
Expired activity
Please go to the PowerPak
homepage and select a course.
Hyponatremia in the Critically Ill Hospitalized Patient
Overview
Hyponatremia is the most common electrolyte imbalance encountered in clinical practice and is especially common in older patients with multiple comorbidities. Defined as serum sodium levels of less than 135 mmol/L or 135 mmEq/L, hyponatremia occurs in up to 30% of hospitalized patients and 7% of patients presenting for ambulatory care.1 Rates are especially high among patients with heart failure (27%),2 cirrhosis (50%)3 and pneumonia (28%)4 and in institutionalized geriatric patients, where it can reach 53%. More than 40% of elderly patients with hyponatremia develop the imbalance during treatment in the hospital. 5 Common in intensive care units, neurosurgical care units and among HIV infected patients, it can also be seen in the outpatient setting among patients with chronic lung disease, on anti-seizure medications, antidepressants and thiazide diuretics.
While the threshold of 135 mmol/L is commonly accepted for hyponatremia, some have suggested revising the definition to serum sodium less than 138 mmol/L, as patients begin to experience increased mortality at this level. By this definition, 38% of hospitalized patients would present with or develop hyponatremia during their stay.6 Others have recommended a more conservative standard of serum sodium less than 130 mmol/L, the point at which patients typically become clearly symptomatic. At this lower standard, the imbalance would affect just 1%-4% of admitted patients.1
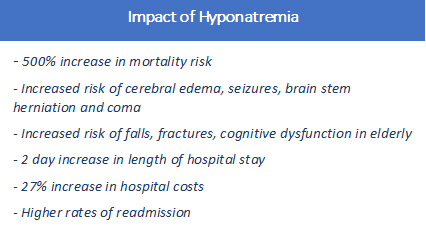
Among hospitalized patients, hyponatremia significantly increases mortality risk, length of stay and cost of care. Hyponatremia doubles the hospital mortality rate and increases the risk of requiring intensive care or mechanical ventilation by nearly 70% compared to patients with normal sodium levels.7 It is an independent prognostic factor for elevated morbidity and mortality in cardiac, hepatic, renal and neurologic conditions among others.8 Among the elderly, even mild, apparently asymptomatic hyponatremia is associated with gait instability, osteoporosis, falls, hip and femur fractures and cognitive dysfunction.9, 10,11 Severe hyponatremia can lead to cerebral edema, seizures, coma and death.
A 2013 study using National Health and Nutrition Examination Survey data found a 500% increase in mortality risk in individuals with hyponatremia compared to those with normal sodium levels, even in the absence of any identified comorbidities or hospitalization. Further, the research suggested that "even small derangements in serum sodium in the general population are clinically significant" and "support the growing body of evidence that mild hyponatremia is not benign."12
Hyponatremia poses a substantial economic as well as clinical burden, with estimated annual cost of treating hyponatremia in the US of $1.6 to $3.6 billion.13 The electrolyte imbalance is also associated with a two-day average increase in hospital length of stay, 27% increase in hospital costs and higher rates of readmission.14,15 Despite the significant risks posed by low serum sodium and ready access to effective therapies, more than 75% of patients hospitalized with hyponatremia leave the hospital still hyponatremic.16
Presentation and diagnosis
Hyponatremia is defined as:
- Mild at serum sodium concentration between 130 and 135 mmol/L
- Moderate at serum sodium concentration between 125 and 130 mmol/L
- Severe (or profound) at serum sodium concentration below 125 mmol/L
Symptoms and signs of hyponatremia depend not only upon the severity of the hyponatremia but also its duration and underlying disease states that might affect the clinical presentation. Patients may present with nausea and vomiting, headache, weakness, delirium and confusion, gait instability, lethargy, weakness, depressed reflexes, irritability, abdominal pain, myalgias, cramps, dizziness, and dysarthria.17 Sudden onset and serum sodium less than 125 mmol/L put patients at high risk of more severe manifestations of cerebral edema and increased intracranial pressure including deep somnolence, seizures, coma, respiratory arrest, brain-stem herniation and death.18 (See Table 1.)
