generic agents it is important to consider several things. We don't necessarily know the dosing, because they're coming on using equivalence data in a generic form. This means they are being approved based on pharmacologic characteristics alone. They don't have the same efficacy studies that we have with the other approved agents, which we know much more closely from the clinical trials for the various fungal infections and empiric antifungal therapy that we've seen and we're all familiar with.
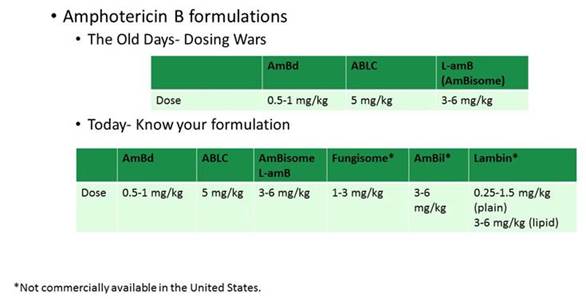
Doses vary for these newer agents. Again, these haven't been proven in clinical trials but are based on best guesses off of their drug-release properties. We see differences in side effects. We really need to be careful about how we deploy these. There are some cases where we can easily interchange between drugs within a class, or where using a generic formulation is no problem, such as the case with fluconazole. But in the case of the lipid amphotericin B products, that's really not true, because all of the lipid carriers are proprietary and differ for each product that's out there. In Section 2, Graphic 3, you can see I've listed some of the differences in dosing for some of the newer drugs, including some of the agents that we do not have here in the United States but that are now available internationally.
Section 2, Graphic 3. Dosing of various amphotericin B formulations. [Abelcet PI 2010; AmBisome PI 2012; Fungisone PI 2009; Jadhav 2012; Lambin PI 2016]
We have learned a lot about antifungal pharmacodynamics over the past several years. Now, more than ever, pharmacodynamics are being used to guide dosing regimens that are deployed for antifungal agents. I just want to take a few moments and go over the key characteristics that are used to guide our dosing principles. [Abelcet PI 2010; Ambisome PI 2012; Fungisone PI 2009; Jadhav 2012; Lambin PI 2016]
Sometimes, when we go in and review patients on antifungal treatments, we see best of intentions lead to poor dosing regimens. And we'll go through some very specific examples in a few minutes. But lying beneath what drives best practices for antifungal therapy are these principles. I will give these correlates to our antibacterial treatments for which we're more familiar with using pharmacodynamic approaches.
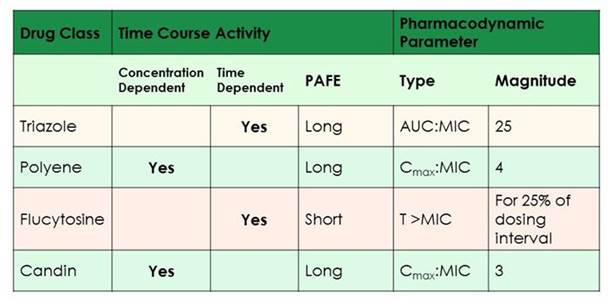
As shown in Section 2, Graphic 4, for the azole class, those drugs are much more similar to our β-lactam antibiotics. Their best pharmacodynamic parameter is the AUC-to-MIC ratio. It seems to be optimized around 25. And that's really consistent for the class. I'll show you some data with the newest agents in just a moment that confirms this. We have the polyenes and the echinocandins. Their dynamic profile is most similar to that of aminoglycosides. So it's the Cmax-to-MIC ratio that we really want to optimize in those agents. Consolidated dosing will work; that's why we can get away with giving larger doses less frequently, as is sometimes done particularly with amphotericin B to minimize toxicity. It is important to point out that with the echinocandins, we do see the ceiling, or Eagle effect. At certain very high doses, we see decreased activity of the drug, so we don't want to drive too high. That's reflected in the optimal pharmacodynamic parameter, here with a Cmax-to-MIC ratio of just 3 for the echinocandins. [Andes 2003]
Lastly, I didn't want to leave out flucytosine. It's a drug that we are seeing used in cryptococcal meningitis. That agent has the closest profile to β-lactams, with time above MIC being the predictor we look to most closely there. It needs to have a concentration above the MIC for the pathogen of about 25% of the dosage interval. [Andes 2003] What we've learned most about flucytosine as of late is we may have been too aggressive in dosing. When the drug was initially trialed it was dosed to the point of toxicity. So we had targets where we wanted to have concentrations of less than 100 mg/kg/d—and that was to avoid toxicity. But what we've learned is we actually probably don't need that much drug to have clinical efficacy. Now we're able to target some lower doses when we're using it to treat our patients with cryptococcal meningitis. [Lepak 2015]
Section 2, Graphic 4. Pharmacokinetic and pharmacodynamics for antifungals
Dosing
Now I want to walk through several of the agents and talk about where we're seeing common mistakes in dosing today in clinical practice. The first of these is fluconazole. I will tell you that it amazes me in my clinical practice how often I see fluconazole misdosed. As I was preparing for this talk, this actually happened to me. I was in a hospital in which we do antimicrobial stewardship. We were looking at their antifungal use, and they had a lot of fluconazole use that we really didn't understand, so we looked at the purchasing data. I asked, "Can I see how many fluconazole 200-mg tablets you bought?" They said, "We don't buy 200-mg tablets and we use 150 mg pretty exclusively for our patients." They hospital staff replied that they use this dosing even for patients with candidemia. We have now really started talking more about appropriate dosing. I think we've done such a good job of talking about which drug we can use to treat for various fungal infections. But we don't spend as much time on the dosing.
The most commonly used dose of fluconazole is the 150-mg dose that's widely used in the community. Never appropriate for inpatients though. And we need to be careful about this and make sure we're doing the right thing. So this really is a major concern for us.
There was a recent publication that looked at fluconazole use for candidemia. It reviewed about 200 cases in ICUs and oncology wards. The investigators found that a fifth of them had inappropriate dosing based on the use of the drug in those particular patients. While dosing may have been based on renal dysfunction, most often it was just inappropriate empiric-dose selection. [Nivoix 2012]
I thought it was important to just highlight the most recent candidemia/invasive candidiasis guidelines that talk about fluconazole use in a load of 800 mg or 12 mg/kg, followed by 400 mg daily. [Pappas 2016] It certainly is a drug that we can use to treat susceptible candidemia. But we need to be sure that we're dosing it correctly, or we will see treatment failures and we will drive resistance. This is a very important concern because we're seeing more and more fluconazole use again. And I just want to highlight that we need to pay attention to how we're dosing this drug in particular.
Another drug that has caused some concerns around dosing is posaconazole. Posaconazole is a triazole; it has an IV formulation available. Section 2, Graphic 5 shows the dosages for posaconazole formulations. When posaconazole was first marketed in the United States, it had a single formulation: it was an oral suspension. In suspension form, posaconazole requires a high-fat meal to have ideal absorption. And it's really unique in that it has a saturable absorption mechanism, where the maximum amount a human will absorb in a day is right around 800 mg. So when we want to optimize concentrations or raise concentrations in a patient because we feel they're not responding appropriately, we can't simply dose escalate and have an increased concentration achieved. What we needed to do with this formulation, instead, was give more frequent smaller doses. So the dose for prophylaxis is a total daily dose of 600 mg, and it was administered as 200 mg 3 times a day. For treatment, we do like to give the full 800 mg. And we could give it 400 mg twice a day, which would really optimize exposure but lose some convenience for our patients. We could also give 200 mg 4 times daily.
Fortunately, there has been the more recent development of delayed-release oral posaconazole tablets. This has changed how we are able to treat patients. It is better absorbed and has a better pharmacokinetic profile. But the dosing isn't the same. So we do see dosing errors as patients are converted between the oral suspension and the delayed-release oral tablets. It's very important to know what formulation of this drug the patients are receiving, even if it's changing between oral formulations.
The standard of care now is definitely the tablets. They've all but completely eliminated the need for oral suspension, which we save for our pediatric patients now. We do everything we can to get the delayed-release oral tablets into our patients. The nice thing about the tablets is that they don't have the same dependence on food for absorption, but we still recommend giving it with a meal, if possible. The IV formulation follows dosing of the oral tablets, not the oral suspension. [Noxafil PI 2014]
Section 2, Graphic 5. Dosing for posaconazole. Adapted from Noxafil PI 2014.

We've seen a lot of errors as patients switch between these formulations and as patients may request a formulation change at their pharmacy when they're filling out patient prescriptions. When we prescribe this for patients, we need to educate them about the importance of this fact: if you need to take a different formulation, we need to change the dose that we're going to give to you. That's not something that's always quite so clear. The switches in formulation are commonly done at the pharmacy. So we need to be aware of that, and we need to educate on that, which is very important.
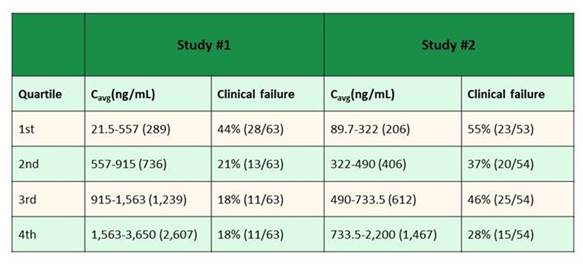
This is another drug that we want to be sure that we dose appropriately to ensure that patients are receiving optimized treatment, especially if we're trying to use it to treat mucormycosis. Posaconazole has some data about treatment success and failure related to serum drug concentrations. I'm going to show you Section 2, Graphic 6 for a whole bunch of reasons, and I hope one thing you walk away from this talk with is an understanding of how we do therapeutic drug monitoring for antifungal drugs. But we do this based on data from a very small number of patients.
So these were data from 2 early clinical trials with posaconazole. And the patients were stratified into quartiles based on the serum drug concentrations. These were random serum drug concentrations that were obtained from patients in either a prophylaxis or treatment study. And you can see that especially if you look at Study 2 (with levels reported in ng/mL), I want to tell you the target in just a minute, which falls in right around 0.5 mcg/mL You can see there was 37% clinical failure below 0.5 mcg/mL, and 46% clinical failure above 0.5 mcg/mL concentration. These are small numbers of patients. These are murky data. And yet we had to draw a line in the sand somewhere in terms of what our target was going to be for serum drug concentration monitoring. So keep this in mind.
Section 2, Graphic 6. Exposure-response relationship for posaconazole. Plasma steady-state average plasma concentrations vs clinical failure rates. Adapted from Jang 2010.
The other thing to know about the way these data were obtained is that for these patients who enrolled in the clinical trials, the primary goal of the study was not to obtain PK data. So when we had patients enrolled in the studies and we thought they'd be a good candidate to have PK monitoring done, we took random concentrations at convenience and they were frozen away and assessed later and compared with the patient's clinical outcome. So, it's important to consider that these recommendations are based on studies done with small numbers of patients that were not designed to be PK studies.
I want to talk next about isavuconazole, the newest antifungal drug we have. This is also an azole antifungal available in both oral and IV formulations. It's given as a prodrug, and the active form that's given is isavuconazonium that is converted to isavuconazole after administration. This drug has some unique features. One is that after a 2-day load, it's administered only once daily. It has a really good bioavailability. We're able to get really good serum drug concentration in our patients. [Cresemba PI 2015]
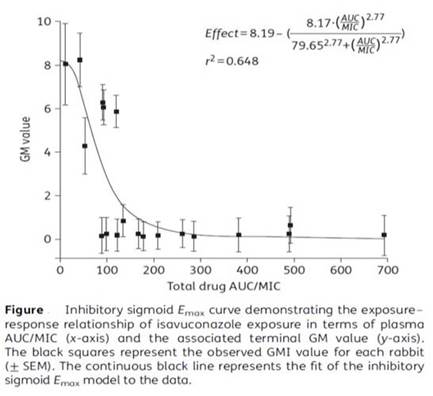
Section 2, Graphic 7 shows a very elegant study from Laura Kovanda. And I like this because it really shows these pharmacodynamic principles in action. So these are data obtained from a rabbit model of pulmonary aspergillosis, but based on Monte Carlo simulation data from serum concentrations obtained from patients during clinical trials with the drug. And what you can see here is that across the X-axis is the total drug AUC-to-MIC ratio. And you might remember earlier that I told you for azoles, the ideal AUC-to-MIC ratio is around 25. What they found here, if you look at galactomannan values on the Y-axis, they drop precipitously, right around the time that the AUC-to-MIC ratio gets to about 30. They saw 50% reduction in the galactomannan index when the AUC-to-MIC ratio reached 30. So that is the ideal target for this drug. And clearly there is a dose-response relationship. We do want to have adequate drug concentrations to assure efficacy with this agent. So the role of therapeutic drug monitoring is starting to emerge, but is less clear for this agent. [Kovanda 2016]
Section 2, Graphic 7. Exposure response relationship of isavuconazole exposure in terms of plasma AUC/MIC and terminal GM value. From Kovanda LL, Petraitiene R, Petraitis V, et al. Pharmacodynamics of isavuconazole in experimental invasive pulmonary aspergillosis: implications for clinical breakpoints. J Antimicrob Chemother. 2016;71(7):1885-1891. ©British Society for Antimicrobial Chemotherapy. With permission; and Jitmuang 2016.
