
Expired activity
Please go to the PowerPak
homepage and select a course.
Anesthesia and Neuromuscular Blockade: A Guide for Hospital Pharmacists
HISTORY AND CLASSIFICATION OF NEUROMUSCULAR BLOCKING AGENTS
Neuromuscular blocking agents (NMBAs) cause paralysis of the muscles; they have been used as adjuncts to general anesthesia for more than a half-century. These medications are used to facilitate tracheal intubations, enable mechanical ventilation, and create an immobile surgical field. Additionally, NMBAs are given in emergency and intensive care settings to select patients.
Curare is one of the oldest agents known to have a paralyzing effect. Its first known use was as the poison on dart tips of South America Indians during game hunting. Centuries later, crude curare was investigated and researchers isolated the active alkaloid d-tubocurarine from biologically authenticated samples of Chondrodendron tomentosum, a tropical plant native to Central and South America. (The compound was named d-tubocurarine because it was stored in tubes.)1
By the early 1940s, d-tubocurarine was refined, purified, and marketed by Squibb Corporation as Intocostrin, the first NMBA for clinical use.2 The practice of administering tubocurarine during surgery was introduced in 1942 when Dr Harold Griffith of Montreal used the drug in a patient undergoing an appendectomy. Griffith reported that it was a safe drug and stated, “anesthesiologists have wished at times that they might be able to produce rapid and complete muscular relaxation in resistant patients under general anesthesia.”1
In 1954, an important study by Beecher and Todd evaluated the administration of 599,548 anesthetic agents in 10 university hospitals. They found that 6 times as many deaths occurred in patients who received “curare” compared to patients who did not receive curare. Many of the deaths were attributed to respiratory depression. The study revealed that anesthesia practice at that time did not have an understanding or appreciation of the pharmacology of NMBAs or muscle relaxants.3 The postoperative effects of these agents had not been recognized and, therefore, anesthesia specialists did not recognize the importance of residual neuromuscular blockade that causes muscle weakness nor the need to antagonize the blockade.
Prior to the discovery of d-tubocurarine, if muscle relaxation was needed, a patient was administered high concentrations of volatile anesthetics. However, this greatly increased the risk of adverse cardiac and respiratory events. Additionally, tracheal intubation was rarely employed before the introduction of NMBAs.1 After the discovery of NMBAs, anesthesia practice was redesigned to keep patients asleep, pain free, and motionless. This triad of anesthesia is commonly referred to as hypnosis, analgesia, and muscle relaxation.
NMBAs produce immobility and relaxation, which are important for airway management and surgical field exposure. At induction, the main goals of neuromuscular blockade are paralysis of the vocal cords and jaw muscles to allow endotracheal intubation and relaxation of respiratory muscles of the diaphragm to allow controlled ventilation.4 This use of NMBAs improves intubation conditions, reduces tissue trauma, and minimizes vocal cord injury and hoarseness. Relaxation of abdominal muscles and the diaphragm facilitates many abdominal and laparoscopic surgeries. NMBAs allow a large operative space, sufficient visualization, access to tight spaces, and low insufflation pressures during laparoscopy. They also minimize potential harmful effects of patient movement during a surgical procedure.5,6
NMBAs are categorized by their mechanism of action at neuromuscular junctions; they are termed depolarizing or non-depolarizing agents (Table 1). Depolarizing agents act like acetylcholine and activate cholinergic receptors at motor end plates to produce depolarization. This depolarization may be observed as fasciculation. Subsequent neuromuscular transmission is inhibited as long as adequate levels of the depolarizing agent remain at the receptor site.2
| Table 1. Neuromuscular Blocking Agents2,6-8 |
Depolarizing
Non-depolarizing |
Succinylcholine
Aminosteroids
Pancuronium
Vecuronium
Rocuronium
Benzylisoquinoliniums
Atracurium
Cisatracurium |
Succinylcholine is the only depolarizing NMBA in clinical use and it is used for intubation. It has a prompt onset and a short duration of action, which are important benefits of the medication. At a dose of 1 mg/kg, succinylcholine produces a profound neuromuscular block in 60 seconds, and, due to its short half-life, neuromuscular strength recovers quickly, starting as little as 3 minutes after administration and normally complete within 12 to 15 minutes. Succinylcholine does not produce residual neuromuscular blockade.7 However, many unwanted side effects are associated with the drug, likely due to its depolarizing action. For example, patients can experience myalgia, which is often more problematic in young, healthy patients with large muscle mass than in older, leaner patients. Other adverse effects of succinylcholine include heart rhythm disturbances (i.e., tachycardia or bradycardia) and increased intragastric, intracranial, and intraocular pressures. Although rare, hyperkalemic dysrhythmias can occur and lead to cardiac arrest and death. When administered with inhalational agents in susceptible patients, succinylcholine can precipitate malignant hyperthermia.2,5,7,8
