
Expired activity
Please go to the PowerPak
homepage and select a course.
Management of Short Bowel Syndrome: The Pharmacist’s Perspective
INTRODUCTION
Intestinal failure is defined as the inability to maintain adequate nutrition and hydration despite normal oral intake because of physical or functional loss of significant portions of the small intestine.1,2 Short bowel syndrome (SBS), one of the most common forms of intestinal failure, occurs when the length of functional small bowel is less than 200 cm in an adult. Common reasons leading to extensive intestinal resection include mesenteric vascular events that compromise intestinal blood flow, recurrent Crohn’s disease, trauma, volvulus, or complications from abdominal surgery.3 Patients with SBS typically experience severe diarrhea, steatorrhea, dehydration, malnutrition, nutrient deficiencies, and electrolyte abnormalities. The extent of malabsorption will vary depending on the length and location of remaining bowel, its functional status, and the length of time since last intestinal resection. The heterogeneity of patients with SBS is further complicated by the lack of a comprehensive database or diagnosis code (ICD-10) to identify and characterize patients accurately or estimate incidence and prevalenceof SBS. The actual incidence of SBS, a rare disorder, is unknown.1-4 Patients with SBS will often complain that the medical community fails to recognize the condition or appreciate its complexity.
The high degree of patient diversity creates a need for clinicians to individualize management of patients with SBS based on underlying gastrointestinal physiology. Medical management of SBS should focus on supportive care and symptom control. Uncontrolled diarrhea in patients with SBS can lead to significant fluid and nutrient loss and difficulty maintaining adequate body weight. Many of these patients are dependent on parenteral nutrition (PN), intravenous (IV) fluids, and/or vitamin and mineral supplementation to maintain adequate nutrition and hydration. Dietary modification and pharmacologic treatment options have critical roles in controlling diarrhea and potentially improving quality of life in patients with SBS. Surgical intervention may also be appropriate for some patients with SBS, including ostomy reversal (to use distal unused bowel), intestinal lengthening procedures, and intestinal transplantation. Patients with SBS challenge clinicians across all health care settings. This article focuses on medical management of adult patients with SBS from the pharmacist’s perspective.
PATHOPHYSIOLOGY
In adults, the normal small bowel is about 300 to 600 cm in length and consists of the duodenum (25–30 cm), the jejunum (160–200 cm), and the remainder is ileum. Because bowel length varies greatly from person to person, it is more informative to classify patients according to remaining rather than resected bowel length. This relies on measurement and documentation by the surgeon performing intestinal resection.2,5
The anatomic site and extent of intestinal resection helps determine the patient’s risk of developing fluid and electrolyte imbalances and/or nutritional deficiencies from malabsorption.3,6,7 The duodenum and jejunum are the primary sites of carbohydrate, protein, and fat absorption, and micronutrient (calcium, phosphorus, iron, and water-soluble vitamin) absorption.
The ileum serves as the primary site for absorption of bile salt, vitamin B12, and magnesium. Removal of more than 100 cm of distal ileum disrupts enterohepatic circulation of bile salts. Bile salts that are not absorbed in the distal ileum enter the colon and stimulate fat and water secretion, causing diarrhea. Fat malabsorption resulting from the bile salt deficiency in turn contributes to steatorrhea.
The distal ileum is also an important regulator of gastric emptying and small bowel transit time. Resection of distal intestine disrupts the release of certain mediators, such as peptide YY (PYY) and glucagon-like peptide-1 (GLP-1). PYY and GLP-1 are involved with slowing gastric emptying and intestinal transit.
The ileum/colon also releases glucagon-like peptide-2 (GLP-2), stimulates mucosal growth, and promotes nutrient and fluid absorption.
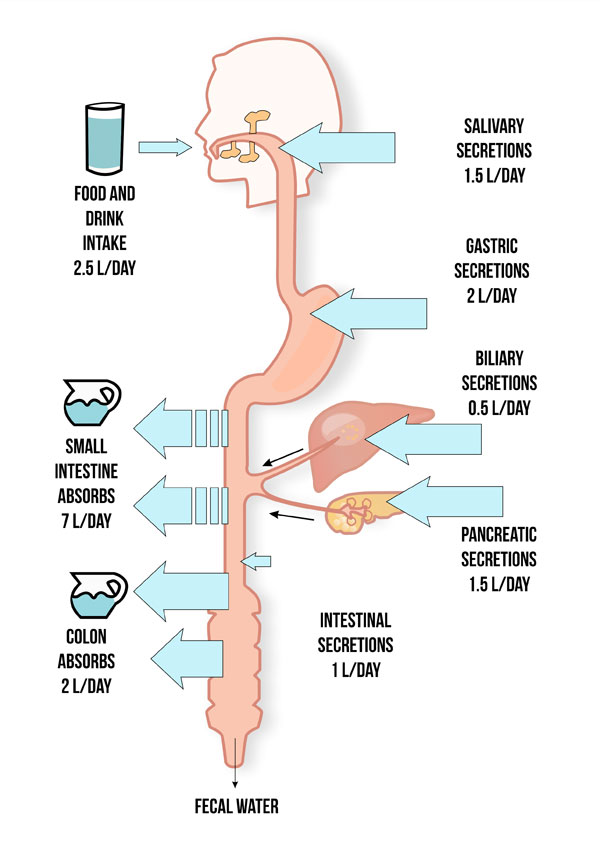
Fluid absorption occurs throughout the GI tract. Normally, individuals ingest 8–9 L/day in fluids, saliva, gastric, biliary, and pancreatic secretions (Figure 1).3,7 The small intestine absorbs about 7 L/day of fluid and the remaining 1–2 L/day pass into and are absorbed by the colon. The colon actually has the capacity to absorb up to 6 L/day of fluid.8 In addition, the colon can generate energy through colonic fermentation of nonabsorbed carbohydrate to produce short-chain fatty acids.9 The presence or absence of an intact colon and whether it is in continuity with the small intestine is important in determining severity of malabsorption. The presence or absence of an ileocecal valve, which links the ileum with the colon, may also be a factor in determining severity of malabsorption since it helps regulate intestinal transit time.6 The ileocecal valve extends the absorption time of nutrients within the intestinal mucosa and also physically prevents retrograde flow of colonic contents and bacteria into the small intestine.