Therapeutic Drug Monitoring
When do we want to do antifungal drug concentration monitoring? I know this sounds kind of a foolish thing to do, but when I worked with our clinical fellows and they want to obtain drug concentrations, one thing I do with each of them is say, 'We'll go ahead and we'll order the concentration. But before we do I want you to think through how you're going to respond to that concentration. And how that concentration is going to change what you're doing in this individual patient." And I often have them write it in and put it in their pocket. They don't have to tell me, they don't have to share. But just to show that sometimes I think we rely on these as a crutch. These can be very valuable tools, but we need to be wise about how we're deploying antifungal drug concentrations. And just measuring a concentration is not going to make a patient's clinical picture change. It may change how we decide to dose the drug. But if someone's truly failing treatment, we need to assess that outside of any drug concentration we have.
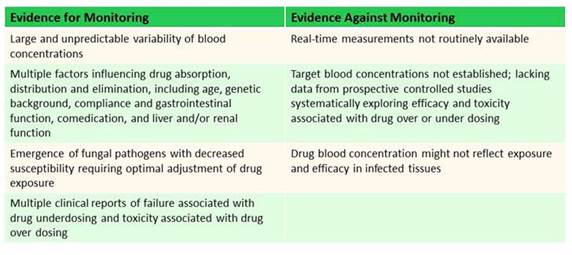
Section 2, Graphic 8 shows some of the pros and cons for antifungal drug concentration monitoring. These are several criteria to consider when we try to identify drugs for which we might want to look at serum concentrations. There's a lot of interpatient variability. They're unpredictable. They have reasons that they may or may not be absorbed well. They have lots of drug-drug interactions, and they also have toxicities that are associated with high concentrations in many cases, especially with the azole antifungals. But there's some reasons not to monitor them. A lot of in-house facilities aren't doing antifungal drug concentrations, and it can take a long time to get a result back. If this is something that you don't have set up for your facility, you want to consider that—will I have the results available in enough time to make a meaningful change for my patient? Regarding the target drug concentrations—I'll show you in a second—there are guidelines out there that talk about these. But, again, these are based on data from a very small number of patients with limited data on their clinical outcome. [Andes 2009] So we need to be careful about being too hard and firm on targeting specific concentrations. And lastly, because we measure serum concentrations and often we're treating invasive disease in the tissues, we don't know concentrations at the local site of infection.
Section 2, Graphic 8. Evidence for and against antifungal fungal concentrations. Reproduced from Andes D, Pascual A, Marchetti O. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother. 2009;53(1):24-34. ©American Society for Microbiology. With permission.
Here are the target drug concentrations that have been proposed in some recent expert recommendations (Section 2, Graphic 9). You can see here, there's a lot of variability. I want to go over some key points. One is that although we have newer azole antifungals that we monitor, we don't routinely monitor drug concentrations for fluconazole. All the other agents have fairly long half-lives. So it takes us a while to get to steady state where it is valuable to obtain serum drug concentrations. Ideally, we'd like to wait for a full 7 days. But you can measure – if you need to and you want a faster answer, right around 4 or 5 days is probably okay to get an early concentration. We monitor troughs with all of our azoles. You can see that we have different targets for prophylaxis, which honestly tends to be a target trough of 0.5 mcg/mL for all the agents. [Andes 2009] The isavuconazole targets haven't been as firmly set, but it looks like they're probably going to be closer to 0.7 mcg/mL for prophylaxis. [Astellas 2015]
Then there are higher target drug concentrations if you're treating an invasive disease. And these tend to be less firm. You can see with posaconazole, the therapy trough is recommended to be anywhere from greater than 0.5 up to 1.5 mcg/mL. I'll tell you that's what we're seeing now that we've switched formulations. We used to struggle with the oral suspension of posaconazole to get anyone above 0.5 mcg/mL. It really was a struggle, and we found ourselves kind of pulling our hair out to optimize that exposure. And now that we've switched to the delayed release tablets, we are having trouble keeping posaconazole concentrations from going outside the top of that range. It has such good bioavailability. But we aren't seeing toxicity in those patients, so we don't always know what to do.
I think that what comes out of this is that we often get these concentrations back, but then no one knows what to do with them. So they've obtained a serum drug concentration, and then they call—we get calls all the time in our offices and say, "I have this concentration. Now, what do I do?" And the immediate question back is how is the patient? We don't treat just a drug concentration. But we really want to make ourselves feel good about that number. But we know that a lot of times, the concentrations we're seeing aren't within those ranges.
Section 2, Graphic 9. Therapeutic drug monitoring targets for varied antifungal agents. Adapted from Andes 2009.
Data from the Fungus Testing Laboratory evaluated the range of samples they received between January 1, 2001 through April, 2013 for serum drug concentrations. Almost 40% to 50% fall below the minimum trough concentration recommendation of 0.5 mcg/mL for prophylaxis. [Wiederhold 2014] And we don't know the indications that these patients were on the drug for, but we know we have low concentrations in a lot of patients; what we don't necessarily know is what to do with it.
Algorithms have been proposed. Here's one that's been published for voriconazole. And what I like about this one is it does clearly factor in how the patient is doing.
- For a concentration that's less than 1 mcg/mL and it's treatment, and there's lack of response—it's worth increasing the dose by 50%. I always have to say remember with voriconazole, it is not linear pharmacokinetics. So we need to be careful by how much we increase the dose. But it's not just the low concentration that drives us to change. It's the low concentration combined with the lack of clinical response
- Similarly, if it's within the range of 1 to 5.5 mcg/mL, no change recommended
- If the level is >5.5 mcg/mL, we do tend to see more toxicity. But again, if you measure concentration and the patient is responding and it comes back at 6.4 mcg/mL, and the patient's not showing signs of toxicity, we don't have to stop the drug [Pascual 2008]
We stop the drug or make drastic changes in the dosing if the patient is having signs and symptoms of toxicity. For example, with voriconazole, we look for liver function test increases, rashes, visual changes, and neurologic disorders if the levels come back high. We don't stop the drug or make dosage adjustments just because of the number. We need to be very cautious about that. Often, we'll hear patients were switched quickly from 1 dosing regimen to the other without showing any signs of lack of clinical efficacy. They may be responding quite well to their treatment, in fact. No need to increase the dose if they're responding well. Always put the patient response first.
Drug-Drug Interactions/Safety
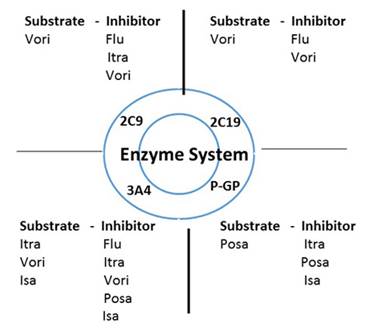
I want to also talk about another key consideration with all of the antifungal drugs. That is drug-drug interactions, which is a particularly important consideration with the azoles. Pretty much all of the azoles are inhibitors of cytochrome P450 3A4; it drives the drug interactions we see with all the immunosuppressant agents, agents used post transplant (Section 2, Graphic 10). But also important to talk about is interactions through P-glycoprotein. This not only can affect drug metabolism of other agents, but also drug absorption involving this pathway. And as a result, drugs that are a substrate, such as posaconazole, could have altered absorption if we use inhibitors or inducers of P-glycoprotein. And the other drugs that affect this tend to be very closely related to those that interact through cytochrome P450 3A4. So rifampin is an inducer, and other common agents are inhibitors. [Brüggemann 2009]
One of the questions that still remains is related to the newest agent we talked about, isavuconazole, which seems to maybe have a less strong inhibition through cytochrome P450 3A4. But I don't know that we have enough clinical data yet to really make firm recommendations. We spend a lot of time debating this based on the early clinical data. It seems to have less of an effect. [Maertens 2015] But we know that with voriconazole and with posaconazole, we learned a lot about drug-drug interactions in the first 3 years that those drugs were on the market that we didn't know at the time they were approved.
Section 2, Graphic 10. Drug interactions by enzyme system. Adapted from Brüggemann 2009.
So my advice is to have caution. Be aware of what's happening. And have a good monitoring plan to be sure that we're appropriately adjusting other drugs and monitoring for toxicity for the azoles that are substrates of the cytochrome enzymes.
We looked back—this is somewhat old, but we looked at Duke at our patients who are on voriconazole. We found that 80% of patients who were receiving voriconazole back in 2002 were getting some drug that was interacting. Some of them were even listed as contraindications. [Macdougall 2003]
So pretty much any time you're using an azole, drug interactions need to be a concern. You're not going to be able to completely avoid interacting drugs, so you need to have a good plan for how you are going to handle the drug interactions and monitor for them. Some clinicians adjust the dosage of the interacting agent whenever they start a new azole. For example, the dose of tacrolimus can be halved when starting a new azole (personal communication, Alison Freifeld, MD).
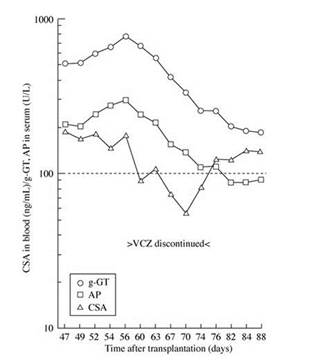
Equally as important to thinking about drug-drug interactions when you start an azole antifungal is to think about drug-drug interactions when you stop an azole antifungal. Section 2, Graphic 11 illustrates a problem with cyclosporine. When voriconazole was discontinued, although they had appropriately dose-reduced the cyclosporine when initiating the voriconazole, but they forgot to increase the dose when they stopped the drug. This patient actually went on to have some rejection as a result. So do not forget about drug interactions when stopping azole antifungals as well. This is a very important consideration. [Groll 2004]
Section 2, Graphic 11. Time course of cyclosporine A (CSA) trough levels, gamma-glutamyltransferase (g-GT) and alkaline phosphatase (AP) values prior, during, and following discontinuation of voriconazole (stable CSA dosing). Reproduced from Groll AH, Kolve H, Ehlert K, Paulussern M, Vormoor H. Pharmacokinetic interaction between voriconazole and ciclosporin A following allogeneic bone marrow transplantation. J Antimicrob Chemother.2014;53(1):113-114. ©British Society for Antimicrobial Chemotherapy. With permission.
Toxicities
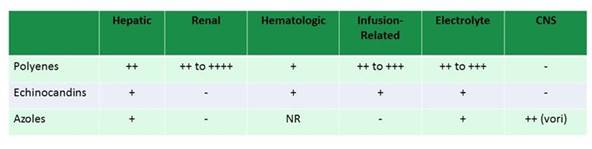
Lastly I want to talk about toxicities. When we look at all the big studies comparing caspofungin to amphotericin B, or voriconazole to amphotericin B followed by fluconazole in Candida infections, adverse events were common causes of the differences between the agents and common causes of clinical failure in the trials. So adverse drug events are something that we need to be cognizant of. We need to be sure that we have a plan to monitor for them and to help our patients through. As shown in Section 2, Graphic 12, all of the azoles and all of the antifungal drugs across the board do have side effects that we need to be careful of. I think amphotericin B is well known for its renal toxicity. The old principles remain true: we can still use hydration to help. Using the lipid formulations does help. I'll talk briefly about the echinocandins in a minute. [Dodds Ashley 2006]
Section 2, Graphic 12. Adverse event profiles of different antifungal therapeutic classes.
The echinocandins all have reported hepatic toxicity. It's not as much of a concern as when the drug was first released, but it does occur. And I think something that I still get a call about, about once every 3 months, is this histamine-mediated reaction that appears just like red-man syndrome to vancomycin. It's disturbing when you see it, but just like the vancomycin, we need to know that we can pause the infusion and slow down the rate and patients will not react with subsequent doses. [Hindahl 2012] So, it's something to be aware of because it still surprises people as it does happen relatively rarely, but if you do get that call, know it's still safe to go ahead and continue administering the echinocandin.