Non-depolarizing muscle relaxants produce neuromuscular blockade by acting as competitive antagonists and competing with acetylcholine. The non-depolarizing agents have longer onsets and durations of action than succinylcholine. Non-depolarizing NMBAs inhibit muscle contraction when they bind at approximately 70% of receptors.6,8 Agents in this category are classified according to their structure: aminosteroids or benzylisoquinoliniums. The agents are further identified by their durations of action: long-acting, intermediate-acting, or short-acting. Duration of action is defined as the time from drug administration to the time when the evoked neuromuscular function of the thumb returns to 25% of baseline. Duration is a factor of dose and plasma concentration, especially for intermediate-acting and long-acting drugs. Vecuronium, pancuronium, and rocuronium are steroidal compounds: pancuronium is the longest acting agent with a duration of 120 to 180 minutes; vecuronium and rocuronium are intermediate-acting agents, with durations of 20 to 50 minutes. Atracurium and cisatracurium are benzylisoquinolinium compounds of intermediate duration: atracurium has a duration of action of 30 to 40 minutes and cisatracurium has a slightly longer duration of 30 to 70 minutes. Cisatracurium is 4 times as potent as atracurium. Non-depolarizing agents do not carry the same risks of complications as succinylcholine.2,4,5,7,8
NMBA SAFETY AND ADMINISTRATION
The overall safety of anesthesia has well been established, but neuromuscular blockade induced by NMBAs has the potential for significant adverse outcomes. In February 2016, the Anesthesia Patient Safety Foundation Newsletter described residual paralysis caused by NMBAs as a patient safety hazard.9
Worldwide, more than 234 million surgeries are performed each year.10 In the United States (U.S.) alone, an estimated 100 million NMBA doses are given for tracheal intubation and optimization of surgical conditions.11 Complete return of neuromuscular function should be achieved at the conclusion of surgery, unless mechanical ventilation is planned. Without reversal of paralysis, residual effects, including hypoxia, weakness, and respiratory complications, can occur, which increases the risks for morbidity and mortality. Even during minimal neuromuscular blockade, respiratory and pharyngeal
muscle function can be affected.6,12,13
In 1979, Viby-Morgensen et al reported that 42% of patients receiving a non-depolarizing NMBA followed by neostigmine at the end of surgery had muscular weakness. While in the recovery room, patients had documented train-of-four ratios (TOFRs) of less than 0.7, which by current standards indicates inadequate return of neuromuscular function. (The TOFR is four stimuli given at a frequency of 2 Hz, potentially causing 4 twitches (T1-T4 ), the ratio of T4:T1 indicated the degree of neuromuscular block.) This phenomenon was later termed as unidentified residual paralysis or residual curarization.14
A more recent study by Murphy et al evaluated 120 patients scheduled for elective gynecologic or general surgery procedures. All patients were classified as having an American Society of Anesthesiologists status of I or II, indicating that they were healthy or had only mild disease with minimal functional limitations. The patients were administered rocuronium for tracheal intubation and maintenance, and, at the end of the surgeries, the neuromuscular blocks were reversed with neostigmine 50 mcg/kg and glycopyrrolate 10 mcg/kg. The reversal was not administered until 2 responses to the train-of-four (TOF) stimulation were visible. Clinicians were also instructed to conduct clinical tests and/or use visual or tactile assessments for residual paralysis. The TOFR was determined with the use of acceleromyography immediately before tracheal extubation and on arrival to the post-anesthesia care unit (PACU). The mean TOFR was 0.67 ± 0.2 immediately before tracheal extubation; in the PACU, a TOFR of less than 0.7 was observed in 9 patients and a TOFR of less than 0.9 was observed in 38 patients. Murphy et al described a period of vulnerability for patients from the time of tracheal extubation to complete recovery; they also concluded that neuromuscular recoveries are seldom complete while patients are in the operating room or through transport time to the PACU. Overall, the authors concluded that residual paralysis occurred in the majority of patients.9 Other recent studies show that residual paralysis occurs in 16% to 42% of patients receiving intermediate-acting NMBAs, revealed by TOFRs of less than 0.7 to 0.8 in the PACU.12