Figure 1. Normal Intestinal Absorption and Secretion
 |
Types of SBS
Patients with SBS can generally be categorized into 3 surgical types: ileocolonic anastomosis, jejunocolonic anastomosis, and end-jejunostomy (Figure 2).6
Figure 2. Surgical Types of Short Bowel Syndrome
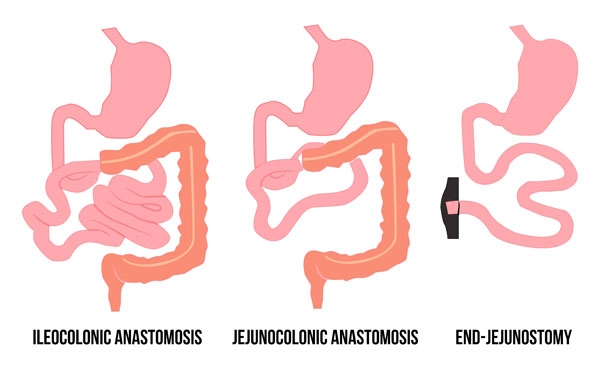 |
In patients with an ileocolonic anastomosis, a portion of the jejunum and/or partial ileum is resected and connected to an intact colon. In this situation, the risk of nutrient deficiency and likelihood of PN dependence is low.6
Patients with jejunocolonic anastomosis have undergone resection of the ileum and sometimes part of the colon with the jejunum connected to the colon. Patients with ileal resection do not do as well as patients with jejunal resection because of a more limited ability to adapt. They manifest diarrhea and are at risk for fat malabsorption, vitamin B12 deficiency, and fat-soluble vitamin deficiency. The likelihood of PN dependence varies, but generally more likely if length of remaining jejunum is less than 60 to 65 cm.6
Patients with an end-jejunostomy have undergone removal of the ileum and part of the jejunum, and the colon has been removed or is out of continuity. A stoma (an incised opening in the abdominal wall for drainage) connects to the jejunal remnant. These patients experience significant nutrient malabsorption and lack the benefits of fluid reabsorption and energy production that normally occurs in the colon. Magnesium deficiency is common since this mineral is normally absorbed in the ileum and colon. Patients with an end-jejunostomy experience impaired vitamin B12 and bile salt absorption, and accelerated gastric emptying and intestinal transit due to altered release of humoral and neural mediators. The likelihood of PN dependence is variable, but generally more likely if length of remaining jejunum is less than 115 cm.6
Intestinal Adaptation
Following extensive small bowel resection, the remaining intestine undergoes a process of adaptation that may result in structural and functional changes.10,11 These changes generally occur within the first 2 years following resection, although changes may continue over a longer period of time under certain conditions.10 The degree of intestinal adaptation that occurs is related to both the extent of resection and portion of bowel resected. As mentioned, the ileum is able to adapt structurally and functionally when jejunum is resected, whereas the jejunum is unable to adapt to the site-specific absorptive function of the ileum. Stimulation of remaining bowel with oral feeding or enteral nutrition is an essential component of intestinal adaptation. Clinicians encourage hyperphagia (consuming abnormally large quantities of food) in patients with SBS because it can enhance intestinal adaptation. The use of growth hormone and intestinal growth factors may also enhance intestinal adaptation.10 Conversely, use of certain medications (i.e., octreotide) may compromise intestinal adaptation.12
MEDICATION ABSORPTION
Pharmacists involved in the management of patients with SBS should be aware of its impact on medication absorption. Essentially all orally administered medications are absorbed in the small intestine, so clinicians must anticipate impaired medication absorption in patients with SBS.12 In addition, malabsorption of medications that rely on enterohepatic circulation may occur when more than 100 cm of ileum is removed. The extent of medication malabsorption is unpredictable and may be inconsistent day-to-day within the same patient. This may be due to variations in intestinal transit time that occur as dietary intake varies. Patients who have stomas may report the presence of unabsorbed tablet or capsule fragments within their ostomy output. Switching from a solid dosage form to an oral chewable or liquid formulation may be beneficial when this occurs, but this recommendation is theoretical and not evidence-based. Prescribers should avoid sustained-release or enteric-coated formulations in patients with SBS because limited intestinal surface area and accelerated transit time may alter the intended pharmacokinetic properties of these formulations.
Ultimately, patients with SBS may require higher than usual medication doses to achieve desired effect.12 Prescribers must titrate medication doses based on therapeutic response and employ measureable outcome, such as blood pressure, drug concentration, or other laboratory value, when possible and appropriate. Prescribers can consider alternative medication administration routes (e.g., transdermal, sublingual, rectal, and subcutaneous) when available. When all other routes of medication administration fail, the IV route may be indicated.