Patients with intracranial disease processes, including tumors, hematoma or resection cavities may be more susceptible to symptoms and signs of hyponatremia at higher levels of serum sodium than those without neurological conditions. Generally, in patients with serum sodium greater than 130 mmol/L, consideration of alternate or additional causes should be considered in the presence of more severe neurological symptoms.19
| Table 1: Hyponatremia Symptoms |
| Classification* |
Serum Sodium |
Neurological Symptoms |
| Severe |
<125 mmol/L |
Vomiting, seizures, obtundation, deep somnolence, respiratory distress, coma |
| Moderate |
<130 mmol/L |
Nausea, delirium or confusion, weakness, disorientation, altered mental status, weakness, depressed reflexes, gait instability or falls |
| Mild or "asymptomatic" |
<135 mmol/L |
Headache, irritability, difficulty concentrating, altered mood, gait irregularities |
| * Current guidelines weigh symptomology as more important in classification than serum sodium levels, although severe symptoms with serum sodium >130 mmol/L should be investigated for other causes. |
Acute or Chronic?
When hyponatremia lasts more than 48 hours, it is considered chronic. Over that period of time, the brain adapts to maintain its volume and symptoms may subside. Careful study employing psychological testing and a thorough history, however, shows that many patients continue to have subtle cognitive deficits, difficulty concentrating, more frequent falls and gait irregularities.11
Given the difficulty of determining the length of time many patients have had hyponatremia upon presentation to a hospital, the guidelines issued by the European society of Intensive Care Medicine, the European Society of Endocrinology and the European Renal Association-European Dialysis and Transplant Association recommend that all patients not definitively known to have developed hyponatremia within the previous 48 hours should be assumed to have the chronic condition. 19
Assessment
In addition to a thorough history, review of medications and recent intravenous fluids,20 and neurological assessment, three laboratory tests help confirm and classify hyponatremia:
- Urine osmolality distinguishes between an inability of the kidneys to appropriately dilute urine (>100 mOsm/kg) or excess hypotonic fluid intake, which may occur in potomania, psychogenic polydipsia and in over-hydrating athletes (≤100 mOsm/kg).
- Serum osmolality enables differentiation between true (hypotonic) hyponatremia and pseudohyponatremia, which is typically a lab error caused by hyperlipidemia or hyperproteinemia interfering with sodium measurement. Serum osmolality will also identify hypertonic hyponatremia caused by hyperglycemia or elevated levels of mannitol, glycine, sucrose and maltose.
- Urinary sodium concentration tells a clinician whether the likely cause is syndrome of inappropriate antidiuretic hormone secretion (SIADH) or salt-wasting syndrome (>30 mmol/l) or is secondary to hypovolemia (≤30 mmol/l).21
Pathophysiology
Hyponatremia develops from aberrations in the intake and retention of water. In the average adult, total body water represents 50% to 70% of total body weight. Approximately two-thirds of total body water is contained within the intracellular compartment, while one-third is distributed in the interstitial and intravascular spaces. Water moves between cellular compartments in response to differences in plasma osmolality, which is largely determined by sodium concentration.22 The most severe symptoms of hyponatremia occur from low plasma sodium concentrations leading to an influx of water into the brain, resulting in swelling of the cells, with neurological sequalae. In extreme cases, the cranium's rigidity limits expansion of the cells and causes cerebral herniation.