Naguib et al conducted a survey and sent questionnaires to members of the Anesthesia Patient Safety Foundation and European Society of Anaesthesiologists to assess their understandings of the incidence of residual paralysis. More than 2500 providers completed the survey. Respondents from the U.S. (64.1%) and Europe (52.2%) estimated the incidence of clinically significant residual neuromuscular weakness to be less than 1% (P < 0.0001). Reversal of neuromuscular blockade was less common among European providers than among those in the U.S. (18% vs 34.2%, respectively; P < 0.0001). Quantitative monitors were less available to clinicians in the U.S. than to those in Europe (22.7% vs 70.2%, respectively; P < 0.0001). Interestingly, 19.3% of providers in Europe and 9.4% of providers in the U.S. did not routinely use neuromuscular monitors. Most respondents stated that neither conventional nerve stimulators nor quantitative TOF monitors should be part of minimum monitoring standards. The study
results suggest that many anesthesia providers are not aware of the extent of residual paralysis and they imply a lack of agreement among anesthesia providers about the best way to monitor neuromuscular function.15
Adverse effects of NMBAs
Patients demonstrating residual neuromuscular blockade or residual paralysis may experience oxygen desaturation, pulmonary collapse, abnormal swallowing, and an increased risk for aspiration and pneumonia. These patients may need tracheal re-intubation, which can cause a delay in discharge. Potentially, respiratory failure can result, which could lead to severe, permanent brain damage or death.12,13 The risk of complications associated with NMBAs increases with older age, the presence of myasthenia gravis, and upper abdominal and thoracic surgeries.
Postoperative side effects or adverse events that are frequently associated with residual neuromuscular paralysis can also be caused by other anesthetic drugs. As such, the anesthesia provider may not be able to clearly identify which agent is causing the effects; this uncertainty contributes to the inability to recognize true residual neuromuscular blockade. When residual neuromuscular blockade is absent, the patient can breathe normally, clear secretions, cough, maintain an airway, and minimize possible aspiration. Because the surgical patient is asleep, drowsy, or remains under anesthesia, return of these important functions cannot be easily detected or assessed by the anesthesia care provider at the end of surgery or early in the recovery period.8,12,13
Monitoring of NMBAs
Until the 1990s, a TOFR of 0.7 or greater was considered adequate muscle recovery. More recent studies have shown that patients continue to have muscular weakness at this ratio, which is now described as residual paralysis. Residual paralysis is currently accepted and defined as a TOFR less than 0.9 at the adductor pollicis – the muscle in the hand that adducts the thumb. Residual neuromuscular blockade occurs either with or without reversal agents, and it may be present at a TOFR up to 0.9.13 Patients with residual paralysis may need tracheal reintubation and further support. A patient will likely have adequate return of neuromuscular function at a TOFR of 0.9 or greater. Quantitative monitoring of the neuromuscular blockade is the only way to assure complete reversal.12,13,16
Neuromuscular function or the degree of residual paralysis can be evaluated in 3 ways: 1) clinical tests that requires a patient’s participation and are normally completed when a patient has emerged from anesthesia; 2) visual or tactile evaluation of TOF or double burst stimulation at the adductor pollicis; and 3) measurement of the TOFR with a technique such as acceleromyography, kinemyography, mechanomyography, or electromyography. Clinical signs or tests used to assess the reversal of neuromuscular blockade include a patient’s ability to lift his or her head or leg for 5 seconds, remove a tongue depressor when manually held, or maintain hand grip strength. Tidal volume (i.e., recovery of breathing) is also included in the assessment of NMBA reversal. These tests require the patient to be awake and cooperative, which is not always possible after a surgical procedure. Further, studies have shown that assessment of residual paralysis cannot be accurately assessed by clinical signs. Kopman et al studied healthy adults who were given an NMBA to reach a TOFR of less than 0.7; the participants’ clinical signs were correlated to the actual TOFRs. Participants could not complete a 5-second head lift until TOFRs reached an average of 0.60, and they were unable to clench a tongue depressor until they returned to a TOFR exceeding 0.85. Participants with a TOFR less than 0.90 were found to have visual disturbances. The study illustrated that the clinical test of a head or leg lift is not adequate for properly assessing the return of normal upper airway function. The clenching of the tongue depressor between the teeth may be better correlated to the return of function to the upper airway, but this method is still limited in its accuracy.13,17