EARLY SBS MANAGEMENT: HOSPITAL TO HOME TRANSITION
Management of SBS in the immediate postoperative period should focus on fluid and electrolyte balance stabilization and nutritional status assessment. Most, if not all, of these patients will require PN for fluid, electrolyte, and nutrition supplementation. As oral diet resumes and advances, patients should expect increased stool output. Pharmacologic management of diarrhea in this acute care setting should include the initiation of antimotility and antisecretory agents. The type of SBS and length of remaining small bowel provides insight into the extent of malabsorption to be anticipated, and can be useful when developing the plan of care and establishing realistic patient expectations.
Parenteral Nutrition Care Plan
During the immediate postoperative period, the plan of care should specify fluid and electrolyte balance stabilization and appropriate nutrition support. The preferred route of nutrition support during this initial stage for patients newly diagnosed with SBS is typically PN. Enteral nutrition's utility is limited at this time because it can often make it more difficult to manage stool output and stabilize fluid and electrolyte balance. Even if a patient resumes oral diet within a few days following surgery, dietary intake may be insufficient to meet fluid and nutrient requirements attributable to malabsorption. The health care team should conduct a nutritional assessment when they initiate PN therapy to estimate protein (generally 1–2 g/kg/d) and calories requirements (25–30 kcal/kg/d).14 This calorie goal, which is modest, maintains weight during the inpatient stay. Further calorie adjustments are typically deferred to the outpatient setting based on achievement of targeted weight goals (e.g., weight gain, weight loss, or weight maintenance).
Patients with SBS are at risk for electrolyte deficiency due to high losses from stool output, especially magnesium and potassium, and metabolic acidosis resulting from loss of acetate. The PN regimen can be formulated to provide maintenance electrolyte requirements (Table 1) and replace ongoing electrolyte losses.14 The health care team should adjust doses based on close laboratory monitoring and intake/output records (Table 2).15 The goal is to stabilize the PN formulation and reduce laboratory monitoring frequency to no more than once weekly; this facilitates discharge to home on PN.
| Table 1. Standard Daily Electrolyte Requirements in Adult PN Formulations.14 |
| Electrolyte |
Dose |
| Sodium |
1–2 mEq/kg |
| Potassium |
1–2 mEq/kg |
| Magnesium |
8–20 mEq |
| Calcium |
10–15 mEq |
| Phosphorus |
20–40 mmol |
| Acetate |
As needed to maintain acid-base balance |
| Chloride |
As needed to maintain acid-base balance |
| Adapted from: Mirtallo J, Canada T, Johnson D, Kumpf V, Petersen C, Sacks G, Seres D, Guenter P. Safe practices for parenteral nutrition. JPEN J Parenter Enter Nutr. 2004;28 (6 suppl):S39–S70. |
| Table 2. Routine Laboratory Monitoring in Patients Receiving PN15 |
| Parameter |
Frequency of Monitoring |
| Acute Care |
Long-term |
| Basic metabolic panel, magnesium, phosphorus |
Daily, until PN advanced to goal and patient stable; then 1–2 times weekly |
Weekly; then decrease frequency when stable |
| CBC with differential |
Baseline; then 1–2 times weekly |
Monthly; then every 2–3 months as stable |
| Liver function: ALT, AST, ALP, total bilirubin |
Baseline; then weekly |
Monthly; then every 2–3 months as stable |
Iron studies: iron, TIBC, ferritin;
25-hydroxyvitamin D |
Not routine |
Baseline (first clinic visit); then every 3–6 months |
| Zinc, copper, selenium, manganese |
Not routine |
Baseline (first clinic visit); then every 6 months |
Adapted from: Kumpf V, Gervasio J. Complications of Parenteral Nutrition. In: Gottschlich MM, ed. The A.S.P.E.N. Nutrition Support Core Curriculum: A Case-Based Approach-The Adult Patient, 3rd ed. Silver Spring, MD: American Society for Parenteral and Enteral Nutrition; 2017.
Abbreviations used: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase; CBC, complete blood count; PN, parenteral nutrition; TIBC, total iron-binding capacity. |
Often the most challenging aspect of the care plan is stabilizing the patient’s fluid status. This requires a multifaceted approach, including efforts to decrease stool output and provide adequate parenteral fluid replacement. Patients with SBS, especially those without a colon or with a colon out of continuity, have impaired ability to reabsorb the high volume of fluid secreted by the GI tract (Figure 1). This contributes to high fluid losses.3,6 Increased thirst is a normal response to this phenomenon, but drinking more fluid can stimulate GI secretions further and worsen net negative fluid balance. Although counterintuitive, implementing a dietary fluid restriction (as low as no more than 1 L daily) often stabilizes fluid balance. Intake and output monitoring, daily weight, and assessment for edema can be used to assess parenteral fluid requirements. A desired endpoint includes adequate urine output (1–2 L/d) and a net positive balance to account for insensible fluid losses.
To prepare for discharge home on PN, the goal is to consolidate all parenteral fluid requirements into the PN formulation and transition from a 24-hour, continuous infusion, to a cycled infusion over 10–12 hours daily that is generally administered overnight.16 Because the PN volume is limited to the size of available bags (4 L), the goal is to stabilize parenteral fluid requirements to less than 4 L daily. In this way, the PN formulation meets the patient's fluid requirements entirely. In addition, WHO recommends providing supplemental IV fluid bags in the home setting to use as needed for dehydration.