A number of physiological processes, including the vasopressin system, thirst and the autonomic nervous system normally regulate both free water intake and retention to maintain the total body water within a narrow range. Osmoreceptors in the anterior hypothalamus respond to rises in osmolality and discharge, causing neurons to release arginine vasopressin (also called vasopressin, AVP, antidiuretic hormone or ADH) into circulation. The higher the osmolality, the more vasopressin is released. Baroreceptors in the aorta, carotid arteries and juxta glomerular apparatus react to decreased arterial blood pressure by stimulating the release of AVP as well, which can occur concurrently with hypo- osmolality. AVP increases renal free water reabsorption, which increases plasma water, reduces plasma sodium concentration and raises blood pressure. AVP acts by binding to the vasopressin type 2 (V2) receptor in the kidney's collecting tubule. As plasma water levels rise, AVP secretion drops and the collecting tubule stops absorbing water. 22
This water regulatory system may fail at several points, resulting in different kinds of hyponatremia. Hypotonic hyponatremia may be classified as hypovolemic, euvolemic or hypervolemic:
- Hypovolemic hyponatremic patients have a diminished total body water and a further diminished total body sodium. Hypovolemia is likely in patients exhibiting vomiting or diarrhea, orthostatic hypotension, dry mucus membranes and decreased skin turgor. Low sodium levels may be caused by vomiting and diarrhea, excessive sweating, third spacing (burns, bowel obstruction, pancreatitis, pulmonary edema, ascites, mast cell disease), non-steroidal anti- inflammatory drugs, thiazide diuretics, furosemide, primary adrenal insufficiency and cerebral salt wasting syndrome.
- Euvolemic hyponatremic patients have an increased total body water but a normal total body sodium. Common causes are syndrome of inappropriate antidiuretic hormone (SIADH), hypothyroidism, glucocorticoid deficiency, stress, certain prescription and illegal drugs (see table), excessive fluid intake and low solute intake.
- Hypervolemic patients with hyponatremia have an increased total body water and a somewhat increased or stable total body sodium. Patients with edema should be assumed to be hypervolemic. Common causes include congestive heart failure, cirrhosis, low arterial blood volume, chronic kidney disease and nephrotic syndrome.23,24
| Table 2: Types of Hyponatremia |
| Type |
Presentation |
Causes |
| Hypovolemic |
Vomiting or diarrhea, orthostatic hypotension, dry mucus membranes, decreased skin turgor |
Gastrointestinal loss via diarrhea and emesis, renal loss from diuretics or adrenal insufficiency, excessive sweating, third spacing, NSAIDs, burns, cerebral salt wasting syndrome |
| Euvolemic |
Assume euvolemia in absence of signs of volume depletion or expansion |
SIADH, hypothyroidism, glucocorticoid insufficiency, reset osmostat, stress, prescription or illegal drugs, excessive fluid intake, low solute intake (anorexia, low sodium diet, "tea and toast" diet) |
| Hypervolemic |
Edema, ascites, pulmonary congestion |
Congestive heart failure, cirrhosis, low arterial blood volume, chronic kidney disease, nephrotic syndrome |
Treatment
Managing hyponatremia remains challenging. Appropriate therapy depends on the underlying etiology, comorbidities, duration of condition, degree of biochemical imbalance and severity of symptoms, not all of which are easily assessed. In addition, recommendations and guidelines for treatment vary based on the authors' relative weighting of concerns about potential overcorrection of hyponatremia and risk of osmotic demyelination versus the risk of cerebral edema from inadequate treatment.
It is essential to remember that patients who have hyponatremia usually have an underlying disorder or process that is dynamic. There is usually an initiation phase, a ramping up of the severity of the underlying disorder leading to the hyponatremia, a maintenance phase and a recovery phase. It is critical to ascertain where the patient might be in the course of the underlying illnesses when initiating and evaluating responses to management of the hyponatremia. For example, some patients with SIADH are diagnosed early on when the disorder has not yet become as severe as it will become over the following few days. Initial management might not seem to be effective because the disorder is worsening. Treating physicians might feel the diagnosis is incorrect when all that is necessary is to be more aggressive in the management of the disease process and the hyponatremia. Conversely, some patients present with hyponatremia after the SIADH has resolved and all that has to be done is to rid the body of the excess water.