Tactile or visual evaluation of a twitch response evoked by electrical stimulations of the peripheral motor nerve is another method for evaluating reversal of NMBAs. A stimulator is applied to the peripheral nerve and the response of the muscle it innervates is observed. Because the site of action of NMBAs is the neuromuscular junction, this method allows for the testing of the degree of the blockade by an externally applied electrical stimulation to a peripheral nerve to cause a contraction. The results of the test with the nerve stimulator are quantified with the TOF count, which requires the provider to count or feel the number of responses to assess the neuromuscular blockade. The TOF count is the most common, but other modes of stimulation can be used, including single twitch, double burst, tetanic, and post-tetanic count. Response in the adductor pollicis correlates to respiratory function and TOF stimulation applied to the ulnar nerve was first used in the late 1970s. Tactile or visual assessment of the TOF count has limitations: while the provider may accurately count the twitches, the fade is difficult to assess with subjective qualitative monitoring.4,13
Both visual and tactile estimates of the TOF have shortcomings, so objective monitoring has been suggested as the most appropriate means of assessing NMBA reversal. The preferred test is a quantitative evaluation of the TOFR: response to peripheral nerve stimulation can be assessed by techniques such as objective myographic measurements. Acceleromyography was first introduced in the mid-1990s, and, along with this technique, kinemyography, mechanomyography, and electromyography are now considered more reliable than the subjective evaluation of the TOF fade for detecting residual blockade. The use of quantitative monitors has been shown to reduce the incidence of residual paralysis; however, the devices are fragile and sensitive to movement.4,12,13,18
NMBA REVERSAL
It is important to understand that neuromuscular monitoring itself does not prevent or eliminate residual paralysis. It can, however, provide a clinician with important and critical information about a patient’s level of neuromuscular recovery and help assess the potential for problems. The anesthesia care provider should make efforts to facilitate patients leaving the operating room with unimpaired muscle strength. The provider should carefully minimize or titrate NMBAs to decrease the level of residual paralysis at the end of surgery. The provider can also expedite recovery of muscle strength by administering a reversal drug. However, not all anesthesia providers choose to use reversal agents in patients receiving NMBAs. Monitoring is useful in determining whether spontaneous recovery has occurred sufficiently to allow administration of reversal agents and to assess the effect of these drugs. The effectiveness of traditional reversal agents is proportional to the level of the blockade, and larger doses of reversal agents are required for deeper blocks.2,4,5,13,18
The principal drugs used as NMBA reversal agents are anticholinesterase medications: neostigmine, edrophonium, and pyridostigmine. Neostigmine is twice as potent as pyridostigmine and 12 times as potent as edrophonium. Neostigmine is also more effective in intense blocks than the other 2 agents.2 Anticholinesterase medications should be administered at a time when the neuromuscular block is not intense. The agents inhibit acetylcholine breakdown to increase the concentration and duration of acetylcholine at the neuromuscular junction. Therefore, these agents have significant parasympathomimetic activity and side effects include tachycardia, bradycardia, nausea, confusion, and constipation. This activity can be reduced by administering antimuscarinic medications such as atropine or glycopyrrolate. However, antimuscarinic agents also have undesirable side effects. For example, atropine causes tachycardia, dry mouth, and blurred vision.2,4,5,13
Administration of an anticholinesterase agent does not remove the NMBA from the patient’s body. Patients undergoing surgery may receive multiple doses of an NMBA and some of these doses may be higher than normal. Even though some patients resume spontaneous breathing with neuromuscular paralysis, not all patients are able to accomplish this. It is always important to ensure that the effects of the NMBA have worn off or are reversed before transferring or discharging a patient.2,4,5,19
Of the 3 anticholinesterase medications, neostigmine is the most commonly used NMBA reversal drug. Neostigmine was introduced in the early 1960s and was intended to be given at the end of surgery to prevent residual paralysis with long-acting NMBAs.2,13 Dosing should be determined by the level of spontaneous recovery at the time of administration, duration of action of the NMBA, and timing of the last dose of the NMBA. Dosing is also based on the depth of the block and the monitoring technique. Normal intravenous doses of neostigmine range from 0.03 to 0.07 mg/kg. A dose of 0.03 mg/kg is used when the TOF stimulus is substantially more than 10% of baseline or when a second twitch is present. A 0.07 mg/kg dose is used for long-acting NMBAs or when the first twitch response is relatively weak (i.e., not substantially more than 10% of baseline). Neostigmine dosing should not exceed 0.07 mg/kg or a cumulative total of 5 mg, whichever is lower. Side effects of neostigmine include cardiac arrhythmias (e.g., bradycardia, tachycardia, atrioventricular block, and nodal rhythm). Nonspecific electrocardiogram changes, cardiac arrest, syncope, and hypotension have also been reported with neostigmine, as well as dyspnea and increased oral, pharyngeal, and bronchial secretions. Respiratory depression, respiratory arrest, and bronchospasm may occur, as well as rash, urticaria, nausea, emesis, flatulence, increased peristalsis, increased salivation, and urinary frequency. An intravenous antimuscarinic agent should be given prior to or concomitantly with neostigmine to help minimize these side effects.2,4,5,13,19