Pharmacologic Treatment Plan
Patients with SBS experience accelerated intestinal motility; it can be treated with opioids or opioid receptor agonists that inhibit intestinal smooth muscle contraction and prolong intestinal transit. First-line choices for antimotility agents in the acute setting are loperamide and diphenoxylate (Table 3).12 Loperamide is a peripherally acting opioid receptor agonist that does not cross the blood-brain barrier and is not associated with undesirable central nervous system effects, such as euphoria, sedation, or addiction. Diphenoxylate crosses the blood-brain barrier, but its coformulation with atropine limits abuse potential by causing unpleasant anticholinergic adverse effects (e.g., dry mouth, tachycardia, dry skin, flushing) at higher doses. Although antimotility agents are commonly prescribed “as needed” in the acute setting for other causes of diarrhea, patients with SBS do better with a scheduled regimen. The medication should ideally be administered 15–30 minutes prior to meals to maximize effect. An additional bedtime dose may help minimize trips to the bathroom and sleep disturbance at night.
| Table 3. Pharmacologic management of diarrhea associated with short bowel syndrome. |
| Etiology |
First-line agent |
Second-line agent |
| Rapid intestinal transit |
Loperamide 2–6 mg po 4 times daily;
maximum daily dose, 16–32 mg* |
Codeine 15–60 mg po 4 times daily |
| Diphenoxylate 2.5–7.5 mg po 4 times daily; maximum daily dose, 20–25 mg |
Opium tincture 0.3–1 mL po 4 times daily |
| Gastric hypersecretion |
Lansoprazole 15–30 mg po 2 times daily
Pantoprazole 20–40 mg po/IV 2 times daily
Omeprazole 20–40 mg po 2 times daily
Esomeprazole 20–40 mg po/IV 2 times daily
Rabeprazole 20 mg po 2 times daily
Dexlansoprazole 30–60 mg po 2 times daily
Famotidine 20–40 mg po/IV 2 times daily
Ranitidine 150–300 mg po/IV 2 times daily
Cimetidine 200–400 mg po/IV QID
Nizatidine 150–300 mg po 2 times daily |
Octreotide 100–250 mcg SC 3 times daily |
Clonidine 0.1–0.3 mg po 2 times daily
Clonidine 0.1–0.3 mg/24 h patch every 7 days |
| Small intestine bacterial overgrowth |
Metronidazole 250 mg po 3 times daily x 7–14 days
Ciprofloxacin 500 mg po 2 times daily x 7–14 days
Rifaximin 200–550 mg po 3 times daily x 7–14 days
Augmentin 500 mg po 2 times daily x 7–14 days
Doxycycline 100 mg po 2 times daily x 7–14 days
Neomycin 500 mg po 2 times daily x 7–14 days |
Reduce/stop acid-reducing agent |
| Prebiotics/Probiotics |
| Steatorrhea |
Pancrelipase, 500 lipase units/kg per meal, maximum dose is 2500 lipase units/kg per meal or 10,000 lipase units/kg/d |
Bile acid replacement |
| *Maximum dosage not well established. Pushing daily dosage up to 32 mg has been suggested because of impaired absorption.25 |
Clinicians employ antimotility agents in this acute postoperative setting to establish initial control of stool output as patients resume start eating again. Typically, prescribers should select 1 agent and start at a low dose (e.g., loperamide 2 mg four times daily or diphenoxylate 2.5 mg four times daily) initially.12 They can escalate the dose in a stepwise approach every few days until the patient achieves the desired response or reaches the maximum recommended dose (Table 3). Further optimization of the antimotility regimen and consideration of second-line agents — including codeine, morphine, and opium tincture — are typically deferred to the outpatient setting.
Antisecretory agents are beneficial in the acute setting because gastric acid release can increase significantly following extensive intestinal resection.17 Secretion of gastric fluid can reach up to 4 L/d, compared with the normal average of 2 L/d, and the sheer volume of secretions contributes to total fecal losses. Inactivation of pancreatic enzymes also is common, contributing to diarrhea and increasing risk of peptic ulcer disease. Proton pump inhibitors (PPIs) and histamine type 2 receptor antagonists (H2RA) are used to treat gastric acid hypersecretion (Table 3).12 All patients who have undergone significant intestinal resection should be started on a PPI or H2RA antagonist during the immediate postoperative period and discharged home on therapy. In the event the oral route fails due to malabsorption, prescribers should consider the IV route; adding an H2RA directly to the PN formulation is an option. When conventional therapy fails to stabilize fluid and electrolyte balance and thereby allow discharge of a patient from the hospital to home, clinicians can consider using octreotide.18
Octreotide, a somatostatin analog, inhibits the release and secretory effects of a wide variety of GI hormones. It reduces the volume of GI secretions and prolongs intestinal transit time, thus reducing diarrhea.12 Use of octreotide is limited by its high cost, inconvenience of subcutaneous injection, rapid development of tachyphylaxis, and adverse effects. Octreotide elevates risk of cholelithiasis (a risk already elevated in patients with SBS), alters glucose levels, and potentially inhibits intestinal adaptation. For these reasons, octreotide is not considered first-line treatment, but may be indicated for short-term use.18
Monitoring Plan
Clinicians must develop an outpatient monitoring plan to identify potential SBS- and PN-related complications and assess response to therapy upon discharge home.16 As with any patient discharged home on PN for the first time, laboratory monitoring is typically performed once weekly and as results stabilize, its frequency decreased (Table 2).15 Patients with SBS are at high risk for developing fluid and electrolyte complications because of high volume stool output and day-to-day fluctuations, especially as dietary intake increases after discharge home. Educating patients and their caregivers on appropriate self-monitoring can help reduce risk of metabolic complications through early detection and prompt intervention if/when problems develop. Written instructions and specific directions that include what/when/how to monitor are recommended.16 It may be helpful to provide monitoring forms or electronic tools to assist the patient with recordkeeping.