Severe symptoms
In patients with severe or moderately severe symptoms, reducing the risk of brain herniation should be the clinician's primary focus. Regardless of the duration of the hyponatremia, both recent American expert panel recommendations and European guidelines recommend prompt, aggressive treatment in the presence of nausea, confusion, headache, vomiting, seizures, deep somnolence, cardiorespiratory distress or coma. Postoperative patients, over-hydrated athletes, and those that have used methylenedioxy-N-methamphetamine (MDMA) can very quickly deteriorate from headache and vomiting to seizures or death from severe cerebral edema.19,24
 For acute hyponatremia with moderately severe to severe symptoms, the guidelines recommend immediate intravenous infusion of 100-150 ml 3% saline over 10-20 minutes and checking serum sodium concentration. Up to three rounds can be administered. The goal is to raise the patient's sodium concentration 5 mmol/L,19 which has been shown to reverse severe symptoms and reduce intracranial pressure by nearly 50% in an hour.25
For acute hyponatremia with moderately severe to severe symptoms, the guidelines recommend immediate intravenous infusion of 100-150 ml 3% saline over 10-20 minutes and checking serum sodium concentration. Up to three rounds can be administered. The goal is to raise the patient's sodium concentration 5 mmol/L,19 which has been shown to reverse severe symptoms and reduce intracranial pressure by nearly 50% in an hour.25
If the target increase is achieved in the first hour and symptoms improve, the guidelines recommend infusing a small volume of 0.9% saline until the cause of the hyponatremia is identified and treatment begun. If symptoms have not improved, clinicians are advised to continue the hypertonic saline solution until symptoms do improve or serum sodium rises 10 mmol/L total or reaches a safe serum sodium level, whichever comes first. For other patients, clinicians will need to address the cause of the hyponatremia by discontinuing drugs, replacing hormones or treating underlying infections, for instance.
For patients with chronic hyponatremia or hyponatremia of unknown duration and severe symptoms, the American panel of experts recommended hypertonic 3% saline infusion with a target of increasing sodium serum levels by 4-8 mmol/L within the first six hours of treatment, with no further increase above 6 mmol/L in a 24-hour period. Patients at high risk of osmotic demyelination syndrome have a lower target of 4-6 mmol/L per day and a limit of 8 mmol/L in any 24-hour period. Those with normal risk of demyelination have an upper limit for increase of sodium concentration of 10-12 mmol/L per day or 18 mmol/L in 48 hours.24
Mild symptoms
Treatment for mildly or moderately symptomatic patients varies by fluid volume.
- For hypovolemic patients, as volume increases, vasopressin will decrease and plasma sodium will normalize. Isotonic saline of 0.9% of 0.5-1.0 mL/kg per hour is generally recommended. 26 Salt tablets may be used as well. Correction of underlying causes (discontinuation of diuretics, replacement of thyroid hormone or mineralocorticoid administration) may result in rapid increase of sodium concentrations, so serum sodium should be monitored every six hours to reduce the risk of demyelination. Monitoring of urine production may alert a clinician to overcorrection if it exceeds 100 mL per hour.27
- For hypervolemic hyponatremia, the first line therapy with mild or moderate symptoms is fluid restriction, which is notoriously difficult to achieve and maintain. Fluid restriction may be prescribed in an amount that is about 500 mL less than the urine output on the prior day. Patients with nephrotic syndrome, chronic kidney disease and cirrhosis may benefit from water and salt restrictions as well as diuretics and albumin. Patients in renal failure will require dialysis.28
- Patients with euvolemic hyponatremia and mild to moderate symptoms also generally start with fluid restriction, as above, and treatment of contributing conditions. Salt and protein should not be restricted. Determining whether a patient is euvolemic or hypovolemic can pose a challenge for clinicians. The most common cause of euvolemic hyponatremia, SIADH involves an unregulated secretion of AVP despite serum hypotonicity. Causes of SIADH include malignancies, pulmonary diseases and central nervous system disorders as well as some genetic mutations and a number of medications. Since most patients with SIADH have low urine output, fluid restriction is frequently intolerable.29 See Table 3 for common causes of SIADH.