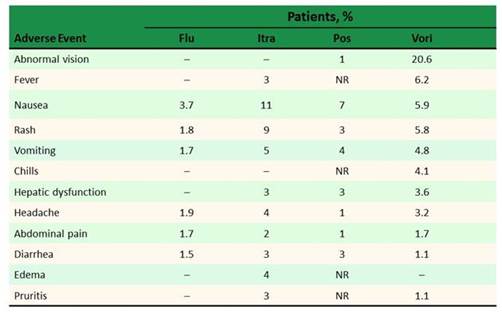
Toxicities with the azoles are variable, as shown in Graphic 13. No 2 azoles are alike in their side effect profile. And you can see that drilled down a little bit more here. We know that voriconazole, in particular, has some unique side effects—one being abnormal vision that can occur as floating lights and true hallucinations in some patients, that are seen in about 30% of our patients when we look at it across the board. Voriconazole also has this phototoxicity reaction that leads to skin abnormalities that we need to be careful of. And that does tend to be seen isolated with voriconazole, which seems to have the worst side effects. Posaconazole has fewer adverse events, but does have hepatic dysfunction, just like any azole antifungal we see. According to Dr Alison Freifeld, azoles are often considered the culprit when there are liver function test elevations, sometimes leading to a class switch. But severe hepatotoxicity is rare. And in the most recent data that came out comparing voriconazole to isavuconazole—treatment emergent adverse events, including skin reactions, ocular disorders, and hepatobiliary disorders were all more common in the voriconazole arm. So, really be careful with voriconazole. Of note, those are all concentration-related side effects when we see them. [Noxafil PI 2008; Vfend PI; Diflucan PI; Sporonox PI]
Section 2, Graphic 13. Adverse event profiles of various azoles. Note that for posaconazole, older data with the oral suspension have been used. Modified from the Noxafil, Vfend, Diflucan, and Sporonox prescribing information.
Summary
I'd like to make a couple points in closing. We have limited agents and we need to be smart about using them. There are a lot of pharmacokinetic and pharmacodynamic considerations. On top of that we need to be good stewards of these antifungal drugs. This includes having a good antifungal formulary that meets the needs of the patients cared for at your facility. You need to be working with your microbiology laboratory to know the infections that you're seeing and not just be making a blind guess about it. You need to have susceptibility testing to guide those decisions. You need to work on good treatment guidelines and protocols. This is one place where we can really do great stewardship by having those in place and working with our colleagues in oncology and in other areas where we commonly see fungal infections to be sure we have best practices in place.
Then it is important to work with frontline providers to provide data and really help drive appropriate antifungal use, because I think that this is going to be so important for us as we go forward, to be sure that we really are being wise about where we're using these drugs and being just as thoughtful about antifungal stewardship as we are about antibacterial stewardship today.
CHANGING PARADIGMS IN IFD MANAGEMENT
THOMAS PATTERSON, MD
Thanks for the invitation. So my challenge is to put a clinical spin on the terrific data and presentations that you've just heard. In other words, how do we put this together in our own practice? Realize now, as clinicians who deal with patients with invasive fungal disease, there are a number of changing paradigms in management that we are now encountering. But I also want to do this in the setting of talking about the evidence gaps, especially for the current recommendations that have recently been published. To do this, I'm going to focus on Candida and how we deal with those resistant strains that you heard about earlier in the presentations. For Aspergillus, how do we manage, not only with the emergence of resistant strains, but also how do we utilize combination therapies and manage salvage and breakthrough? And then finally for Mucor, how the new agents fit into our armamentarium?
Current Challenges in the Management of Invasive Candidiasis
So first with Candida spp, the recommendations have been clear to say that the echinocandins have been very useful therapies for improving outcomes with patients with candidemia and invasive Candida infections. [Andes 2012; Pappas 2016] We know that some measures, like removing catheters and early diagnosis, will also improve the outcome. We also recognize that higher mortality occurs with some species, such as C tropicalis. [Andes 2012] But we also know that mortality decreases when echinocandins are chosen as upfront therapy. The reason for the decreased in mortality are many. But it's really led to the recommendation that the echinocandins be used as primary therapy in most patients in the recent IDSA guidelines that Dr Pappas chaired. [Pappas 2016]
On the other hand, it's a complicated world. Section 3, Graphic 1 shows a case timeline for a patient that we treated in San Antonio a few years ago. [Lewis 2013] This was a patient who didn't have an extensive prior medical history. She was diabetic, but hadn't been treated with prior antifungal therapies. She was admitted to hospital with a urinary tract infection. After admission, she had a complicated course and developed renal failure, and she had multiple intravenous lines and a central catheter in place. She developed a Candida glabrata bloodstream infection a couple of weeks into her stay.
She was begun on fluconazole therapy prior to the blood culture becoming positive. Luckily, it wasn't the dose 150 mg/day that Dr Dodds Ashley discussed, but it was a lower dose (200 mg/day) that was chosen by her primary team due to her renal failure. Blood cultures had been drawn, but they were not yet positive and we didn't have a rapid diagnostic test. By the time that the cultures were positive 2 days later, the patient was—by the guidelines—changed to an echinocandin, in her case micafungin. The cultures were subsequently identified the next day as Candida glabrata.
She was treated with micafungin, as that was the echinocandin we used – but it could have been obviously any of the other echinocandins, and she was continued on micafungin therapy. However, her blood cultures continued to be positive. Subsequently we did do susceptibility testing on her blood culture isolates. As you can see from this case report, we found an unusual occurrence in her culture results: the baseline C. glabrata isolate–shown on the left hand side of the column--was a very susceptible strain with a low MICs early on, but less than 2 weeks into her therapy, the isolate was found to have an MIC of 0.5 mcg/mL.
The rapid development of resistance on therapy is fortunately an unusual occurrence. However, what isn't unusual is that the isolate with echinocandin resistance not only resulted in failure of therapy but was also reported as having decreased susceptibility to fluconazole, which, in this case, resulted in a multi-drug resistant strain. Because of the high MIC, we sequenced the FKS2 gene and found a mutation that would predict the potential for clinical failure with an echinocandin. In fact, what happened in this patient, is she didn't respond to echinocandin therapy.
An extensive search for a source was undertaken, but none was identified. She was treated with higher doses of fluconazole, but eventually she had to be treated with liposomal amphotericin B. That is something that, in the past, we would have rarely had to undertake in the management of invasive candidiasis.
Section 3, Graphic 1. Case presentation in the therapy of candidemia. From Lewis JS 2nd, Wiederhold NP, Wickes BL, Patterson TF, Jorgensen JH. Rapid emergence of echinocandin resistance in Candida glabrata resulting in clinical and microbiologic failure. Antimicrob Agents Chemother. 2013;57:4559-4561. ©American Society for Microbiology. With permission.

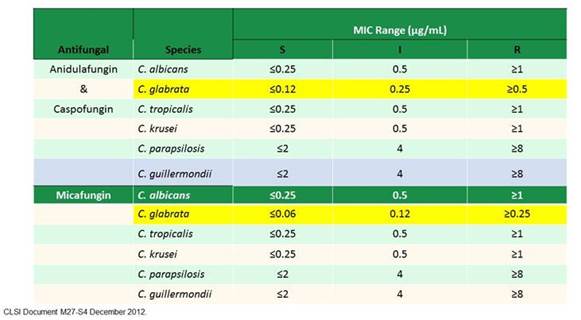
It is important, though, to review the revised breakpoints to emphasize the points that Maiken has already made. You see that now the breakpoints for susceptibility are specific for the individual species (Section 3, Graphic 2) and are individualized for specific drugs, which you'd obviously think would be appropriate. You can see that for Candida glabrataisolates with MICs of 0.25 mcg/mL or greater to micafungin, the isolates are reported as 'resistant,' which correlates with the presence of wild-type mutations that are likely to be present, as was the case in the resistant isolate from our patient.
Section 3, Graphic 2. Revised CLSI breakpoints for Candidaspp. From CLSI Document M27-S3, December, 2012.
Recently, several nice publications have highlighted the importance of these issues. Early papers that showed this included a study done by Pham and colleagues in Shawn Lockhart's group at the CDC. [Pham 2014] In that study in the United States, they found echinocandin resistance rates of about 3%. Some hospitals will definitely have higher rates of resistance. But, importantly, multi-drug resistance occurs with fluconazole resistance detected in about a third of those isolates that are echinocandin resistant. So that's really important for clinical management.
The 2016 IDSA invasive candidiasis guidelines recommend use of liposomal formulations for resistant isolates. However, several area of limited evidence remain: Do you choose liposomal amphotericin therapy initially in some patients? That's not really known and this is a very data-limited area.
The guidelines also recommend that fluconazole susceptibility testing be undertaken for virtually all bloodstream isolates. The guidelines also suggest that echinocandin susceptibility testing be undertaken, certainly in high-risk patients, probably for Candida glabrata and Candida parapsilosis isolates from sterile sites (at least), and for patients with prior echinocandin therapy. Clearly the evidence for the benefit isn't really known. The potential harm from this approach is added cost. But another potentially significant harm is not detecting isolates with echinocandin or even multi-drug resistance. Because of those risks the current IDSA guidelines make that a strong recommendation, but because there is limited evidence to support its use, it's not really very strongly data-driven (ie, low quality evidence). [Pappas 2016]
Aspergillus Susceptibility Testing (Question/Response from Live Meeting)
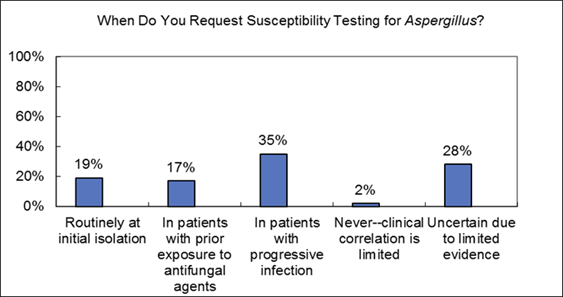
Commentary on live audience response: I think progressive infection is certainly an indication for susceptibility and I think a really important scenario to consider it. I think for areas of the world with higher rates of resistance susceptibility more routinely is indicated and in your own hospitals, especially for epidemiologic purposes, you may need to do it more.
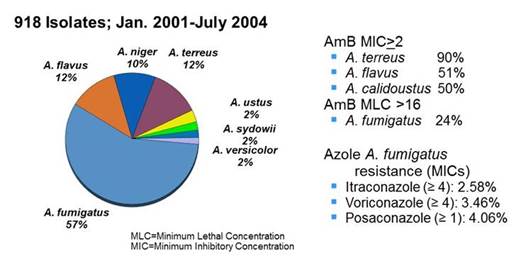
Here, in San Antonio we do have the Fungus Testing Laboratory, which has been mentioned. Section 3, Graphic 3 shows a couple of important findings from their experience as a reference lab: One is that Aspergillus fumigatus makes up the majority of the isolates– but like with Candida,where Candida albicans has become less frequent, other non-fumigatusisolates are increasingly reported—perhaps due to prior therapies and more immunosuppressed patients. As you can see, elevated MICs are not unusual for polyenes, but not only for species which we think of routinely for intrinsically resistant species like Aspergillus terreusand Aspergillus calidoustus, but also others with decreases susceptibility like Aspergillus terreus. But you don't see as much fungicidal activity as we would think that you might against even some of the Aspergillus fumigatus strains. Importantly in the Fungus Testing Lab, there are elevated MICs detected to itraconazole, voriconazole, and posaconazole—with very limited data to date with isavuconazole. The resistance rates for these azoles range from around 3 to 4%. However, these numbers probably look a little higher on this slide because of the breakpoints that are shown. [Sutton 2004; Verweij 2007; Wiederhold 2016]
Section 3, Graphic 3. Aspergillus spp. analyzed in the San Antonio Fungus Testing Laboratory. Adapted from Sutton 2004; Verweij 2007; Weiderhold 2016.
It is really important though, as you've heard earlier in the talks, to recognize that resistance has become an important problem. Initially some of the data showed the emergence of some resistant strains with prior long-term azole therapies, which was especially important among patients in the UK that were chronically treated with azoles, (Section 3, Graphic 4). [Bueid 2010]
Section 3, Graphic 4. Azole resistance frequency in A fumigatus by patient cases, 1997-2009. Overall azole resistance per each year is shown next to each row as a percentage. Adapted from Bueid 2010.

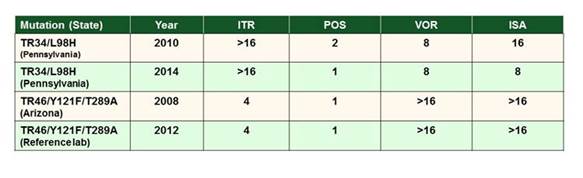
However, and even more significantly in my opinion, have been the reports of the emergence of resistant isolates that have not been associated with prior azole therapy. This isolates have been to have mutations in the target cyp51A gene in combination with a base-pair tandem repeat in the gene promoter (TR34/L98H & TR 46/Y121F/T289A). Specific hot-spot mutations in the target gene include G54, L98, G138, M220, and G440. [Verweij 2007] Other resistance mechanisms outside this target site have been identified, including overexpression of CYP51B [Buied 2013] and changes in the cdr1B efflux transporter. [Fraczek 2013] Moreover, there are now pan-azole-resistant isolates described, in both cavitary disease but also in azole-naïve patients. [van Ingen 2015; Verweij 2015] Resistance has clearly been associated with very high mortality in these patients, making it a really significant clinical problem, especially in western Europe.
Now, these isolates have been reported from all over the world. Recently they've been reported in the United States and interestingly, in Colombia. You know, Maiken talked about the tulip fields in Holland and it's possible association with agricultural fungicides.