Another limitation of neostigmine is that it is only efficient after the beginning of spontaneous recovery. Large doses of anticholinesterases, if administered when there is no neuromuscular block, can cause neuromuscular dysfunction. Smaller doses of anticholinesterase agents are needed if recovery from the block is almost complete.2,4,5,16,20,21 A concerning drawback related to neostigmine is its inability to reverse profound blockade due to a dose ceiling effect.4
A new reversal agent, sugammadex, was introduced into the U.S. market in December of 2015. Sugammadex is a modified gamma cyclodextrin compound that belongs to a new class of drugs called selective relaxant binding agents. These agents have been used as solubilizing agents in the food, pharmaceutical, chemical, and environmental industries since the 1950s. Sugammadex is the first cyclodextrin to be used as a therapeutic agent.22-24 It comprises 8 sugars arranged in a ring (Figure 1).25 Sugammadex has a hydrophobic cavity and a hydrophilic exterior: the hydrophobic interactions trap an NMBA (i.e., rocuronium or vecuronium) into the cyclodextrin cavity; polar side chains attached to the ring hold the drug in place. A water-soluble guest-host complex results. The generic name “sugammadex” is derived from “su,” referring to the sugar molecule, “gamma,” referring to the gamma core of 8 glucose units, and “dex,” referring to cyclodextrin.
| Figure 1. Sugammadex Molecular Structure25 |
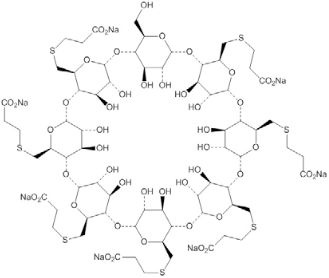 |
Sugammadex quickly, effectively, and safely reverses neuromuscular blockade from steroidal NMBAs by encapsulating the agents. The binding of rocuronium by sugammadex in the plasma leads to a decrease in free (unbound) NMBA. This causes a concentration gradient of the NMBA between the neuromuscular junctions and the plasma, which produces a lower degree of neuromuscular block. Sugammadex does not bind to the benzylisoquinolinium agents (i.e., atracurium or cisatracurium), nor does it bind to succinylcholine.22,23
Sugammadex does not exert action at the nicotinic or muscarinic receptor sites since it is not an acetylcholinesterase inhibitor like other reversal agents. Compared to anticholinesterase agents, sugammadex offers the benefit of not inducing muscarinic side effects such as bradycardia and bronchoconstriction. Therefore, sugammadex’s unique mechanism of action of encapsulating unbound drug provides adequate reversal without the adverse side effects associated with reversal agents such as neostigmine. This allows the added advantage of not needing an antimuscarinic drug to counteract the effects of the anticholinesterase drugs. The side effects of antimuscarinic drugs are, therefore, also avoided.22,23
Sugammadex is not metabolized or excreted by the liver; it is excreted unchanged by the kidneys. Anaphylaxis, hypersensitivity reactions, and marked bradycardia after administration of the drug have been reported, but these effects are rare. Bradycardia resulting in cardiac arrest within minutes of administration of sugammadex has occurred. Other side effects of sugammadex include vomiting, pain at the injection site, nausea, hypotension, and headache. No data on the use of sugammadex in pregnant women are available; likewise, it is not known if sugammadex is present in breast milk or if it effects breast-fed infants of women receiving the drug. Currently, in the U.S., sugammadex is only indicated for use in adults; a pediatric indication is available in other countries. The only clinically significant interactions reported with sugammadex are with toremifine and hormonal contraceptives. The interaction with toremifine may result in delayed recovery, and patients taking hormonal contraceptives should be instructed to use an additional non-hormonal method of contraception for 7 days following the administration of sugammadex.24,25
All doses of sugammadex should be based on total body weight. The dose of sugammadex (Table 2) is 2 mg/kg if spontaneous recovery has occurred up to at least the reappearance of the second twitch in response to TOF stimulation. A dose of 4 mg/kg should be used when recovery has reached 1 to 2 post-tetanic counts and there is no twitch response to TOF stimulation. A dose of 16 mg/kg can be used for immediate reversal of rocuronium;25 this dose has not been studied in vecuronium-induced neuromuscular blockade.