Agents including demeclocycline (off label), lithium and urea may be used, but have weak support for generalized use and, in the case of lithium and demeclocycline, some significant side effects including renal and hepatic toxicity.26 Vasopressin receptor antagonists are newer therapies approved for patients with euvolemic and hypervolemic hyponatremia. See below for discussion.
| Table 3: Causes of Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH)30 |
| Malignancies |
Pulmonary Disorders |
CNS Disorders |
Drugs |
Other |
| Carcinomas, leukemia, lymphoma, neuroblastoma, mesothelioma, Ewing sarcoma, thymoma |
Abscess, acute respiratory failure,
Aspergillosis, asthma, atelectasis, bronchitis, COPD, cystic fibrosis, empyema, fibrosis, pneumonia, positive pressure ventilation, sarcoidosis, tuberculosis |
Acute psychosis, Porphyria, brain abscess, cerebellar and cerebral atrophy, delirium tremens, epilepsy, encephalopathy, encephalitis, Guillain-Barre syndrome, head trauma, hydrocephalus,
Multiple sclerosis, perinatal hypoxia, Rocky Mountain spotted fever,
Schizophrenia, subarachnoid hemorrhage, subdural hematoma, ventriculoatrial shunt obstruction |
Acetylcholine,
antineoplastics, barbiturates, bromocriptine, carbachol, chlorpropamide, clofibrate, cyclopropane, dibenzazepine, halothane, haloperidol, histamine, isoproterenol, lorcainide, MDMA/Ecstasy, opiates, oxytocin, nicotine, nitrous oxide, NSAIDs, phenothiazine, thiopental, MAOIs, SSRIs, tricyclic antidepressants, vasopressin analogs |
Exercise-induced hyponatremia, HIV, genetic mutation, general anesthesia, idiopathic, nausea, pain, stress |
Osmotic Demyelination Syndrome
The most current guidelines take a more conservative approach to raising serum sodium in patients with severe hyponatremia than earlier recommendations, largely in an effort to reduce the number of patients who suffer from central or osmotic demyelination syndrome following treatment. Central demyelination is a complex disorder that results from neuroinflammation as a consequence of a rapid correction of hyponatremia. A sharp increase in sodium concentration does not allow the brain sufficient time to reverse the adaptations made in chronic hyponatremia, particularly the extrusion of solutes.31 Patients at greatest risk of osmotic demyelination syndrome are those with chronic hyponatremia with serum sodium below 120 mmol/L and those with alcoholic cirrhosis, malnutrition, severe hypokalemia, liver disease and severe burns.32
Osmotic demyelination syndrome may occur several days after the rapid rise in serum sodium in a patient with chronic hyponatremia. These symptoms and signs may include mutism, dysarthria, lethargy, obtundation, behavioral changes, movement difficulties, spasticity, dysphagia, impaired gag reflex and pseudobulbar palsy and death.32,33
In the event serum sodium rises too quickly or too high, providers should immediately take steps to reduce the risk of demyelination. Active reduction in serum sodium through administration of 2-4 mcg of desmopressin with oral water or intravenous 5% dextrose given at 3 mL/kg per hour every eight hours in patients who have experienced an unexpectedly rapid increase in serum sodium or have exceeded daily limits of increase can mitigate risk.26 (See Table 4.)
| Table 4: Osmotic Demyelination Syndrome |
| Risk Factors |
Symptoms |
- Chronic hyponatremia with serum sodium below 120 mmol/L
- Alcoholic cirrhosis, liver disease, severe burns, severe hypokalemia, malnutrition |
- Symptom improvement followed by emergence within a few days of new neurological deficits
- Behavioral changes, mutism, altered consciousness, lethargy
- Movement difficulties, spasticity, dysarthria, pseudobulbar palsy, dysphagia, impaired gag reflex |
Use of Vaptans
Vasopressin receptor antagonists, also known as "vaptans," bind to the vasopressin receptor and thus prevent binding of vasopressin, directly addressing the cause of SIADH. Two drugs in this class—conivaptan and tolvaptan—have received U.S. Food and Drug Administration (FDA) approval for patients with euvolemic hyponatremia and patients with euvolemic or hypervolemic hyponatremia who do not respond to fluid restriction. Both are absolutely contraindicated in hypovolemic hyponatremia.34 They are primarily used in moderate to mild cases of hyponatremia as hypertonic saline remains the treatment of choice for severe hyponatremia.