Daily weight is a useful self-monitoring parameter. The health care team should instruct patients to measure weight at the same time every day, typically in the morning after finishing the PN infusion. Daily weight can be used to assess fluctuations in fluid status and to assess appropriateness of the PN calorie dose.
Strict intake (including oral fluid consumption) and output (urine plus stoma) monitoring requires effort by the patient to record data accurately, but can be especially useful in assessing fluctuations in fluid status and appropriateness of the PN fluid volume. Once fluid status stabilizes, patients can decrease the frequency of intake and output monitoring to an as-needed basis.
Self-monitoring guidelines should include specific thresholds describing when and how to contact a health care professional. Examples of when it may be reasonable for the patient to contact a clinician include weight gain/loss exceeding 1 pound (2.2. kg) per day for 2 consecutive days and increase/decrease in urine or stoma output by more than 500 mL per day for 2 consecutive days. When these parameters reflect dehydration or risk of dehydration, the attending health care provider can instruct the patient/caregiver to administer supplemental IV fluid in the home setting and possibly avoid hospital readmission.
LONG-TERM SBS MANAGEMENT
The long-term management of patients with SBS should focus on enhancing absorptive capacity of the remnant GI tract and minimizing complications. Intestinal rehabilitation is a term used to describe the process of maximizing oral intake of nutrients and fluid to sustain adequate nutrition and hydration balance. Optimally, intestinal rehabilitation combines targeted nutrient and hydration interventions with adjunctive medication therapy to decrease dependence on PN/IV therapy.
As mentioned above, most patients newly diagnosed with SBS are discharged home from the hospital on PN until able to sustain adequate nutrition and hydration with diet modification alone. Clinicians can transition some patients who can sustain their nutrition status but cannot sustain their hydration/electrolyte balance with diet modification from PN to IV fluid/electrolyte therapy. Many patients will remain chronically dependent on PN/IV therapy, which can be associated with serious complications and negatively impact quality of life.19 Complications may be related to the central venous access device (e.g., central line associated bloodstream infections, thrombosis)20 or metabolic complications (e.g., PN-associated liver disease, renal failure, metabolic bone disease).15,21 See supplemental resources for further discussion of PN-related complications.2,15,18,20,21 The complications, quality of life burden, and high cost associated with PN/IV are all reasons to promote therapy weaning whenever possible. Intestinal rehabilitation centers that specialize in the management of patients with intestinal failure are available, including those with SBS-associated intestinal failure.
Nutrition and Fluid Optimization
Diet modification is the cornerstone of SBS management because of diet's fundamental role in promoting intestinal adaptation and maximizing absorptive capacity of remaining bowel.13,22,23 An experienced dietitian should provide dietary education to patients following intestinal resection and continue as the process of intestinal adaptation evolves. Patients should be encouraged to eat small, frequent, high-calorie meals and snacks throughout the day with the goal of consuming at least twice the calories of a standard diet.13,22,23 If the colon is present, a diet high in complex carbohydrate is recommended since the colon allows for fermentation of unabsorbed carbohydrate to small chain fatty acids. Patients should restrict dietary fat because unabsorbed fatty acids can lead to increased fluid loss from the colon and exacerbate diarrhea. Patients with intact colons should also restrict oxalate-containing foods (spinach, rhubarb, purslane, buckwheat, amaranth, almonds, coco, dark chocolate, tea and coffee, parsley, and chives) because of the risk of forming calcium oxalate renal stones. Oral calcium supplementation with meals is recommended in these patients as a way to bind oxalate in the gut lumen and prevent absorption.2
Patients without colons have a limited capacity to salvage energy from unabsorbed carbohydrate and therefore do better with foods that are high in fat. Eating salty foods and using table salt liberally is also recommended, especially for those without a colon, because sodium enhances intestinal absorption.
All patients with SBS should avoid simple carbohydrates and caffeine because both can worsen diarrhea. Patients should also avoid consuming beverages concurrently with meals and snacks, as these can accelerate transit time and worsen diarrhea. Many patients with SBS complain of thirst, which can be problematic since fluid intake may increase GI secretions and worsen stool output. Oral rehydration solutions (ORS) can be used to maximize intestinal fluid absorption and help maintain adequate hydration by providing a balanced ratio of sodium, glucose, and water. Patients should be encouraged to sip on these solutions throughout the day with a goal intake of 1 to 2 L per day, while avoiding sugar-containing beverages and hypotonic fluids (e.g., water). Commercial ORS products are available, as are less expensive options that involve mixing contents of simple, readily available ingredients.23
In conjunction with diet modification, optimization of the PN/IV regimen should focus on achieving/maintaining appropriate body weight and nutritional status; preventing/treating dehydration; and preventing/treating micronutrient imbalance (electrolytes, minerals, vitamins). Target weight is a useful guide to identify PN calorie requirements. Since the amount of energy absorbed from dietary intake for a given patient is not readily apparent, the health care team needs to individualize each patient's PN calorie dose based on achievement of target weight.16 If a patient with SBS is underweight when discharged home from the hospital, the PN calories may need to be incrementally increased to promote weight gain. PN calories are typically increased at intervals of every 1 to 2 weeks in the home setting, as needed, to promote a desired weight gain of 1 to 2 pounds (2.2. to 4.4 kg) per week.