In San Antonio, we looked at azole resistance in the United States, in data from the Fungus Testing Lab. Dr. Nathan Wiederhold and colleagues recently screened A fumigatus isolates with elevated azole MICs in from their lab. As shown in Section 3, Graphic 5, they actually found 4 strains that had the tandem repeat mutation. This was the first time it had been reported in the United States. Curiously, some of those pre-dated the first isolates for those specific mutations described in Europe. So clearly, it's been around in the United States for a long time. [Wiederhold 2016] There's a report here at this meeting from MD Anderson Cancer Center, where isolates were screened using an assay that was also described earlier in this symposium (the 4-well screen). These results showed a significant number of pan-resistant isolates at MD Anderson Cancer Center. Clearly, there is a very high-risk population there, and this finding could have significant clinical implications. [Tatara 2016]
Section 3, Graphic 5. Mechanisms of azole resistance in Aspergillus fumigatus in the United States. Adapted from Wiederhold 2016.
So what are the recommendations and guidelines for susceptibility testing? There's one paper from an expert panel led by Paul Verweij that provides guidance, not really a guideline, as shown in Section 3, Graphic 6. They basically encourage the microbiologic diagnosis, obviously to identify the organisms to the species complex level to make sure you know what you're really dealing with. Then in areas with high rates of resistance, you consider susceptibility testing. And as well, also previously mentioned, you might even consider multiple isolates. It's reasonable advice but certainly not much data to support those choices. [Verweij 2015] The recent IDSA Aspergillus guidelines also recommend species identification and, in the case of isolates with atypical growth or when there is concern about resistance, that susceptibility testing be performed. [Patterson 2016]
Section 3, Graphic 6. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Adapted from Verweij 2015.

The gaps in recommendations for Aspergillus susceptibility testing are pretty clear, but limited availability of testing in many of your medical centers is a big factor. Often local testing is just not timely in its return, and so you end up using reference labs, which, hopefully, are timely, but it's obviously not as convenient as really being local. And again, expert opinion has driven this scenario. But just like with Candida, obviously there's a number of potential harms with this misdetection of resistance.
The most common answer in the audience response question was that you would choose testing in progressive infection. But it would really be interesting, as well, to see how many of you routinely do susceptibility testing for your Aspergillus species.
One of the key points from the new invasive aspergillosis guidelines is an emphasis on establishing a firm diagnosis. The recent IDSA guidelines still recommend that tissues and fluids be submitted for both simultaneous histopathology/cytological and culture examination. Serum and BAL galactomannan is recommended for diagnosis of IA in adult and pediatric patients, particularly in hematologic malignancy/HSCT populations. Serum β-D-glucan can be used for diagnosis as well in these high-risk populations but are not specific for Aspergillus. PCR is not recommended for routine use but that recommendation is based at least in part on the lack of FDA-cleared assays that can be used locally in the US. If PCR testing is utilized, the panel recommended that those results be interpreted in the context of the entire clinical and diagnostic evaluation. [Patterson 2016]
Therapeutic Management
The IDSA guidelines for invasive aspergillosis were just recently published. As shown in Section 3, Graphic 7, I've summarized some of the recommendations from the 2016 version. Note that voriconazole still has a central role and remains as recommended primary therapy in most patients, due to the extensive experience and a number of studies evaluating voriconazole in the context of various host risk groups. However, other agents, including liposomal amphotericin and isavuconazole, are recommended as alternative primary therapy in some patients, especially where voriconazole is not tolerated or may be contraindicated.
Section 3, Graphic 7. Therapeutic strategies for different types of Aspergillus infections per the 2016 IDSA aspergillosis guidelines. Adapted from Patterson 2016.
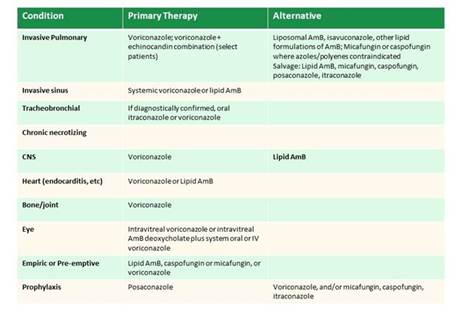
As mentioned, one change in these guidelines is the inclusion of isavuconazole for primary therapy for IPA. Section 3, Graphic 8 shows some of the pivotal trial data for this indication, showing comparable efficacy for isavuconazole with voriconazole (with the differences in adverse event rates previously discussed by Libby). [Maertens 2016] There's a lot more data since this initial trial—in Mucor and other rare molds that has subsequently been published. In some patients, isavuconazole may be an appropriate alternative, especially if the change is due to an adverse event to voriconazole.
Section 3, Graphic 8. Voriconazole vs isavuconazole: Treatment of invasive aspergillosis and other molds (ITT Population). Adapted from Maertens 2016.
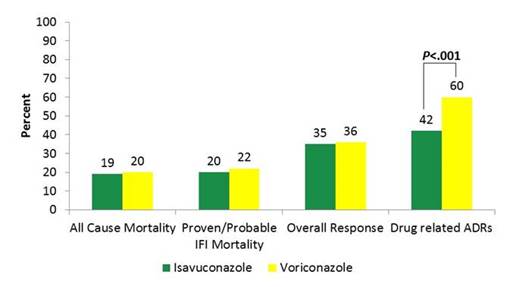
Role of Combination Therapy (Question/Response from Live Meeting)
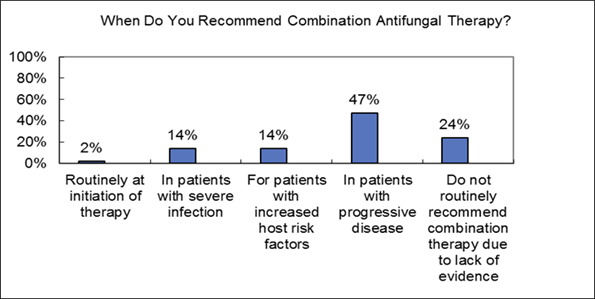
Commentary on live audience response: So again your answer is in patients with progressive disease for almost half of you. Interestingly, only a small proportion chose "routinely at initiation of therapy." Maybe I shouldn't have put in the routinely part, but even for initiation of therapy, it's a small percentage.
So let's look at the data from a large randomized trial that Dr. Kieren Marr and the Mycoses Study Group have published that has been discussed for a number of years, which looked at combination therapy with voriconazole alone compared to voriconazole plus anidulafungin – 2 agents that were chosen for the trial. Overall, as you also likely know, the primary endpoint of improved survival for statistical significance wasn't met, but was favorable to the combination therapy arm, with decreased mortality for the combination arm compared to voriconazole alone. However, the P value didn't meet that statistical mark that was set prior to the study (Section 3, Graphic 9, left panel). [Marr 2015]
On the other hand, the pre-determined sub-analysis did show significant differences, with combination therapy having a significant lower overall mortality vs. that in those who received voriconazole monotherapy. So who are these patients? These were the patients with what was felt by the investigators to be early disease (right panel)—those patients with diagnosis established by galactomannan rather than cultures. They weren't the patients with progressive disease or those with higher rates of predicted failure. So I think the evidence doesn't really support what our practice mostly ends up to be (ie, use of combination therapy in progressive patients and in higher risk patients). [Marr 2015]
So the randomized clinical trial failed to meet that pre-determined endpoint. But were there harms? Specifically, what were the potential harms of the combination therapy? And was the real harm just in not doing it? Cost is obviously one of the issues, and there could be side effects, which you've heard from Libby's talk earlier, as well in addition to the lack of potential efficacy with the combination therapy approach.
In the new guidelines we mentioned from the IDSA and in most other current guidelines, combination antifungal therapy, usually with voriconazole and an echinocandin, is recommended for consideration in some patients but not for routine use as initial therapy in all patients. [Patterson 2016] The use of combination therapy in patients with disseminated or progressive disease seems reasonable from a clinical perspective, but the evidence is pretty limited to support that approach.
Section 3, Graphic 9. Combination therapy for invasive aspergillosis. Left panel shows overall results, right panel shows subgroup analysis based on GM positivity. Adapted from Marr 2015.
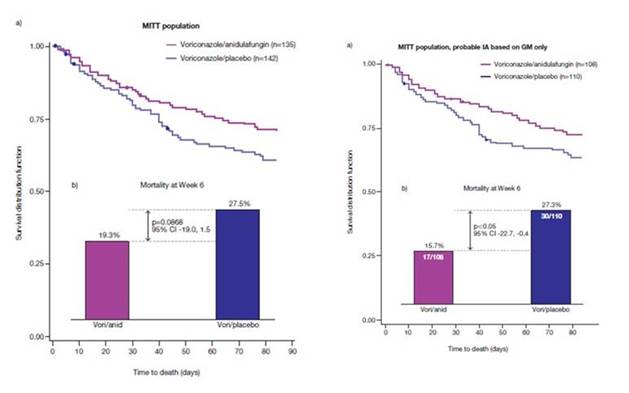
Management of Breakthrough Infections
So what about a breakthrough? Section 3, Graphic 10 shows an example of a breakthrough infection for a patient who has undergone an allogeneic stem cell transplant who is on voriconazole prophylaxis. Obviously again, you didn't get much data here. But the patient is now on empiric cefepime and has a BAL that's galactomannan positive at an OD of 0.5.
Section 3, Graphic 10. Case study of a patient with a breakthrough infection.

Management of Breakthrough Infections (Question/Response from Live Meeting)
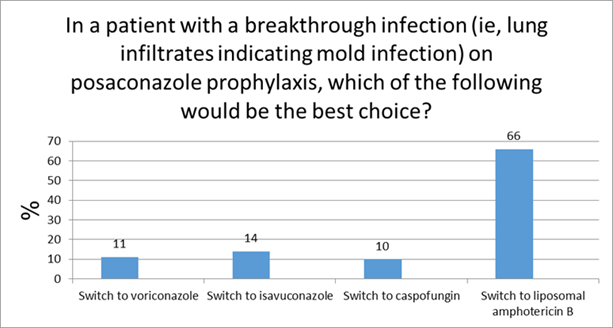
Commentary on live response: So most of you – about half again—are going to switch to liposomal amphotericin, but there were a lot of varied responses with a number of different options. A few would go with combinations, but kind of a mixed picture.
So let's move ahead then to think about how to really approach this. I think one of the really important things to think about is: what does the evidence show that the likely etiological agent of the breakthrough infection would really be? And there's a number of choices.
Professor Maschmeyer and I did an opinion piece a couple of years ago that looked at data on breakthroughs in this sort of setting. Here are some of the things we often think about when approaching such a patient in our practices: [Maschmeyer 2014]
- Second IFI due to pathogens resistant to the antifungal agent given
- Development of resistance in previously susceptible fungi
- Fungal growth in a compartment where insufficient antifungal drug concentrations are achieved (eg, CNS)
- Nonadherence of patients given oral antifungal agents
- Failure of the antifungal agent due to drug-drug interactions or bioavailability
So what were the organisms associated with breakthrough that we found in the literature? As shown in Section 3, Graphic 11, it's really mostly the usual suspects— Candida, Aspergillus spp. Obviously it would not be Candida in this sort of scenario. You do see some resistant organisms, and some are listed there. But it turns out resistance to the azoles is really uncommon in that setting. It can happen, but it's not really very common in those patients.
Section 3, Graphic 11. Etiology of breakthrough IFIs in patients receiving broad-spectrum azole prophylaxis. Adapted from Maschmeyer 2014.

Therapeutic drug monitoring (TMD) for breakthroughs, I think, is really important. Clearly you've seen the data earlier in Libby's presentation. We don't know so much about isavuconazole, and I think the role of TDM with the new formulations of posaconazole still remains unclear because of the significantly better levels that we're seeing. For instance, I can tell you that we are seeing higher posaconazole concentrations in our reference lab since the introduction of the newer formulations. For example, before December 2013, only 26.9% of the samples we received had concentrations ≥1 mcg/mL. [Wiederhold 2014] Since December 2013, 46.7% of the samples we received had concentrations ≥1 mcg/mL (personal communication, Nathan Wiederhold). Whether or not the really high levels are going to be untoward in some patients is not clear. While some preliminary research suggested that there might be the potential for cardiac and liver abnormalities associated with high levels of posaconazole, that has not been supported by more recent data. [Pettit 2015] And I think most people's experiences with higher levels of posaconazole really backs that up; the relationship between high levels and adverse events aren't so clear. While I agree with Libby's general take on therapeutic drug monitoring, at our institution we use slightly different target trough concentration ranges. [Chau 2014; Laverdiere 2014; Jung 2014] There's just no strong evidence to be too dogmatic about exactly what levels should be or exactly what time these levels ought to be drawn.
The management of breakthroughs is also a little controversial and not very data driven. But Georg Maschmeyer and I did come up with a suggested algorithm (Section 3, Graphic 12). Obviously you can imagine if you've been on posaconazole and you have something that looks like a yeast breakthrough, then you'd probably take a different path than if you had lung infiltrates, where you're probably going to switch to liposomal therapy. Same thing for breakthrough infections on voriconazole, where liposomal therapy is going to be much more attractive. [Maschmeyer 2014]
Section 3, Graphic 12. A strategy for managing breakthrough infections in patients who have received posaconazole or voriconazole prophylaxis. Adapted from Maschmeyer 2014.