| Table 2. Dosing Guidelines for Sugammadex25 |
| Dose |
Time of administration* |
NMBA to be reversed |
| 2 mg/kg |
When spontaneous recovery has reached the reappearance of the second twitch in response to TOF stimulation |
Rocuronium, vecuronium |
| 4 mg/kg |
When spontaneous recovery of the twitch response has reached 1 to 2 post-tetanic counts and there are no twitch responses to TOF stimulation |
Rocuronium, vecuronium |
| 16 mg/kg |
When there is a clinical need to reverse neuromuscular blockade quickly (approximately 3 minutes) after administration of a single dose of 1.2 mg/kg of rocuronium |
Rocuronium only |
*Monitor for twitch responses to determine the timing and dose for administration of sugammadex. Administer as a single bolus injection; may be given over 10 seconds into existing intravenous line.
Abbreviations: NMBA, neuromuscular blocking agent; TOF, train-of-four |
The main advantages of sugammadex when compared to established reversal agents (i.e., anticholinesterase agents) are a faster recovery time, efficient reversal of deep levels of neuromuscular blockade, and no need for antimuscarinic drugs.5,9,19 Blobner et al directed a randomized, multicenter, parallel group trial involving 98 adult patients: 49 patients received sugammadex 2 mg/kg and 49 received neostigmine 50 mcg/kg and glycopyrrolate 10 mcg/kg at the reappearance of the second response of the TOF following the last dose of rocuronium. Neuromuscular blockade was assessed using acceleromyography and TOF mode of stimulation. Sugammadex was associated with a significantly faster return to a TOFR of 0.9, with a time to recovery of 1.5 minutes; time to recovery of a TOFR of 0.9 with neostigmine/glycopyrrolate was 18.6 minutes.26
In another multicenter, randomized study, researchers reviewed the recovery time of patients receiving intubation and maintenance doses of vecuronium. Patients were randomized to receive either sugammadex 2 mg/kg or neostigmine 50 mcg/kg and glycopyrrolate 10 mcg/kg. As with the other study, the reversal agents were administered at the reappearance of the second response of the TOF after the last dose of vecuronium. Neuromuscular blockade was assessed using acceleromyography and TOF mode of stimulation. The patients in the sugammadex arm of the study had a significantly faster reversal of the block: the mean times to recovery of a TOFR of 0.9 were 2.7 minutes with sugammadex and 17.9
minutes with neostigmine/glycopyrrolate.27
Surgeons frequently request complete or profound relaxation of a patient until the end of a procedure. The anesthesia care provider may be hesitant to administer additional doses of an NMBA to provide extended paralysis because doing so will likely delay recovery and transfer of the patient to the PACU. Acetylcholinesterase inhibitors cannot adequately or rapidly reverse profound blocks. However, sugammadex, at a dose of 4 mg/kg, could be used in such cases to provide a rapid reversal; it has been shown to effectively reverse profound block induced by rocuronium.19,22-25
Lemmon was the first researcher to compare the safety and efficacy of sugammadex and neostigmine in the reversal of a profound block caused by vecuronium. In a phase III multicenter, randomized, parallel group trial, patients were allocated to receive sugammadex 4 mg/kg (n = 47) or neostigmine 70 mcg/kg and glycopyrrolate 14 mcg/kg (n = 36) at 1 to 2 post-tetanic count. The mean time to recovery of a TOFR of 0.9 was 15 times faster with sugammadex than with neostigmine/glycopyrrolate (4.5 minutes vs 66.2 minutes). Neither group experienced any serious adverse events.28
Della Rocca et al conducted a large, multisite, prospective, non-randomized observational study of adult patients undergoing abdominal surgery with a shallow or deep neuromuscular block. A total of 359 patients were enrolled to determine the time from the start of neostigmine or sugammadex administration to the recovery of a TOFR of 0.9. All patients received rocuronium; 207 patients received sugammadex (44 patients with deep block and 163 patients with shallow block) and 150 patients received neostigmine (8 patients with deep block and 142 patients with shallow block). Sugammadex reversal was significantly faster than neostigmine reversal. Shallow block recovery times for sugammadex and neostigmine were 2.2 minutes and 6.9 minutes, respectively, and recovery times for deep block were 2.7 minutes and 16.2 minutes, respectively.29