The vaptans promote excretion of water only rather than water and solutes as seen in diuretics. These drugs result in a reversible, transient nephrogenic diabetes insipidus leading to an aquaresis, or free water clearance, that effectively increases the serum osmolarity and serum sodium concentration.34 The bulk of the water excreted comes from intracellular water. Notably, vaptans do not require fluid restriction to be effective in most cases, although thirst may lead to increased fluid intake.35 Both agents appear to safely reverse hyponatremia; no patients in the clinical trials for conivaptan or tolvaptan experienced osmotic demyelination.
Because of concerns about potential adverse effects associated with a rapid increase in sodium levels and the need for titration, the FDA issued a "black box warning" indicating that tolvaptan should be started in the hospital with close monitoring of serum sodium.36 The FDA also noted that about 9% of participants in the clinical trials for conivaptan experienced an increase of serum sodium in excess of the recommended 12 mmol/L in 24 hours, though none had evidence of osmotic demyelination or other permanent neurologic sequelae.37
Serum sodium levels return to normal within 2-4 days in most patients treated with vaptans. Patients with ongoing or active SIADH may require continued therapy, while those with SIADH that has resolved typically require one to two days of treatment. Because a rapid rise in serum sodium has been seen in 8%-10% of patients,38 physicians should monitor serum sodium and volume every four to six hours during administration and not restrict access to water. Patients at high risk of osmotic demyelination will need more frequent monitoring.
Conivaptan is administered intravenously and reversibly binds to both V1 and V2 receptors. It is particularly useful in the ICU setting but can be utilized on general medical floor under close supervision. It is administered as 20 mg loading dose in 30 minutes, followed by continuous intravenous infusion of 20 mg for 24 hours and increasing to 40 mg for up to four days, if needed. See Table 5 for treatment summary.
Clinical trials found that conivaptan safely raised serum sodium levels in 70% to 80% of treated patients. Peak aquaresis occurs at two to four hours, producing a 700% increase in urine flow rate and reduction in urinary osmolality. The aquaretic effect continues for 12 hours. A trial of intravenous conivaptan in euvolemic patients found the drug effectively raised mean serum sodium in 24 hours. An open-label multicenter trial found that increases in serum sodium persisted at 34 days after four days of treatment with conivaptan.39,40
Conivaptan may increase the risk of toxicity of several statins and other medications with strong CYP3A4 inhibitors including midazolam, digoxin and amlodipine. Co-administration of conivaptan with ketoconazole, intraconazole, ritanovir, indinavir or clarithromycin can increase conivaptan's potential toxicity and is contraindicated.39,40
Tolvaptan is administered orally and binds to the V2 receptor. Effective doses range between 15-60 mg daily. Tolvaptan can be used in the outpatient setting for up to 30 consecutive days. Two randomized clinical trials, SALT-1 and SALT-2, compared tolvaptan to placebo with favorable results in euvolemic and hypervolemic patients with mean baseline plasma sodium concentrations of 128 mmol/L.41 Patients were not required to restrict fluid intake. Those taking tolvaptan experienced a greater increase in serum sodium concentration, with the sharpest rise seen in those with the lowest baseline levels. Comparison of baseline and end of study results of the SF-12 Mental Component Score readings demonstrated a symptomatically beneficial improvement. In less than 2% of cases, serum sodium concentration increases exceed the maximum recommended rate of 0.5 mmol/L per hour.34 Tolvaptan may cause abnormal liver function tests when used in high doses and, as a result, this drug should be continued with caution, if at all, in patients with hepatic dysfunction.
| Table 5: Vasopressin Receptor Antagonists |
| Drug |
Route |
Receptor |
Effective Doses |
Duration |
| Conivaptan |
IV |
V1 and V2 |
20 mg loading dose, followed by 20-40 mg/day |
4 days |
| Tolvaptan |
Oral |
V2 |
15-60 mg/day |
30 days |
| Source: Adapted from N Engl J Med. 2007. 356:2064-2072 |
Collaboration in care
While guidelines and recommendations assist in diagnosis and treatment, clinicians must continue to employ their experience, clinical judgment and knowledge of the individual patient to ensure quality care and positive outcomes. In addition, a team approach to hyponatremia may provide the best results.