PN calories can be stabilized once patients reach their target weights, or reduced if the exceed their target weights. As patients begin absorbing sufficient protein/energy from dietary (or enteral) intake alone, PN calorie requirements decrease. It may be possible to decrease PN frequency and provide PN-free nights. The health care team will rely on laboratory values (Table 2) and self-monitoring parameters (e.g., intake/output, frequency and consistency of stool output, weight) to determine IV fluid/electrolyte requirements at this stage and when/if it is appropriate to stop IV supplementation. Eventually, PN may be discontinued, but some patients will continue to require IV fluid and/or electrolytes to maintain adequate hydration and electrolyte balance.
During the process of PN/IV weaning, oral supplementation of vitamins and minerals is necessary to prevent and treat micronutrient deficiency states. Because of malabsorption, oral vitamin and mineral dosage requirements may be vastly higher than normal. Most patients will require oral multivitamin, calcium, and vitamin D supplementation for life to prevent micronutrient deficiency and associated disorders, such as metabolic bone disease.13,22,23 Although effective repletion of most vitamins and minerals can be achieved by the oral route, oral magnesium supplementation is problematic in patients with SBS, especially those with an end-jejunostomy. These patients typically experience high magnesium losses from the GI tract, and poor oral magnesium absorption. They tolerate oral magnesium salts poorly since these can exert a laxative effect.22 In addition, the majority of patients with SBS receive PPIs, which have been associated with hypomagnesemia.24 Unfortunately, some patients will continue to require IV magnesium supplementation even after PN ends. Patients with resection of the terminal ileum will require vitamin B12 supplementation, typically with a monthly subcutaneous injection.
Pharmacologic Therapies
Adjunctive use of targeted medications are integral to the intestinal rehabilitation process and decreasing dependence on PN/IV therapy. The initiation of antimotility and antisecretory agents in the acute care setting remains important and should be fine-tuned in the outpatient setting. Loperamide and diphenoxylate continue to serve as first-line antimotility agents and dosages should be escalated in a stepwise manner every few days, until desired benefits achieved, adverse events occur, or the maximum dosage is reached (Table 3).12 It is anticipated that patients with SBS will require higher doses to achieve desired effect compared with those with an intact bowel. Loperamide is typically well tolerated at high doses and patients with SBS may benefit and tolerate doses up to 32 mg daily.25 Stool and urine output monitoring is a useful parameter to assess benefit. If no benefit is achieved, the medication should be stopped. If adverse events occur at higher doses, such as abdominal cramping/discomfort or nausea, dose reduction may help alleviate the symptoms. Some patients experience partial but incomplete response with combined loperamide and diphenoxylate at maximum tolerated doses; adding a second-line antimotility agent and escalating the dose in the same stepwise manner is reasonable.
Codeine, opium tincture, and morphine preparations have been used as second-line agents because of their potent opioid receptor activity. These agents prolong transit time, but have higher abuse risk. Patients develop tolerance to their CNS effects, but do not develop tolerance to constipating effects.26 This property may minimize risk for abuse because it allows for a stable opioid dose and no need to escalate dose to maintain effect when used over a long period of time. For all antimotility agents, clinicians should instruct patients to take the medication 15 to 30 minutes before meals/snacks and at bedtime as needed. Patients should also be provided with guidelines on dosage adjustments because responses to the medication may vary with diet alterations or changes in the course of the disease.
An H2RA or PPI is recommended during the first 6 months after extensive intestinal resection to address gastric acid hypersecretion.18 Even though this response is typically transient (weeks to months), patients with SBS often need to use antisecretory agents (primarily PPIs) long-term to avoid worsened stool output that follows discontinuance. Clinicians and patients need to decide together whether to continue long-term PPI therapy based on the potential for adverse events, including bacterial overgrowth, hypomagnesemia, pneumonia, osteoporosis, and kidney disease. Another antisecretory agent to consider in patients with SBS is clonidine, an alpha-2-adrenergic agonist.27 Clonidine acts on enteric neurons to reduce gastric acid and intestinal fluid secretion and inhibit GI motility. Its availability in a transdermal preparation is an advantage for patients with SBS. Unfortunately, patients with SBS may not tolerate clonidine well because of its antihypertensive effects and risk of orthostatic hypotension. Adverse effects and expectation of only modest response make clonidine a second-line agent for treatment of diarrhea in patients with SBS.
Gastric acid hypersecretion can denature pancreatic enzymes and lead to fat malabsorption. A trial of pancreatic enzyme replacement therapy for fat malabsorption may be warranted (Table 3).12 In addition, fat malabsorption may be caused by an interruption in bile acid recycling and decrease in bile acid pool that occurs following extensive resection of the distal ileum. A trial of bile acid supplements may be warranted for this reason, but they are only available as oral dietary supplements and not strictly regulated by the U.S. Food and Drug Administration (FDA).28 Patients should take pancreatic enzyme and bile acid replacement therapy with meals to enhance digestion and stop therapy if they see no benefit.12
Cholestyramine, a bile acid-binding resin, may be of benefit in a select group of patients with ileal resection less than 100 cm and colon in continuity.12,25 Since bile salts are absorbed primarily in the distal ileum, these patients may experience spillover of bile salts into the colon, where they are metabolized by bacteria to lithocholic acid and induce a secretory diarrhea. Bile acid-binding resins form insoluble complexes with bile salts and reduce diarrhea in these patients. However, more extensive ileal resection is more common. As noted above, patients with extensive ileal resection experience a decrease in bile acid pool and use of a bile acid-binding resin may worsen fat malabsorption. Therefore, cholestyramine should be used with caution in patients with SBS and clinicians and patients should monitor for steatorrhea, fat-soluble vitamin deficiencies, and decreased efficacy of concomitant medications that result from impaired absorption.