In 2010, a systematic review was published that evaluated randomized controlled trials comparing sugammadex and neostigmine/glycopyrrolate for the reversal of moderate and profound neuromuscular blocks induced by vecuronium and rocuronium. The review included an economic assessment of the use of the reversal agents that aimed to determine the reductions in recovery times associated with the agents and calculate the value of this time. Three trials indicated that sugammadex produced more rapid recovery from moderate and profound neuromuscular blockade than neostigmine/glycopyrrolate. The results indicated that, if reduction in recovery time associated with sugammadex can be replicated in practice, sugammadex would be cost-effective. However, this would not apply if the time savings are achieved in the recovery room rather than the operating room. The economic assessment was based on costs in the United Kingdom and, therefore, may not apply to other countries. Still, the authors concluded that sugammadex is potentially cost-effective.30
Sugammadex may be an optimal reversal agent for patients with neurodegenerative diseases, myotonic syndromes, and musculoskeletal disorders. Patients with diseases such as myasthenia gravis, dermatomyositis, muscular dystrophy, myotonic dystrophy, spinal muscular atrophy, and amyotrophic lateral sclerosis present particular challenges to anesthesia care providers and careful anesthetic management is required. Several case reports have shown the benefit of sugammadex in patients with these conditions.31,32
In one report, a patient with spinal muscular atrophy received a single dose of rocuronium. The patient appeared to have a sensitivity to the NMBA and experienced residual paralysis for almost 2
hours after the NMBA was administered. The patient received sugammadex and recovered to a TOFR of 0.90 in 69 seconds. The authors described the reversal as very efficient.31 In another report, a 59-year-old male with myotonic dystrophy presented for surgery. (Patients with this disease have a high perioperative risk for profound respiratory and cardiac depression from the anesthetic and they may also have unpredictable responses to NMBAs.) The patient received 30 mg of rocuronium intraoperatively. Post-surgery, the patient did not show a return of TOF response. Sugammadex 150 mg was administered and the TOF showed 4 equal twitches. The patient was extubated and he was awake
and sitting within 10 minutes.32
CONCLUSION
There is a high incidence of postoperative residual paralysis in patients undergoing surgical procedures, and this paralysis may lead to serious adverse events. Monitoring of neuromuscular blockade and muscle strength is, therefore, important for patients receiving an NMBA. Although residual paralysis is a common postoperative complication, pharmacists may not be aware of the frequency and potential morbidity and mortality associated with the use of NMBAs. Additionally, reversal agents, both the anticholinesterases and the new selective relaxant binding agent, have unique dosing regimens that are based on the monitoring and assessment of neuromuscular function. In order to conduct medication use evaluations, prepare pharmacy budgets, and recommend dosing, pharmacists need to understand the use of these agents. The new selective relaxant binding agent, sugammadex, has several advantages over anticholinesterase drugs, including rapid response to recovery of a TOFR of 0.9 or greater, no need for antimuscarinic agents, and effectiveness in profound neuromuscular blocks.
REFERENCES
- Raghavendra T. Neuromuscular blocking drugs: discovery and development. J R Soc Med. 2002;95(7):363-367.
- McManus MC. Neuromuscular blockers in surgery and intensive care, part 1. Am J Health Syst Pharm. 2001;58(23):2287-2299.
- Beecher HK, Todd DP. A study of deaths associated with anesthesia and surgery: based on a study of 599,548 anesthesias in ten institutions 1948-1952, inclusive. Ann Surg. 1954;140(1):2-35.
- McManus MC. Neuromuscular blockers in surgery and intensive care, part 2. Am J Health Syst Pharm. 2001;58(24):2381-2395.
- Naguib M, Lien CA, Meistelman C. Chapter 34: Pharmacology of Neuromuscular Blocking Drugs. in Miller RD, ed. Miller's Anesthesia. 8th ed. Philadelphia, PA: Elsevier Saunders; 2015:958-994.
- Kopman AF, Naguib M. Laparoscopic surgery and muscle relaxants: is deep block helpful? Anesth Analg. 2015;120(1):51-58.
- Hunter JM. New neuromuscular blocking drugs. N Engl J Med. 1995;332(25):1691-1699.
- Appiah-Ankam J, Hunter J. Pharmacology of neuromuscular blocking drugs. Contin Ed Anaesth Crit Care Pain. 2004;4(1):2-7.