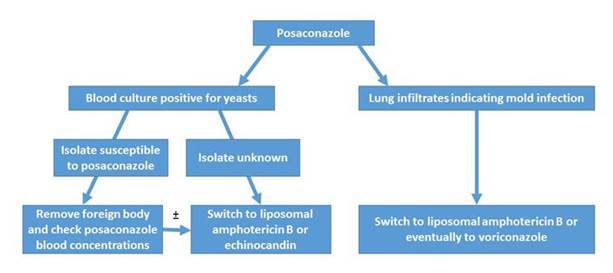
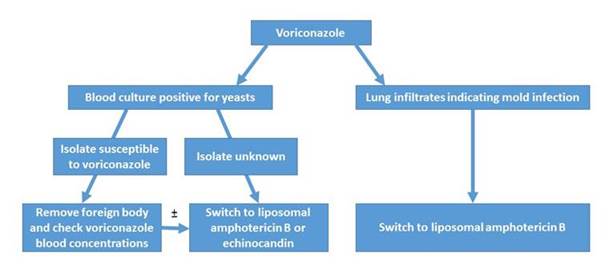
But in fact, most of this is actually practical 'guidance' versus evidence-based 'guideline' recommendations. These would be very weak strength of recommendations and the quality of evidence is low, as well. So that's something I think to keep in mind when reading these approaches. Obviously, for breakthroughs, you have to individualize these approaches and consider a number of different issues with adding other agents. These include the severity of illness, the extent of disease, as well as the possibility of a new pathogen. Some would say that a change of class of drug is almost always indicated. But again, the data are a little conflicting in those settings, where a change within the same class can be useful. Another issue is to consider is tapering the immunosuppression, if appropriate.
Management of Mucormycosis
So let me talk briefly about Mucor. Although it remains an uncommon infection in most centers, when it does occur, it's usually associated with high morbidly and mortality. We can see it emerging in a number of settings, including patients who have received prior therapy such as voriconazole or the echinocandins.
But we also see it in settings like trauma – burns, but also following natural disasters, tornadoes, and such, where we see these horrible infections. [Andresen 2005] For example, Section 3, Graphic 13 shows lesions associated with mucormycosis in a trauma victim but has also been associated with injury following tsunamis. [Andresen 2005]
Section 3, Graphic 13. Top panel. Multifocal cutaneous mucormycosis complicating a traumatic wound infection. Bottom panel. Apophysomyces elegans, cotton blue stain. This pathogen can cause these types of infections. Images courtesy of Thomas Patterson, MD.
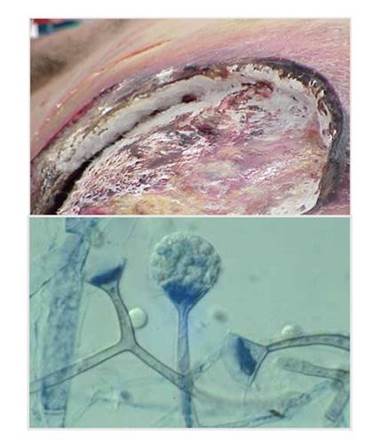
In terms of therapeutic options, the data with posaconazole have been out there for a while, including a small study from Greenberg and colleagues that found 79% survival in the 24 patients studied. [Greenberg 2006] Posaconazole is not approved in the US for primary therapy. Lipid amphotericin remains a drug that's used frequently in this setting. Isavuconazole has been recently approved for this indication. [Cresemba PI] With isavuconazole, the data in invasive mucormycosis are shown in Section 3, Graphic 14. It's a very small data set, but it's basically one that shows that with both primary therapy and salvage therapy, mortality rates are favorable compared with the other agents in historical registries—certainly liposomal amphotericin and standard amphotericin B. [Astellas 2015; Marty 2016]
Section 3, Graphic 14. Isavuconazole for mucormycosis. Data adapted from the Astellas isavuconazonium FDA briefing document 2015.
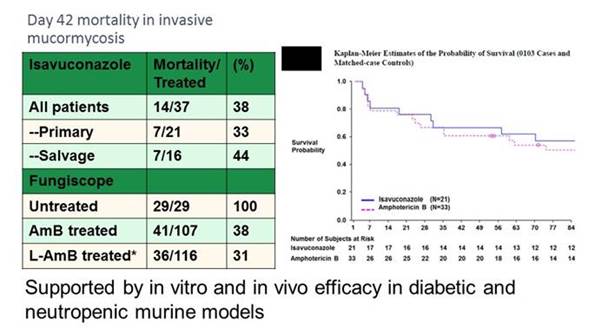
The critical thing about Mucor is to make sure you give therapy early. Data from MD Anderson shows that if you delay therapy from time of diagnosis, you're really likely to have an unfavorable outcome. For patients with hematologic malignancy and mucormycosis who had therapy initiated within 6 days of diagnosis, the mortality rate at 12 weeks was 49%. The rate was nearly doubled (83%, P=.008) for those who had therapy initiated at more than 6 days from diagnosis. [Chamilos 2008]
Combination therapy seems attractive for Mucor. Let's go back to the MD Anderson data from Dimitrios Kontoyiannis; they have a huge experience with this disease, so they were most likely to be able to notice trends in real-world use. In fact, combination therapy didn't produce better outcomes—at least in their center—when given to those patients with hematologic malignancies. [Kyvernitakis 2015]
Well, what would really be the problem in giving combinations for something that has such highly lethality? And this is an example, in the next few slides, of caution that I think is deserved in some of these cases. So the iron chelator story with mucormycosis is interesting, since strong data in preclinical models showed that deferasirox, an iron chelator that didn't allow the organism to take up the iron in model systems combined with lipid amphotericin, produced significantly better outcomes. [Ibrahim 2006; Ibrahim 2007] A few case reports showed the same thing, as did a small trial. [Reed 2006]
On the other hand, when it was taken into a clinical study, this randomized trial, which is complicated, basically showed very poor results, especially in those patients with hematologic malignancies (Section 3, Graphic 15). In fact, the deferasirox-treated patients had poorer outcomes than the placebo-treated patients. So, the trial had to be stopped, and it really wasn't recommended to continue. [Spellberg 2012a]
Section 3, Graphic 15. Time to death of patients randomized to deferasirox vs placebo (P=.02). From aSpellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67(3):715-722. ©British Society for Antimicrobial Chemotherapy. With permission.
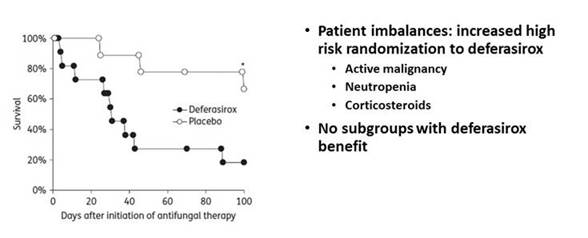
So now there are a number of potential candidates for combination therapy in invasive mucormycosis (Section 3, Graphic 16). The echinocandins might be used as an immunomodulatory agent, especially in the diabetic population. So we don't recommend deferasirox now because of the increased mortality. But the underlying thought is maybe the issue with the Spellberg study was that it was used predominantly in hematologic patients, where it didn't really help. Could there be an advantage in other patient types, such as those with diabetes? [Spellberg 2012b]
Section 3, Graphic 16. Drug candidates for combination therapy for mucormycosis. Reprinted with permission from bSpellberg B, Ibrahim A, Roillides E, et al. Combination therapy for mucormycosis: why, what, and how? Clin Infect Dis.2012;54(Suppl 1):S73-S78. ©Infectious Diseases Society of America. With permission.
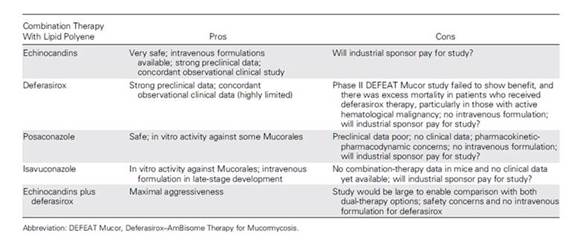
So the challenge in trying to adopt evidence-based therapy for Mucor is that we don't yet have strong comparative data. We also have a severely immunosuppressed population that varies in the severity of illness and extent of disease. There are limited randomized trial data, as I mentioned, only a little bit of data for posaconazole and isavuconazole. In the 2013 joint ESCMID/ECMM recommendations for management of mucormycosis, Cornely and colleagues recommended lipid or liposomal amphotericin B for primary therapy and posaconazole for salvage; they did not support deferasirox in hematologic malignancy patients (outside of trials and noted marginal support in diabetes mellitus. [Cornely 2014] There are a number of new options (including isavuconazole), but we still don't know the best options overall.
Summary
In summary, there are evidence gaps in IFD recommendations. We have "expert opinion" evidence regarding susceptibility testing in Aspergillus spp. as well as the management of drug-resistant Candidaspp. But even in the areas where we have randomized trials, there's still some limited evidence, like in combination therapies (ie, early disease vs progressive/severe infection) and newer therapies (such as azole therapy or multidrug combinations for Mucorales). We also need individualized management strategies, particularly when approaching breakthrough infections. I believe additional evidence is needed for guideline recommendations, but clinical judgments and interpretations will still be required. Thank you.
INTERDISCIPLINARY CASE DISCUSSION
Case 1: 60-year-old woman with uterine cancer
- This patient is admitted with fever and hypotension
- She has a chronic indwelling Foley catheter and has received multiple courses of systemic antibacterial therapy for recurrent urinary tract infections
- Blood and urine cultures on admission are positive for yeast, subsequently identified as Candida glabrata, with a fluconazole MIC of 16 mcg/mL, "susceptible dose-dependent." The micafungin MIC is 0.5 mcg/mL
- Fluconazole 400 mg daily was begun on admission. But on Day 3, the patient has not improved clinically
- Repeat blood cultures and T2Candida® Panel remain positive for yeast
- The CT scan of abdomen and pelvis reveals left-sided mild hydronephrosis with stranding, but otherwise normal
CASE 1 (Question/Response from Live Meeting)
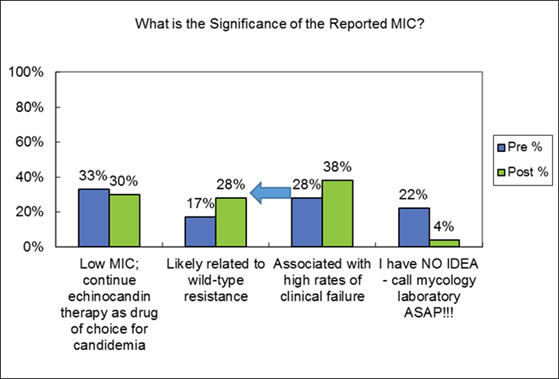
Commentary on Live Audience Response: Let's see your answers. It looks like we're kind of evenly divided across the answers, except only 4% indicating "I have no idea." Tom, how would you answer this question?
THOMAS PATTERSON, MD:
OK, so a little bit of a trick question, I would say. Because it is a low MIC compared to the prior breakpoint that was previously 2, but now the breakpoint is lower. And 0.5 is clearly associated with wild-type resistance.
Answer 3 (associated with high rates of clinical failure) isn't true, though; it's probably not really associated with high rates of clinical failure. But it's associated with some clinical failure. And so because of that, I think you wouldn't choose that as the answer.
PETER PAPPAS, MD:
I'll address this since I'm supposed to know something about the treatment of this disorder. But I will be the first to admit, this is a data-free zone. What do you do in individuals who have higher MICs, that are right at the threshold, right at the breakpoint? And since we don't know how these individuals really respond, we don't have a mountain of data on these patients.
If we're going to abide by these breakpoints, the logical thing is to say that this is really a micafungin-resistant organism, certainly right at the threshold. And amphotericin B lipid formulation is probably the right answer—until we gather more data. But the data are just not out there. Nobody's reporting large numbers of these patients and how they respond to treatment. Do any of you have any other comments on this?
MAIKEN ARENDRUP, MD:
All I have to comment is that basically this patient failed therapy. So you have culture positive, so how much more evidence would you like in this case?
THOMAS PATTERSON, MD:
Right, she failed fluconazole. So I don't think going up on the dose is really the right answer. The question really is whether or not most of us would go to micafungin if we didn't have that data. But now we have data that this individual has a resistant organism, so you skip over the micafungin and go to amphotericin B.
MAIKEN ARENDRUP, MD:
I would definitely do so. It was also molecularly confirmed.
PETER PAPPAS, MD:
I think this is probably the correct answer.
CASE 2: 45-year-old man with allogeneic stem cell transplant
Here's a 45-year-old man. He's +190 days for acute myelogenous leukemia (AML) on his long-term secondary voriconazole prophylaxis following presumed invasive pulmonary aspergillosis (IPA).