Clinical pharmacists can also significantly contribute to care of patients with hyponatremia by identifying patients at risk and the source of hyponatremia, monitor serum sodium concentration and advise on the selection of appropriate therapeutic agent, dosage and duration. Because many drugs may contribute to hyponatremia, a careful review of prescribed medications may identify the cause of the electrolyte imbalance and contribute to prompt resolution.
The importance of collaborative care to ensure best practices in hyponatremia can be seen in the results of study at a teaching hospital that found that of 104 patients with hyponatremia, just 26% had plasma osmolality tested, 27% had urine osmolality assessed and 10% had urinary sodium measured. Nearly half of the patients had diagnoses inconsistent with the clinical data and a third experienced significant errors in management—which doubled the mortality rates compared to those whose hyponatremia was properly treated. The authors concluded that "severe hyponatraemia is a serious condition, but its investigation and evaluation is often inadequate."42 As this study illustrates, everyone on the patient's care team needs to be vigilant about proper diagnosis, treatment and monitoring of hyponatremia. Collaboration of physicians, pharmacists and other involved clinicians provides the necessary attention this serious condition deserves and improves both care and outcomes for the many patients affected by it.
REFERENCES
- Hawkins RC. Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta. 2003 Nov;337(1-2):169-72.
- Bettari L, Fiuzat M, Shaw LK, et al. Hyponatremia and long-term outcomes in chronic heart failure—an observational study from the Duke Databank for Cardiovascular Diseases. J Card Fail. 2012; 18: 74–81.
- Angeli P, Wong F, Watson H. et al. Hyponatremia in cirrhosis: results of a patient population survey. Hepatology. 2006; 44: 1535–1542.
- Nair V, Niederman MS, Masani N, Fishbane S: Hyponatremia in community-acquired pneumonia. Am J Nephrol. 2007, 27: 184-90.
- Cumming K, Hoyle GE, Hutchison JD, Soiza RL. Prevalence, incidence and etiology of hyponatremia in elderly patients with fragility fractures. PLoS One. 2014 Feb 5;9(2):e88272.
- Wald, R., Jaber, B.L., Price, L.L. et al. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med. 2010; 170: 294–302.
- Zilberberg MD, Exuzides A, Spalding J, et al. Hyponatremia and hospital outcomes among patients with pneumonia: a retrospective cohort study. BMC Pulm Med. 2008 Aug 18; 8:16
- Jao GT, Chiong JR. Hyponatremia in acute decompensated heart failure: mechanisms, prognosis, and treatment options. Clin Cardiol. 2010. 33(11):666-671.
- Soiza RL, Talbot HS. Management of hyponatremia in older people: old threats and new opportunities. Ther Adv Drug Saf. 2011;2(1):9-17.
- Usala RL, Fernandez SJ, Mete M, et al. Hyponatremia is associated with increased osteoporosis and bone fractures in a large US health system population. J Clin Endocrinol Metab. 2015;100(8):3021-3031.
- Renneboog, B., Musch, W., Vandemergel, X. et al. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119:71
- Mohan S, Gu S, Parikh A, Radhakrishnan J. Prevalence of hyponatremia and association with mortality: results from NHANES. Am J Med. 2013 Dec;126(12):1127-37.e1.
- Boscoe A, Paramore C, Verbalis JG. Cost of illness of hyponatremia in the United States. Cost Eff Resourc Alloc. 2006;4:10.
- Callahan MA, Do HT, Caplan DW, Yoon-Flannery K. Economic impact of hyponatremia in hospitalized patients: a retrospective cohort study. Postgrad Med. 2009 Mar;121(2):186-91.
- Deitelzweig S, Amin A, Christian R, et al. Health care utilization, costs and readmission rates associated with hyponatremia. Hosp Pract. 2013;41:89-95.
- Greenberg A, Verbalis JG, Amin AN, et al. Current treatment practice and outcomes. Report of the hyponatremia registry. Kidney Int. 2015;88(1): 167-177.