- Stoelting RK. Monitoring of neuromuscular blockade: what would you expect if you were the patient? The Official Journal of the Anesthesia Patient Safety Foundation. 2016;30(3):45-47.
- Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372(9633):139-144.
- Grosse-Sundrup M, Henneman JP, Sandberg WG, et al. Intermediate acting non-depolarizing neuromuscular blocking agents and risk of postoperative respiratory complications: prospective propensity score matched cohort study. BMJ. 2012;345:e6329.
- Murphy GS, Szokol JW, Marymont JH, et al. Residual paralysis at the time of tracheal extubation. Anesth Analg. 2005;100(6):1840-1845.
- Plaud B, Debaene B, Donati F, Marty J. Residual paralysis after emergence from anesthesia. Anesthesiology. 2010;112(4):1013-1022.
- Viby-Mogensen J, Jorgensen BC, Ording H. Residual curarization in the recovery room. Anesthesiology. 1979;50(6):539-541.
- Naguib M, Kopman AF, Lien CA, et al. A survey of current management of neuromuscular block in the United States and Europe. Anesth Analg. 2010;111(1):110-119.
- Brull SJ, Prielipp RC. Reversal of neuromuscular blockade: "identification friend or foe". Anesthesiology. 2015;122(6):1183-1185.
- Kopman AF, Yee PS, Neuman GG. Relationship of the train-of-four fade ratio to clinical signs and symptoms of residual paralysis in awake volunteers. Anesthesiology. 1997;86(4):765-771.
- Donati F. Residual paralysis: a real problem or did we invent a new disease? Can J Anaesth. 2013;60(7):714-729.
- Fuchs-Buder T, Meistelman C, Raft J. Sugammadex: clinical development and practical use. Korean J Anesthesiol. 2013;65(6):495-500.
- Hammermeister KE, Bronsert M, Richman JS, Henderson WG. Residual neuromuscular blockade (NMB), reversal, and perioperative outcomes. The Official Journal of the Anesthesia Patient Safety Foundation. 2016;30(3):74-75.
- Brull S, Naguib M, Miller RD. Residual neuromuscular block: rediscovering the obvious. Anesth Analg. 2008;107(1):11-14.
- Donati F. Sugammadex: a cyclodextrin to reverse neuromuscular blockade in anaesthesia. Expert Opin Pharmacother. 2008;9(8):1375-1386.
- Jahr JS, Miller JE, Hiruma J, et al. Sugammadex: a scientific review including safety and efficacy, update on regulatory issues, and clinical use in Europe. Am J Ther. 2015;22(4):288-297.
- Partownavid P, Romito BT, Ching W, et al. Sugammadex: a comprehensive review of the published human science, including renal studies. Am J Ther. 2015;22(4):298-317.
- Bridion [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2015.
- Blobner M, Eriksson LI, Scholz J, et al. Reversal of rocuronium-induced neuromuscular blockade with sugammadex compared with neostigmine during sevoflurane anaesthesia: results of a randomized, controlled trial. Eur J Anaesthesiol. 2010;27(10):874-881.
- Khuenl-Brady KS, Wattwil M, Vanacker BF, et al. Sugammadex provides faster reversal of vecuronium-induced neuromuscular blockade compared with neostigmine: a multicenter, randomized, controlled trial. Anesth Analg. 2010;110(1):64-73.
- Lemmens HJ, El-Orbany MI, Berry J, et al. Reversal of profound vecuronium-induced neuromuscular block under sevoflurane anesthesia: sugammadex versus neostigmine. BMC Anesthesiol. 2010;10(1):15.
- Della Rocco G, Pompei L, Pagan de Paganis C, et al. Reversal of rocuronium induced neuromuscular block with sugammadex or neostigmine: a large observational study. Acta Anaesthesiol Scand. 2013;57(9):1138-1145.
- Paton F, Paulden M, Chambers D, et al. Sugammadex compared with neostigmine/glycopyrrolate for routine reversal of neuromuscular block: a systematic review and economic evaluation. Br J Anaesth. 2010;105(5):558-567.
- Vilela H, Santos J, Colaço J, et al. Reversal of neuromuscular blockade with sugammadex in a patient with spinal muscular atrophy type III (Kugelberg-Welander syndrome). J Anesth. 2012;26(2):306-307.
- Baumgartner P. Rocuronium and sugammadex in myotonic dystrophy. Anaesth Intensive Care. 2010;38(5):959-960.
Back to Top