The patient is on empiric vancomycin and cefepime for sustained fever in the recent development of progressive pulmonary infiltrates, and a bronchoalveolar lavage (BAL) galactomannan that's positive with an optical density of 0.6. The corresponding serum galactomannan (GM) is 0.5. All cultures and cytology are negative. The patient is non-neutropenic and receives prednisone and cyclosporine for graft-vs-host disease (GvHD) of the gut and skin.
CASE 2 (Question/Response from Live Meeting)
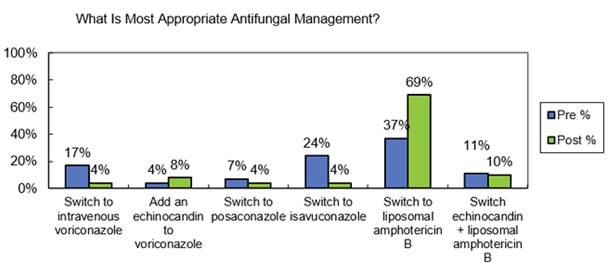
Commentary on Live Audience response. Okay, let's see where you come out here, see what most of you would do. Two thirds of you are going to switch to liposomal amphotericin B. This person has broken through voriconazole presumably.
They have evidence now of probable invasive aspergillosis based on those numbers. I mean, if you adhere to the definition strictly, they've got positive serology, they have an infiltrate. They're an at-risk patient, they've had it before and seem to have broken through voriconazole. The group would go with amphotericin B.
I think the temptation would be to just simply add another agent, such as an echinocandin. But in this group, two thirds have gone to amphotericin B. Tom?
THOMAS PATTERSON, MD:
I might add that in the Aspergillus guidelines group, there was a lot of discussion over this. And some people felt that confirming diagnosis is really key. And I think that's really important to emphasize. But some would argue that you could also switch, maybe to posaconazole or isavuconazole in that setting. And as we move forward, those may be reasonable choices.
PETER PAPPAS, MD:
Right; again we have no new data on which to base this. This is where you get to the point that Tom is making about this: expert opinion and just experience with this disorder really is going to be what drives many of our thoughts. Maiken, what do think about the certainty of that diagnosis? Are you okay with that?
MAIKEN ARENDRUP, MD:
No, not at all. A BAL of 0.6 is really low. In the Maerten's study, 0.5 and below could rule out aspergillosis. But 3 and above could rule aspergillosis in. So we are really in the low end, it could just as well be contamination.
The same is more or less true for the serum value. Both of course, given the fact that the patient is on voriconazole, I would really like to know if that patient is perhaps failing with or without a therapeutic level.
PETER PAPPAS, MD:
Here's where our TDM has to come in and we have not provided you with that. Let's go to the last question.
CASE 3: 32-year-old woman with type 1 diabetes
This is the last case, a 32-year-old woman with long-standing type 1 diabetes is admitted for progressive right facial numbness and sinus pressure for 3 weeks. Conventional antibiotics for sinusitis have not helped. She denies fever or pain. She has a long history of poor diabetic control. Exam reveals a necrotic right hard palate (in image) and right mild proptosis. On the suspicion of invasive fungal sinusitis, ENT takes her to the operating room. Biopsy confirms a suspicion of mucormycosis.
CASE 3 (Question/Response from Live Meeting)
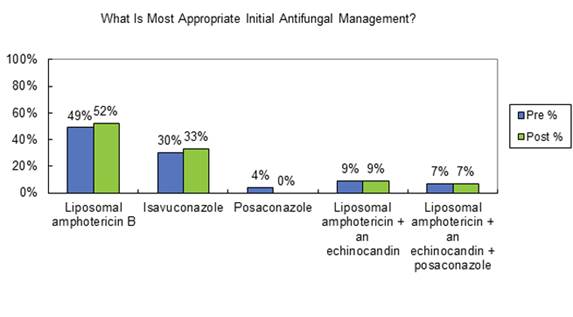
Commentary on live answer responses: Let's see where you fall out here. Most of you are taking the traditional approach, 50% taking the liposomal amphotericin approach. A third would go to the newly approved isavuconazole. And then the rest, a combination approach. Nobody chose posaconazole as an initial therapy —and it's not approved for that.
PETER PAPPAS, MD:
I think this really does reflect, frankly, how many of us feel about it. No one really knows. There will not be a comparative trial of this disorder. And much of the data that you are going to see published is going to be salvage data, single-arm data, noncomparative data. So it's imperfect in many ways.
SECTION 4: QUESTION AND ANSWER SESSION
Q: do you advocate for tdm with azoles?
PETER PAPPAS, MD:
I think the answer for most of us, I think for most situations, it is 'Yes, we would advocate for TDM.'
I think the question that was maybe not as clearly answered, Libby, was when. Are you a trough person? I mean, patients come in, they've taken their medication at 8 in the morning, they're in clinic at 10:00. Am I really going to wait until 7 that afternoon to draw a trough? Or do I just simply want to get a level?
ELIZABETH DODDS ASHLEY, PharmD:
That's a great question. And I think the answer is ideally we'd like trough concentrations. But the truth is these drugs have very long half-lives and there's not a lot of variability across the dosing interval. So if you want the concentration, we always just go ahead and draw it. We know that it was taken not as a true trough, but there really isn't that much variability. So randoms seem to be okay.
And if you go back to the clinical data that all of our targets are based on, they're actually all random. They're very rarely true troughs in those clinical trials with the data obtained. So we always get it.
PETER PAPPAS, MD:
They are random, that is for sure.
MAIKEN ARENDRUP, MD:
Maybe one comment, we quite often see that when the level, for instance, for voriconazole—that fluctuates a bit more definitely than posa does. But sometimes, when the level is high, when we call out to the physician it often turns out that the patient just received the medication. And I think that is very important to be aware of. Because if you dose de-escalate based on such a level, then you will go too low.
ELIZABETH DODDS ASHLEY, PharmD:
Yes, absolutely.
MUCORMYCOSIS QUESTIONS
3. When do you do antifungal susceptibility testing? And how relevant is it for Mucorales? These azoles are not active against every species. And so, how important is that?
MAIKEN ARENDRUP, MD:
I think in our country we always do it. But that's clearly less susceptible to the azoles than the other ones. And speciation of these organisms is not very easy. I have to say for myself, when we started to do ITS sequencing, we found that not all our identifications were correct in the past. Even though when you're a reference lab you have the privilege of naming your fungus. But therefore, I think that it's definitely helpful to do a susceptibility test. It will at least tell you where on the scale you are.
THOMAS PATTERSON, MD:
There can be differential susceptibility among agents of Mucorales. For example, Rhizopus arrhizusmay display different susceptibility patterns than Rhizopus delemar,which may be a sub-species of arrhizus,but it may be less susceptible to some of the drugs than others. Now with multiple choices of drugs for Mucorales, susceptibility testing may help guide therapy.
Q: What about combination therapy for invasive pulmonary aspergillosis specifically. Who uses it and when? Real briefly, Tom.
THOMAS PATTERSON, MD:
I think that where we use it is mostly like you chose for your answer, in more progressive disease. That's not what we have the data for, which is why I showed it in that way. I think you might take really high-risk patients that you're really worried that are going to do poorly, and might treat them sooner with combinations than others.
But another one of these questions is do you think the data are specific for voriconazole and anidulafungin? No, I really don't. But again, that's the data in the area, although the other agents would make sense.
MAIKEN ARENDRUP, MD:
I think another situation where you can consider it is if you are in an area where you have a high rate of resistance. Because then you would sort of cover the organism until you have a susceptibility test.
I guess we all agree that voriconazole—or perhaps, isavuconazole—is the best agent if the organism is susceptible. And therefore, you might want to use a combination until you know if it is or is not.
PETER PAPPAS, MD:
My response to the combination question is that we, as a rule, use monotherapy as a starting point. And we offer combination therapy to patients because of progressive disease or lack of perceived initial response to therapy.
The question about the selection of the azole or the selection of the echinocandin —you know, it's approved or the data was published looking at voriconazole plus anidulafungin. That was generated for no other reason other than Pfizer was a sponsor, and anidulafungin was their agent.
I think that in reality, the combination of voriconazole and any of the echinocandins has been used and seems to be effective. Would the same apply to the combination of isavuconazole, or even posaconazole with an echinocandin? Probably so.
But there are no data specifically to support that. But I don't think a person would be wrong.
Q: Can you really dose posaconazole at 300 mg a day, both orally and IV, even though the oral is only 50% absorbed?
ELIZABETH DODDS ASHLEY, PharmD:
So it's somewhat similar to when voriconazole was approved and we used weight-based dosing for IV and a fixed dosing orally. You know, it is somewhat based on early PK studies. But the exposure seems adequate. I think we're seeing higher—I think we actually have higher bioavailability than 50%. I know that's what reported, but based on what we're seeing in terms of concentration in our patients, I believe it's higher with those oral tablets. So it really is based on achievable concentration data. So it may seem counterintuitive, but that's how the doses were drafted.
Q: (From Peter Pappas). And I'd like to ask you a question that came up very recently and I had to respond to it in a CID letter to the editor. What about purely weight-based dosing for fluconazole in obese patients? This is a sticky wicket, for sure.
ELIZABETH DODDS ASHLEY, PharmD:
It sure is, yes. So fluconazole is a very water-soluble drug, and adipose tissue is about 30% water. So we don't—we need to fully dose based on the total body weight in our very obese patients. So we keep that in mind as one consideration.
The other thing: I think we forget that fluconazole also has toxicity. And when we start using higher doses—I've personally been involved in cases that use up to 1.2 grams a day of fluconazole in obese patients. And we've had to do that to treat some particularly sequestered infections.
But you can see toxicities that we forget happening. We see alopecia, we see more drug-drug interactions. When you exceed daily dosages of 400-600 mg a day routinely with fluconazole, you get drug inhibition much more like that, more akin to what you see with posaconazole in cytochrome 3A4 than you see with the more traditional fluconazole doses.
So we need to be careful, but also keep in mind that fluconazole is very water soluble. So it's not full adipose tissue we need to fill.
Q: What about this Aspergillus lateral flow device? I think the question was related to that. Is it approved? Are you using it? And if so, how?
MAIKEN ARENDRUP, MD:
Well, it's approved in Europe. The cryptococcal LFD is approved by FDA, as well. But I think the Aspergillus test is undergoing approval. And the test that is used— not in Denmark, but in Europe, is the lateral flow device. But being a reference center, we use the galactomannan test in order to have the numerical values. Also because it's more likely that the primary centers use the lateral flow device, and then could have some kind of confirmation or numeric value by the index of the galactomannan value.
Q: Libby, I'd like to ask you, how do the azoles compare in terms of their QT prolongation potential? You know, they all have it; we've all been increasingly aware of it, especially when we add azoles and azithromycin and other similar types of agents. Do they differ one from the other?
ELIZABETH DODDS ASHLEY, PharmD:
I think they do differ. I think the jury is still out completely. I think we continue to collect data on it. And we also struggle, just like we do with the quinolones with meaningful prolongation of the QT and then moxifloxacin prolongs the QT but doesn't seem to be associated with a significantly higher rate of events.
We see more QT prolongation with older agents like fluconazole. However, I never let my guard down on that with any of the agents because we've seen it with all of them. And I think we get lulled into a sense of comfort if we think there's one that's safer than the others because they all have the risk.
Q: What about this Aspergillus lateral flow device? I think the question was related to that. Is it approved? Are you using it? And if so, how?
MAIKEN ARENDRUP, MD:
Well, it's approved in Europe. The cryptococcal LFD is approved by FDA, as well. But I think the Aspergillus test is undergoing approval. And the test that is used— not in Denmark, but in Europe, is the lateral flow device. But being a reference center, we use the galactomannan test in order to have the numerical values. Also because it's more likely that the primary centers use the lateral flow device, and then could have some kind of confirmation or numeric value by the index of the galactomannan value.
Q: Libby, I'd like to ask you, how do the azoles compare in terms of their QT prolongation potential? You know, they all have it; we've all been increasingly aware of it, especially when we add azoles and azithromycin and other similar types of agents. Do they differ one from the other?
ELIZABETH DODDS ASHLEY, PharmD:
I think they do differ. I think the jury is still out completely. I think we continue to collect data on it. And we also struggle, just like we do with the quinolones with meaningful prolongation of the QT and then moxifloxacin prolongs the QT but doesn't seem to be associated with a significantly higher rate of events.
We see more QT prolongation with older agents like fluconazole. However, I never let my guard down on that with any of the agents because we've seen it with all of them. And I think we get lulled into a sense of comfort if we think there's one that's safer than the others because they all have the risk.
Q: Here's a question about Candida glabrata, candidemia in chorioretinitis —the unanswerable question. Kind of like Candida glabrata urinary tract infections, what a tough situation. What's your appropriate treatment in those? And I assume that they mean in this question—those that are fluconazole resistant.