- Olsson K, Ohlin B, Melander O. Epidemiology and characteristics of hyponatremia in the emergency department. Eur J Intern Med. 2013;24:110-116.
- Edmonds NZ. Pathophysiology, impact, and management of hyponatremia. J Hosp Med. 2012;7(Suppl 4):S1-S5.
- Spasovski G, Vanholder R, Allolio B, et al. Hyponatraemia Guideline Development Group. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol. 2014 Feb 25;170(3):G1-47.
- Haskal R. Current issues for nurse practitioners: hyponatremia. J Am Acad Nurse Practitioners. 2007; 19:563-579.
- Simon E. Hyponatremia. Medscape. 2016 July 26.
- Freda BJ. Hyponatremia and hypernatremia. Cleveland Clinic. August 2010.
- Decaux G, Musch W. clinical laboratory evaluation of the syndrome of inappropriate secretion of antidiuretic hormone. CJASN. 2008 July;3(4):1175-1184.
- Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 Suppl 1):S1-S42.
- Koenig, M.A., Bryan, M., Lewin, J.L. III et al. Reversal of transtentorial herniation with hypertonic saline. Neurology. 2008; 70: 1023–1029
- Verbalis JG, Grossman A, HÖybye C, et al. Review and analysis of differing regulatory indications and expert panel guidelines for the treatment of hyponatremia. Curr Med Res Opin. 2014;30:1201-1207.
- Braun M, Barstow C, Pyzocha N. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015 Mar 1;91(5):299-307.
- Pfenning CL, Slovis CM. Sodium disorders in the emergency department: A review of hyponatremia and hypernatremia. Emerg Med Pract. 2012;10:1-26.
- Laville M, Burst V, Peri a, Verbalis J. Hyponatremia secondary to the syndrome of inappropriate secretion of antidiuretic hormone (SIADH): therapeutic decision-making in real-life cases. Clin Kidney J. 2013 Nov;6(Suppl 1):i1-20.
- Thomas CP. Syndrome of Inappropriate Antidiuretic Hormone Secretion. Medscape. 2016 Oct 29.
- Allenman AM. Osmotic demyelination syndrome: Central pontine myelinolysis and extrapontine myelinolysis. Semin Ultrasound CT MR. 2014;35:153-159.
- Mount DB. The brain in hyponatremia: Both culprit and victim. Semin Nephrol. 2009;29:196– 215.
- Soupart A, Decaux G. Therapeutic recommendations for management of severe hyponatremia: Current concepts on pathogenesis and prevention of neurologic complications. Clin Nephrol. 1996;46:149–69.
- Robertson G. Vaptans for the treatment of hyponatremia. Nat Reviews Endo. 2011 March;7:151-161.
- Sherlock M, Thompson C. The syndrome of inappropriate antidiuretic hormone: current and future management options. Eur J Endocrinol. 2010 June 1;162:S13-18.
- Torres A, Wickham E, Biskobing D. Tolvaptan for the management of SIADH: lessons learned in the titration of dose. Endocr. Pract. 2011 Jul-Aug;17(4):397-100.
- U.S. FDA. Safety: Vaprisol (conivaptan) injection. Detailed view: safety labeling changes approved by FDA Center for Drug Evaluation and Research (CDER)-May 2010 and February 2012. February 2012.
- Li-Ng M, Verbalis J. Conivaptan: evidence supporting its therapeutic use in hyponatremia. Core Evid. 2009;4:83-92.
- Ghali J, Farh J, Daifallah S, Zabalawi H, Zmily H. Conivaptan and its role in the treatment of hyponatremia. Drug Des Devel Ther. 2009;3:253-268.
- Hline S, Pham PT, Pham PT, aung M, Pham PM, Pham PC. Conivaptan: a step forward in the treatment of hyponatremia? Ther Clin Risk Manag. 2008 Apr;4(2):315-326.
- Schrier R, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2 -receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099-2112.
- Rhoney D. Cost issues in hyponatremia. Hosp Pharm. 2011;46(12 Suppl 2):S30-38.
Back to Top