Small intestine bacterial overgrowth (SIBO) may contribute to diarrhea in patients with SBS.12 Resections of the terminal ileum and/or ileocecal valve may allow anaerobic colonic bacteria to migrate into the small intestine. Other patients with SBS may be at risk because of blind loops of bowel that allow intestinal contents to stagnate. In addition, acid suppression therapy can increase risk of SIBO because gastric acid normally reduces the concentration of ingested bacteria.
Anaerobic bacteria overgrowth can deconjugate bile salts and lead to fat malabsorption. Release of bacterial toxins can cause mucosal inflammation, possibly increasing intestinal permeability and causing loss of fluid. Symptoms of SIBO can include gas, bloating, abdominal discomfort, and worsening foul-smelling stool output. Because testing for this condition is difficult, most clinicians treat patients who are at risk and manifest symptoms empirically. A variety of oral antibiotics agents that target anaerobic bacteria or provide broad spectrum coverage have been utilized for a treatment course of 7 to 14 days (Table 3). Repeat courses are often necessary in patients with SBS on account of the ongoing presence of risk factors. Rotation of antibiotic agents and inclusion of antibiotic-free intervals may decrease development of resistant strains and improve long-term success in managing SIBO.12,13 Although clinical studies are lacking, some preclinical evidence suggests adding prebiotics and probiotics to the antibiotic regimen may be beneficial for treating SIBO or used alone for reducing risk of SIBO recurrence.13
Growth Factor Therapy
The recent availability of intestinal growth factor therapy to treat SBS offers another targeted pharmacologic approach to achieving PN/IV optimization. Teduglutide is a recombinant human glucagon-like peptide-2 (GLP-2) analog approved for use in adult patients with SBS who are dependent on parenteral support. GLP-2 is naturally produced in the L-cells of the distal small intestine and colon, and helps regulate normal intestinal function and absorption. The drug reduces the volume of parenteral nutrition (PN)/IV fluid requirements and the number of PN/IV infusion days safely and effectively.
Two phase 3 studies demonstrated that PN/IV volume requirements decreased by a mean of 2.5–4.4 L/wk from baseline after 24 weeks of teduglutide.29,30 Patients who received tedugludide demonstrated mucosal hypertrophy, characterized by increased villus height and crypt depth when compared with placebo. In an open-label extension of one of the trials, a mean reduction of PN/IV volume by 4.9 L/week from baseline was documented at 52 weeks. The majority of patients (68%) achieved at least a 20% reduction of PN/IV volume.31 Some patients successfully achieved independence from PN/IV therapy. The algorithm used for PN/IV volume reduction in these trials required that urine output increase over baseline but remain within the target range of 1 to 2 L/d.
The recommended dose of teduglutide is 0.05 mg/kg subcutaneously once daily. The most common adverse events reported in clinical trials were abdominal pain; digestive symptoms including nausea, vomiting and abdominal distension; upper respiratory tract infection; and stoma complications. A colonoscopy prior to initiation of therapy, at 1-year follow-up, and at least every 5 years is recommended because of the potential risk of accelerated neoplastic and colon polyp growth. Additional precautions and monitoring are recommended to assess for potential development of intestinal obstruction, gallbladder, biliary and pancreatic disease, and fluid overload. Laboratory monitoring should include an amylase, lipase, bilirubin, and alkaline phosphatase within 6 months of starting therapy and every 6 months. Clinicians should monitor patients taking concomitant oral medications, especially those with a narrow therapeutic index, for potential increased drug absorption and need for dose reduction.
FDA requires a risk evaluation and mitigation strategy (REMS) program to inform prescribers and patients of potential risk with this therapy. The REMS includes patient counseling information and a medication guide. Optimal use of teduglutide requires a coordinated effort by experienced health care professionals to ensure proper safety monitoring and to promote efficacy by incorporating appropriate strategies for PN/IV weaning.32
Summary
Short bowel syndrome is a complex medical condition that poses significant challenges to pharmacists involved in the care of these patients. Goals of care should be individualized based on functional capacity of remaining GI anatomy. Diet modification is the cornerstone of SBS management. Medications can be used to control high stool output and minimize fluid and electrolyte losses; treat suspected or actual SIBO; reduce fat malabsorption; and enhance intestinal absorption and decrease PN/IV volume requirements in patients with SBS. When patients with SBS are unable to sustain adequate fluid and hydration status with the combined approach of dietary modification, oral nutritional supplements, and medication therapy, then PN/IV therapy is indicated and may be required for the life of the patient.
References
1. O’Keefe SJD, Buchman AL, Fishbein TM, et al. Short bowel syndrome and intestinal failure: Consensus definitions and overview. Clin Gastroenterol Hepatol. 2006;4(1):6-10.
2. Pironi L. Definitions of intestinal failure and the short bowel syndrome. Best Pract Res Clin Gastroenterol. 2016;30(2):173-185.