PETER PAPPAS, MD:
Fortunately, the ophthalmologists have gotten on board and helped us with their vitrectomies. So debridement and installation of amphotericin is probably critical to their cure.
The treatment of the systemic infection with an echinocandin or amphotericin, whichever is most appropriate, is not so much the question here. The problem is getting drug into that compartment, which doesn't appear to be very penetrable for echinocandins or amphotericin, for that matter.
So it is absolutely important to have the ophthalmologists and the clinician working hand in glove. The vitrectomy is important. They like to use voriconazole because of ophthalmologic data suggesting voriconazole is the drug of choice in all ocular fungal infections.
It's not, obviously, in a case like this. So amphotericin would be used and it would be administered either subconjunctival or intraocularly.
MAIKEN ARENDRUP, MD:
What about flucytosine in combination? The eye is very susceptible to flucytosine; it penetrates the barrier very well.
PETER PAPPAS, MD:
It is. It would make sense. I'm not aware of any data looking at that, but it should get into that space, and you would expect it to be active.
Q: If you could just briefly opine as to how the lipid (AmB) agents were approved without any clinical trials.
THOMAS PATTERSON, MD:
I don't know that they were approved with no clinical trials; I think that they were approved as equivalents. Which is really how posaconazole was approved—the new formulation, as equivalents. And you basically show that they have equal activity. But as she mentioned, there are just a lot of questions out there.
Q: How can newer diagnostics be used to influence antifungal use (ie, to improve antifungal stewardship)?
MAIKEN ARENDRUP, MD:
In general, you mean? Well, I hope the people would take testing up and then reducing antifungal use basically.
ELIZABETH DODDS ASHLEY, PharmD:
I think one of the biggest challenges is working with the clinicians to be comfortable with discontinuing in cases where we can—you know, we used to have a lot of uncertainty and continue empiric antifungal therapy for long periods of time. But now that we have good negative predictive value—but getting people to trust that, it takes some work with the stewardship. But I think there's a lot of opportunity.
THOMAS PATTERSON, MD:
Well, there are really strong data from the group in Australia, who've really shown having local availability of diagnostic tests can really be an effective tool. Not only to increase your number of confirmed cases, but also to decrease inappropriate and empirical use.
PETER PAPPAS, MD:
But you have to be willing to stop the antifungal when your diagnostic test comes back negative, unless there are compelling reasons to continue it clinically.
Q: What about the long half-life of isavuconazole? Does that bother you at all with respect to drug-drug interactions?
ELIZABETH DODDS ASHLEY, PharmD:
We certainly take it into consideration when we are stopping the drug and we have to put patients on immunosuppressants. We will lag a bit before we will increase doses back to baseline. But it's not uncommon that we do that with some of the other azoles with long half-lives, as well. Sometimes with posaconazole we have to have a similar consideration, although it's just a little bit shorter time.
Q: In your experience, are there specific physician groups or hospitals that are driving misuse of antifungals, in particular? Is there a group that you target?
ELIZABETH DODDS ASHLEY, PharmD:
I think in every hospital, it is different. I think a guiding principle is nobody gets up any day of the week and goes in and tries to use antifungals inappropriately.
So I think that in different places, it can be different groups. I've worked in hospitals where it's inappropriate widespread empiric use in the ICU because of misinformation about how they may prevent candidemia in that population. In other places, it might be prolonged empiric therapy on oncology wards.
So it is not like there is a single group that is a constant violator. I think you have to look at your antifungal use, find out where it's happening, know it's happening locally, and then have a local intervention to fix it. It's not a one size fits all, and there's not consistently one group that's doing it.
PETER PAPPAS, MD:
Is it feasible for most hospitals, in places like the US, to offer multiple fungal diagnostics? Or is that asking too much?
Peter Pappas, MD:
Maiken, I'm going direct that question. Do you think it's feasible? Admittedly, you live in a unique situation. You're in a small country, 3 to 5 million people, you could have a large reference laboratory. Things are fairly controlled.
MAIKEN ARENDRUP, MD:
I think that over the past years, the laboratories in Denmark have really taken up lots of testing. And the level has been improved a lot. We run, for instance, the National Surveillance Program for Fungemia. And in the first years, there were quite a number of the identifications that were wrong. But after MALDI-TOF was introduced in all centers, all the identifications are correct.
So yes, the diagnoses have really gone up and it's also very important because we see a shift in species distribution. So if you go like 10-15 years back, we have 15% glabrata. But today we have more than 30%.
So there's no debate or questioning about that. We use too much antifungals. And actually, a large proportion of that is used in primary healthcare centers, which is an area where we haven't really focused on. Two thirds of our fluconazole use in Denmark is used in primary healthcare. For what—why on earth they put on fluconazole in the primary healthcare center?
Q: But the question is whether it is feasible for regional hospitals to offer a large array of diagnostics. Or does that have to be done—I think the question is does that really have to be done? Or should it be done in a reference laboratory or a regional laboratory? Not everybody can afford MALDI-TOF, T2, etc, etc. So where do you draw the line? Or maybe what should an average lab offer?
MAIKEN ARENDRUP, MD:
I think it depends on the patient population for the hospital. I mean, if you take care of hematological patients, for instance, you need to have access to diagnostics. And the way it's done in Denmark is there are centers that have that. They all do galactomannan antigen and antibody testing. They all have MALDI-TOF.
And then it's quite easy in our country to even transfer some samples to central labs because we have a nationwide microbiology database. So as soon as a result in any of the laboratories is finalized, then it goes into this nationwide database. So any physician, any microbiologist can look up all data that is done in micro labs in Denmark just like that. So we have a total overview also. And that is just really helpful.
Q: How do you treat pulmonary Mucor? If and when do you switch to oral posaconazole or isavuconazole?
THOMAS PATTERSON, MD:
That's a really hard question for the last one of the night. How long to treat and when to switch? Really, we don't know. It depends. If your patient is doing great, if they're really not that sick and you can take oral therapy early on, people are going to give it really soon. If they're really sick and doing poorly, a longer time. So I think it's a really, really tough question.
REFERENCES
- Abelcet prescribing information. Gaithersburg, MD: Sigma-Tau Pharmaceuticals, Inc; 2010.
- Adler-Moore JP, Gangneux JP, Pappas PG. Comparison between liposomal formulations of amphotericin B. Med Mycol. 2016;54:223-231.
- AmBisome prescribing information. San Dimas, CA: Gilead Sciences, Inc; 2012.
- Andes D. In vivo pharmacodynamics of antifungal drugs in treatment of candidiasis. Antimicrob Agents Chemother. 2003;47:1179-1186.
- Andes D, Pascual A, Marchetti O. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother. 2009;53:24-34.
- Andes DR, Safdar N, Baddley JW, et al; Mycoses Study Group. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54:1110-1122.
- Andresen D, Donaldson A, Choo L, et al. Multifocal cutaneous mucormycosis complicating polymicrobial wound infections in a tsunami survivor from Sri Lanka. Lancet. 2005;365:876-878.
- Arendrup MC, Chryssanthou E, Gaustad P, Koskela M, Sandven P, Fernandez V. Diagnostics of fungal infections in the Nordic countries: we still need to improve! Scand J Infect Dis. 2007;39:337-343.
- Arendrup MC, Schierbeck J, Reiter N, Krarup KB, Andersen JS.Performance evaluation of the T2Candida Panel in the ICU setting-data from an ongoing study. Abstract 025. Presented at the ASM Microbe Meeting; June 16-20, 2016; Boston MA.
- Astellas. Isavuconazonium. Invasive Aspergillosis and Invasive Mucormycosis: Advisory Committee Briefing Document. Northbrook, IL: Astellas. January 22, 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiInfectiv eDrugsAdvisoryCommittee/UCM430748.pdf. Accessed June 15, 2016.
- Astvad KM, Jensen RH, Hassan TM, et al. First detection of TR46/Y121F/T289A and TR34/L98H alterations in Aspergillus fumigatus isolates from azole-naive patients in Denmark despite negative findings in the environment. Antimicrob Agents Chemother. 2014;58:5096-5101.
- Badali H, Vaezi A, Haghani I, et al. Environmental study of azole-resistant Aspergillus fumigatus with TR34/L98H mutations in the cyp51A gene in Iran. Mycoses. 2013;56:659-663.
- Bader O, Weig M, Reichard U, et al; MykoLabNet-D Partners. cyp51A-Based mechanisms of Aspergillus fumigatus azole drug resistance present in clinical samples from Germany. Antimicrob Agents Chemother. 2013;57:3513-3517.
- Brüggemann RJ, Alffenaar JW, Blijlevens NM, et al. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis. 2009;48:1441-1458.
- Bueid A, Howard SJ, Moore CB, et al. Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J Antimicrob Chemother. 2010;65:2116-2118.
- Buied A, Moore CB, Denning DW, Bowyer P. High-level expression of cyp51B in azole-resistant clinical Aspergillus fumigatus isolates. J Antimicrob Chemother. 2013;68:512-514.
- Castanheira M, Messer SA, Rhomberg PR, Pfaller MA. Antifungal susceptibility patterns of a global collection of fungal isolates: results of the SENTRY Antifungal Surveillance Program (2013). Diagn Microbiol Infect Dis. 2016;85:200-204.
- Centers for Disease Control. Guidelines for prevention of intravascular catheter-related infections, 2011. Available at http://www.cdc.gov/hicpac/BSI/01-BSI-guidelines-2011.html. Accessed August 2, 2016.
- Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47:503-509.
- Chau MM, Kong DC, van Hal SJ, et al. Consensus guidelines for optimising antifungal drug delivery and monitoring to avoid toxicity and improve outcomes in patients with haematological malignancy, 2014. Intern Med J. 2014;44:1364-1388.
- Chen Y, Wang H, Lu Z, et al. Emergence of TR46/Y121F/T289A in Aspergillus fumigatus isolate from a Chinese patient. Antimicrob Agents Chemother. 2015;59:7148-7150.
- Chowdhary A, Kathuria S, Xu J, et al. Clonal expansion and emergence of environmental multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR34/L98H mutations in the cyp51A gene in India. PloS One. 2012;7(12):e52871.
- Clinical Laboratory Standards Institute. M27-S4. Reference method for broth dilution antifungal susceptibility testing of years; fourth informational supplement; December 2012.
- Cornely OA, Arikan-Akdagli S, Dannaoui E, et al; European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group; European Confederation of Medical Mycology. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect. 2014;20(Suppl 3):5-26.
- Denning DW, Park S, Lass-Florl C, et al. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis. 2011;52:1123-1129.
- Dodds Ashley ES, Lewis R, Lewis JS, Martin C, Andes D. Pharmacology of systemic antifungal agents. Clin Infect Dis. 2006;43:S28-S39.
- aEigl S, Prattes J, Lackner M, et al. Multicenter evaluation of a lateral-flow device test for diagnosing invasive pulmonary aspergillosis in ICU patients. Crit Care. 2015;19:178-187.
- bEigl S, Prattes J, Reinwald M, et al. Influence of mould-active antifungal treatment on the performance of the Aspergillus-specific bronchoalveolar lavage fluid lateral-flow device test. Int J Antimicrob Agents. 2015;46:401-405.
- European Committee on Antimicrobial Susceptibility Testing and International Society of Human and Animal Mycology. FungiMAP app. Available at https://itunes.apple.com/us/app/fungimics/id1095747789?mt=8. Accessed August 2, 2016.
- European Committee on Antimicrobial Susceptibility Testing. Antifungal Agents: Breakpoint tables for interpretation of MICs. Version 8.0, valid from 2015-11-16. Available at http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/Antifung al_breakpoints_v_8.0_November_2015.pdf Accessed August 2, 2016.
- European Committee on Antimicrobial Susceptibility Testing. Data from the EUCAST MIC distribution website. Accessed August 3, 2016.
- Fraczek MG, Bromley M, Buied A, et al. The cdr1B efflux transporter is associated with non-cyp51a-mediated itraconazole resistance in Aspergillus fumigatus. J Antimicrob Chemother. 2013;68:1486-1496.
- Fraczek MG, Kirwan MB, Moore CB, Morris J, Denning DW, Richardson MD. Volume dependency for culture of fungi from respiratory secretions and increased sensitivity of Aspergillus quantitative PCR. Mycoses. 2014;57:69-78.
- Fungizone prescribing information. Princeton, NJ: Bristol-Myers Squibb Company; 2009.
- Greenberg RN, Mullane K, van Burik JA, et al. Posaconazole as salvage therapy for zygomycosis. Antimicrob Agents Chemother. 2006;50:126-133.
- Groll AH, Kolve H, Ehlert K, Paulussen M, Vormoor J. Pharmacokinetic interaction between voriconazole and ciclosporin A following allogeneic bone marrow transplantation. J Antimicrob Chemother. 2004;53:113-114.
- Hagiwara D, Takahashi H, Fujimoto M, et al. Multi-azole resistant Aspergillus fumigatus harboring Cyp51A TR46/Y121F/T289A isolated in Japan. J Infect Chemother. Published online Feb 16, 2016.
- Hindahl CB, Wilson JW. Flash pulmonary oedema during anidulafungin administration. J Clin Pharm Ther. 2012;37:491-493.
- Ibrahim AS, Edwards JE Jr, Fu Y, Spellberg B. Deferiprone iron chelation as a novel therapy for experimental mucormycosis. J Antimicrob Chemother. 2006;58:1070-1073.
- Ibrahim AS, Gebermariam T, Fu Y, et al. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J Clin Invest. 2007;117:2649-2657.
- Jadhav MP, Shinde VM, Chandrakala S, et al. A randomized comparative trial evaluating the safety and efficacy of liposomal amphotericin B (Fungisome™) versus conventional amphotericin B in the empirical treatment of febrile neutropenia in India. Indian J Cancer. 2012;49:107-113.
- Jitmuang A, Panackal AA, Williamson P, Bennett JE, Dekker JP, Zelazny AM. Performance of the cryptococcal antigen lateral flow assay in non-HIV-related cryptococcosis. J Clin Microbiol. 2016;54:460-463.
- Jung DS, Tverdek FP, Kontoyiannis DP. Switching from posaconazole suspension to tablets increases serum drug levels in leukemia patients without clinically relevant hepatotoxicity. Antimicrob Agents Chemother. 2014;58:6993-6995.
- Kidd SE, Goleman E, Meis JF, Slavin MA, Verweij PE. Multi-triazole-resistant Aspergillus fumigatus infections in Australia. Mycoses. 2015;58:350-355.
- Kovanda LL, Desai AV, Lu Q, et al. Isavuconazole population pharmacokinetic analysis using non-parametric estimation in patients with invasive fungal disease: results from the VITAL study. Antimicrob Agents Chemother. 2016;60:4568-4576.
- Kovanda LL, Petraitiene R, Petraitis V, et al. Pharmacodynamics of isavuconazole in experimental invasive pulmonary aspergillosis: implications for clinical breakpoints. J Antimicrob Chemother. 2016;71:1885-1891.
- Kuipers S, Brüggemann RJ, de Sévaux RG, et al. Failure of posaconazole therapy in a renal transplant patient with invasive aspergillosis due to Aspergillus fumigatus with attenuated susceptibility to posaconazole. Antimicrob Agents Chemother. 2011;55:3564-3566.
- Kurzyk EM, Nawrot U, Mroczynska M, et al. Detection of clinical Aspergillus fumigatus isolates resistant to triazoles. Poster presented at 7th Trends in Medical Mycology; October 9-12, 2015; Lisbon, Portugal. Abstract P006.
- Kyvernitakis A, Torres HA, Jiang Y, Chamilos G, Lewis RE, Kontoyiannis DP. Initial use of combination treatment does not impact early survival of 106 patients with hematologic malignancies and mucormycosis: a propensity score analysis. Presented at: ICAAC/ICC 2015; September 17-21, 2015; San Diego, CA.
- Lambin prescribing information. Mumbai, India: Sun Pharma. Available at https://www.healthkonnect.com/drugs/details/med543fa911f28. Accessed August 12, 2016.
- Laverdiere M, Bow EJ, Rotstein C, et al. Therapeutic drug monitoring for triazoles: a needs assessment review and recommendations from a Canadian perspective. Can J Infect Dis Med Microbiol. 2014;25:327-343.
- Lavergne RA, Morio F, Favennec L, et al. First description of azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation in France. Antimicrob Agents Chemother. 2015;59:4331-4335.
- Le Pape P, Lavergne RA, Morio F, Alvarez-Moreno C. Multiple fungicide-driven alterations in azole-resistant Aspergillus fumigatus, Colombia, 2015. Emerg Infect Dis. 2016;22:156-157.
- Lepak AJ, Andes DR. Antifungal pharmacokinetics and pharmacodynamics. Cold Spring Harb Perspect
- Med. May 2015;5:a019153.
- Lewis JS 2nd, Wiederhold NP, Wickes BL, Patterson TF, Jorgensen JH. Rapid emergence of echinocandin resistance in Candida glabrata resulting in clinical and microbiologic failure. Antimicrob Agents Chemother. 2013;57:4559-4561.
- Lockhart SR, Frade JP, Etienne KA, et al. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob Agents Chemother. 2011;55:4465-4468.
- Lourens A, Jarvis JN, Meintjes G, Samuel CM. Rapid diagnosis of cryptococcal meningitis by use of lateral flow assay on cerebrospinal fluid samples: influence of the high-dose “hook” effect. J Clin Microbiol. 2014;52:4172-4175.
- Macdougall C, Gouivea-Pisano J, Johnson MD, Perfect JR, Dodds Ashley ES. An evaluation of usage and safety of voriconazole following its introduction at a tertiary care medical center. 43rd ICAAC. September 14-17, 2003; Chicago, Ill. M-983.
- Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760-769.
- Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162:81-89.
- Marty FM, Ostrosky-Zeichner L, Cornely OA, et al; VITAL and FungiScope Mucormycosis Investigators. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16:828-837.
- Maschmeyer G, Patterson TF. Our 2014 approach to breakthrough invasive fungal infections. Mycoses. 2014;57:645-651.
- Mellado E, Garcia-Effron G, Alcázar-Fuoli L, et al. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involved a combination of cyp51A alterations. Antimicrob Agents Chemother. 2007;51:1897-1904.
- Mortensen KL, Mellado E, Lass-Flörl C, Rodriguez-Tudela JL, Johansen HK, Arendrup MC. Environmental study of azole-resistant Aspergillus fumigatus and other aspergilli in Austria, Denmark, and Spain. Antimicrob Agents Chemother. 2010;54:4545-4549.
- Mortensen KL, Fernandez V, Chryssanthou E, Gaustad P, Sandven P, Cavling Arendrup M. Nordic external quality assessment program in medical mycology (NEQAMM). The Nordic Reference Group on Methods in Medical Mycology. Report 2007.
- Mylonakis E, Clancy CJ, Ostrosky-Zeichner L, et al. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: a clinical trial. Clin Infect Dis. 2015;60:892-899.
- Nivoix Y, Launoy A, Lutun P, et al. Adherence to recommendations for the use of antifungal agents in a tertiary care hospital. J Antimicrob Chemother. 2012;67:2506-2513.
- Noxafil FDA Briefing Document.
- Noxafil manufacturer’s prescribing information. Whitehouse Station, NJ: Merck & Co., Inc; 2014.
- Noxafil Oral Suspension, manufacturer’s prescribing information. Whitehouse Station, NJ: Merck & Co., Inc; 2008.
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-e50.
- Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46:201-211.
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1-e60.
- Pelaez T, Monteiro MC, Garcia-Rubio R, Bouza E, Gomez-Lopez A, Mellado E. First detection of Aspergillus fumigatus azole-resistant strain due to Cyp51A TR46/Y121F/T289A in an azole-naive patient in Spain. New Microbes New Infect. 2015;6:33-34.
- Pettit, Miceli M, Rivera C, et al. Multicenter study of posaconazole delayed-release tablet serum level and association with hepatic and cardiac toxicity. Presented at the ASM Microbe Meeting; June 19, 2016;Boston, MA.
- Pham CD, Iqbal N, Bolden CB, et al. Role of FKS mutations in Candida glabrata: MIC values, echinocandin resistance, and multidrug resistance. Antimicrob Agents Chemother. 2014;58:4690-4696.
- Prigitano A, Venier V, Cogliati M, De Lorenzis G, Esposto MC, Tortorano AM. Azole-resistant Aspergillus fumigatus in the environment of northern Italy, May 2011 to June 2012. Eurosurveillance. 2014;19(12):pii=20747.
- Rath PM, Buchheidt D, Spiess B, Arfanis E, Buer J, Steinmann J. First reported case of azole-resistant Aspergillus fumigatus due to the TR/L98H mutation in Germany. Antimicrob Agents Chemother. 2012;56:6060-6061.
- Reed C, Ibrahim A, Edwards JE Jr, Walot I, Spellberg B. Deferasirox, an iron-chelating agent, as salvage therapy for rhinocerebral mucormycosis. Antimicrob Agents Chemother. 2006;50:3968-3969.
- Richer SM, Smedema ML, Durkin MM, et al. Improved diagnosis of acute pulmonary histoplasmosis by combining antigen and antibody detection. Clin Infect Dis. 2016;62:896-902.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycoses: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Rüchel R, Schaffrinski M. Versatile fluorescent staining of fungi in clinical specimens by using the optical brightener Blankophor. J Clin Microbiol. 1999;37:2694-2696.
- Seyedmousavi S, Hashemi SJ, Zibafar E, et al. Azole-resistant Aspergillus fumigatus, Iran. Emerg Infect Dis. 2013;19:832-833.
- Snelders E, van der Lee HAL, Kuijpers J, et al. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 2008;5:1629-1637.
- aSpellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67:715-722.
- bSpellberg B, Ibrahim A, Roillides E, et al. Combination therapy for mucormycosis: why, what, and how? Clin Infect Dis. 2012;54(Suppl 1):S73-S78.
- Springer J, White PL, Hamilton S, et al. Comparison of performance characteristics of Aspergillus PCR in testing a range of blood-based samples in accordance with international methodological recommendations. J Clin Microbiol. 2016;54:705-711.
- Steinmann J, Hamprecht A, Vehreschild MJGT, et al. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother. 2015;70:1522-1526.
- Stensvold CR, Jørgensen LN, Arendrup MC. Azole-resistant invasive aspergillosis: relationship to agriculture. Curr Fungal Inf Reports. 2012;6:178-191.
- Sutton D, Fothergill AW, Rinaldi MG. Aspergillus in vitro antifungal susceptibility data: new millennium trends. Presented at Advances Against Aspergillosis. 2004; September 9-11, 2004; San Francisco, CA. Abstr. 16.
- Tatara AM, Albert ND, Verweij PE, et al. Increase in pan-triazole resistance to Aspergillus-active triazoles in patients with hematologic malignancies. Presented at the ASM Microbe Meeting; June 19, 2016; Boston, MA.
- Thornton CR. Development of an immunochromatographic lateral-flow device for rapid serodiagnosis of invasive aspergillosis. Clin Vaccine Immunol. 2008;15:1095-1105.
- Valerio M, Rodriguez-Gonzalez CG, Muñoz P, Caliz B, Sanjurjo M, Bouza E; COMIC Study Group (Collaborative Group on Mycoses). Evaluation of antifungal use in a tertiary care institution: antifungal stewardship urgently needed. J Antimicrob Chemother. 2014;69:1993-1999.
- Vallabhaneni S, Cleveland AA, Farley MM, et al. Epidemiology and risk factors for echinocandin nonsusceptible Candida glabrata bloodstream infections: data from a large multisite population-based candidemia surveillance program, 2008-2014. Open Forum Infect Dis. 2015;2(4):ofv163.
- van der Linden JWM, Arendrup MC, Warris A, et al. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis. 2015;21:1041-1044.
- van der Linden JWM, Camps SMT, Kampinga GA, et al. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis. 2013;57:513-520.
- van Ingen J, van der Lee HA, Rijs AJ, Snelders E, Melchers WJ, Verweij PE. High-level pan-azole-resistant aspergillosis. J Clin Microbiol. 2015;53:2343-2345.
- Verweij PE, Ananda-Rajah M, Andes D, et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Update. 2015;21-22:30-40.
- Verweij PE, Mellado E, Melchers WJ. Multiple-triazole-resistant aspergillosis. N Engl J Med. 2007;356:1481-1483.
- White PL, Parr C, Thornton C, Barnes RA. Evaluation of real-time PCR, galactomannan enzyme-linked immunosorbent assay (ELISA), and a novel lateral-flow device for diagnosis of invasive aspergillosis. J Clin Microbiol. 2013;51:1510-1516.
- White PL, Barnes RA, Springer J, et al. Clinical performance of Aspergillus PCR for testing serum and plasma: a study by the European Aspergillus PCR initiative. J Clin Microbiol. 2015;53:2832-2837.
- White PL, Wiederhold NP, Loeffler J, et al. Comparison of nonculture blood-based tests for diagnosing invasive aspergillosis in an animal model. J Clin Microbiol. 2016;54:960-966.
- Wiederhold NP, Pennick GJ, Dorsey SA, et al. A reference laboratory experience of clinically achievable voriconazole, posaconazole, and itraconazole concentrations within the bloodstream and cerebral spinal fluid. Antimicrob Agents Chemother. 2014;58:424-441.
- Wiederhold NP, Gil VG, Gutierrez F, et al. First detection of TR34 L98H and TR46 Y121F T289A Cyp51 mutations in Aspergillus fumigatus isolates in the United States. J Clin Microbiol. 2016;54:168-171.
- Wu CJ, Wang HC, Lee JC, et al. Azole-resistant Aspergillus fumigatus isolates carrying TR34/L98H mutations in Taiwan. Mycoses. 2015;58:544-5
.
Back to Top