3. Jeejeebhoy KN. Short bowel syndrome: A nutritional and medical approach. Can Med Assoc J. 2002;166(10):1297-1302.
4. Buchman AL. Etiology and initial management of short bowel syndrome. Gastroenterolog.y 2006;130(2 Suppl 1):S5-S15.
5. Jeppesen PB. Spectrum of short bowel syndrome in adults: Intestinal insufficiency to intestinal failure. JPEN J Parenter Enter Nutr. 2014;38(suppl 1):8S-13S.
6. Tappenden KA. Pathophysiology of short bowel syndrome: Considerations of resected and residual anatomy. JPEN J Parenter Enter Nutr. 2014;38(suppl 1):14S-22S.
7. Kiela PR, Ghishan FK. Physiology of intestinal absorption and secretion. Best Pract Res Clin Gastroenterol 2016;30(2):145-159.
8. Debongnie JC, Phillips SF. Capacity of the human colon to absorb fluid. Gastroenterology. 1978;74(4):698-703.
9. Royall D, Wolever TM, Jeejeebhoy KN. Evidence for colonic conservation of malabsorbed carbohydrate in short bowel syndrome. Am J Gastrenterol. 1992;87(6):751-756.
10. Tappenden KA. Intestinal adaptation following resection. JPEN J Parenter Enter Nutr.
2014;38(suppl 1):23S-31S.
11. Neelis EG, Olieman JF, Hulst JM, et al. Promoting intestinal adaptation by nutrition and medication. Best Pract Res Clin Gastroenterol. 2016;30(2):249-261.
12. Kumpf VJ. Pharmacologic management of diarrhea in patients with short bowel syndrome. JPEN J Parenter Enteral Nutr. 2014;38(suppl 1):38S-44S.
13. Matarese LE. Nutrition and fluid optimization for patients with short bowel syndrome. JPEN J Parenter Enteral Nutr. 2013;37(2):161-170.
14. Mirtallo J, Canada T, Johnson D, Kumpf V, Petersen C, Sacks G, Seres D, Guenter P. Safe practices for parenteral nutrition. JPEN J Parenter Enter Nutr. 2004;28(6 suppl):S39-S70.
15. Kumpf V, Gervasio J. Complications of Parenteral Nutrition. In: Gottschlich MM, ed. The A.S.P.E.N. Nutrition Support Core Curriculum: A Case-Based Approach—The Adult Patient, 3rd ed. Silver Spring, MD: A.S.P.E.N., 2017 :345-360.
16. Kumpf VJ, Tillman EM. Home parenteral nutrition: Safe transition from hospital to home. Nutr Clin Pract. 2012;27(6):749-757.
17. Windsor DW, Fejfar J, Woodward DA. Gastric secretion after massive small bowel resection. Gut. 1969;10(10):779-786.
18. Pironi L, Arends J, Bozzetti F, et al. ESPEN guidelines on chronic intestinal failure in adults. Clin Nutr. 2016;35(2):247-307.
19. Kelly DG, Tappenden KA, Winker MF. Short bowel syndrome: Highlights of patient management, quality of life, and survival. JPEN J Parenter Enteral Nutr. 2014;38(4):427-437.
20. Pittiruti M, Hamilton H, Biffi R, et al. ESPEN guidelines on parenteral nutrition: Central venous catheters (access, care, diagnosis and therapy of complications). Clin Nutr. 2009;28(4):365-377.
21. Kumpf VJ. Parenteral nutrition-associated liver disease in adult and pediatric patients. Nutr Clin Pract. 2006;21(3):279-290.
22. Wall EA. An overview of short bowel syndrome management: Adherence, adaptation, and practical recommendations. J Acad Nutr Diet. 2013;113(9):1200-1208.
23. Parrish CR. The clinician’s guide to short bowel syndrome. Pract Gastroenterol. 2005;29:67-106.
24. Markovits N, Loebstein R, Halkin H, et al. The association of proton pump inhibitors and hypomagnesemia in the community setting. J Clin Pharmacol. 2014;54(8):889-895.
25. Bechtold ML, McClave SA, Palmer LB, et al. The pharmacologic treatment of short bowel syndrome: new tricks and novel agents. Curr Gastroenterol Rep. 2014;16(7):392.
26. Schiller LR. Review article: anti-diarrhoeal pharmacology and therapeutics. Aliment Pharmacol Ther. 1995;9(2):87-106.
27. McDoniel K, Taylor B, Huey W, et al. Use of clonidine to decrease intestinal fluid losses in patients with high-output short-bowel syndrome. JPEN J Parenter Enteral Nutr. 2004;28(4):265-268.
28. Heydorn S, Jeppesen PM, Mortensen PB. Bile acid replacement therapy with cholysarcosine for short-bowel syndrome. Scand J Gastroenterol .1999;34(8):818-823.
29. Jeppesen PB, Gilroy R, Pertkiewicz M, et al. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60(7):902-914.
30. Jeppesen PB, Pertkiewicz, Messing B, et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143(6):1473-1481.
31. O’Keefe SJ, Jeppesen PB, Gilroy R, et al. Safety and efficacy of teduglutide after 52 weeks of treatment in patients with short bowel intestinal failure. Clin Gastroenterol Hepatol. 2013;11(7):815-823.
32. Seidner DL, Schwartz LK, Winkler MF, et al. Increased intestinal absorption in the era of teduglutide and its impact on management strategies in patients with short bowel syndrome-associated intestinal failure. JPEN J Parenter Enter Nutr. 2013:37(2):201-211.
Back Top