
Expired activity
Please go to the PowerPak
homepage and select a course.
New Therapeutic Options for Multiple Sclerosis: An Update for Specialty and Managed Care Pharmacy
INTRODUCTION TO MULTIPLE SCLEROSIS
Epidemiology and Pathophysiology
Multiple sclerosis (MS) is a chronic, autoimmune disease affecting the central nervous system
(CNS), more specifically the myelin sheath covering of nerve fibers in the brain and spinal cord.
Inflammation and immune activity, including T and B lymphocytes, macrophages, destructive
cytokines, antibodies, and complement, will result in demyelination and damage to axons that
correlates with disability.1 Worldwide prevalence is estimated to be 23 million people who have
been diagnosed with MS, while United States (U.S.) prevalence is estimated to be 400,000
people who have been diagnosed with MS. Symptom onset and diagnosis occurs typically
between the ages of 20 and 50 years of age; women are 2 to 3 times more likely to be affected
than men.2,3 People of Northern European descent are most commonly affected; however, MS
can affect any ethnic group. Additionally, MS is more common in colder climates, higher than a
40° latitude.2
Associated Factors2
Vitamin D is thought to play a role in the development and progression of disease. Higher levels
of vitamin D may reduce disease activity for people with MS. Ensuring that patients with MS
have adequate levels of vitamin D has been recommended as part of routine care.
Research has also shown that smoking increases a person’s risk for developing MS and has been
associated with more severe disease and more rapid disease progression. Smoking cessation has
been associated with a slower rate of disability progression.
The association between MS and several infectious factors, such as measles, canine distemper,
human herpesvirus-6, Epstein-Barr Virus, and Chlamydia pneumonia, has also been investigated,
although no single infectious process has been identified as a trigger.
Although MS is not hereditary, genetic factors have been studied because having a first-degree
relative with MS does increase an individual’s risk of developing the disease. Additionally, the role of hormones has also been studied because pregnancy seems to have a protective effect
against MS relapses.
Clinical Presentation
Disease presentation is variable and characterized by nonspecific symptoms, including optic
neuritis or other visual symptoms, gait issues, muscle spasticity or weakness, slurred speech, or
cognitive changes, bladder or bowel dysfunction, or fatigue. Often, patients will seek help for a
specific symptom prior to being evaluated by a neurologist specifically for MS. For example, a
patient who develops eye pain and vision loss may present to their ophthalmologist prior to
ultimately being diagnosed with optic neuritis. Optic neuritis, for some individuals, may be the
first indication of MS that results in a diagnostic evaluation. The diagnostic criteria are further
described below.4
Clinically Isolated Syndrome (CIS)
The term “clinically isolated syndrome” (CIS) has been used to describe the first episode of
neurologic symptoms that lasts at least 24 hours and has been caused by inflammation and
demyelination in one or more sites in the CNS. A person with CIS may or may not progress to
develop MS.5
TYPES OF DISEASE
Patients can be classified as having any 1 of the 4 types of MS and often the boundaries between
categories may be blurred, so diagnosis can be complicated.
Relapsing Remitting (RRMS)
The most common type of MS, affecting 85% of patients, is known as RRMS. In RRMS,
patients experience the worsening of preexisting symptoms or the onset of new symptoms for
periods longer than 24 hours without concomitant fever, which are known as relapses of MS.
These are contrasted by symptom-free periods, known as remissions, where the patient’s
symptoms partially or completely disappear. Before the development of disease-modifying
medications, 50% of patients with RRMS commonly developed secondary-progressive MS
(SPMS) after 10 to 15 years.2
Secondary Progressive (SPMS)
SPMS is a progression of RRMS. The disease steadily progresses, and can present with or
without clear-cut relapses. Approximately 50% of patients progressed to SPMS after 10 years
with RRMS. However, since the development of disease-modifiying medications, this incidence
is believed to have decreased.
Primary Progressive (PPMS)
PPMS is another form of MS that is relatively rare, affecting approximately 10% of patients.
This disease course is characterized by a steady decline, without clear-cut relapses.2 To make this
diagnosis, the individual demonstrates 1 year of disease progression and 2 of the following:
positive brain magnetic resonance imaging (MRI), positive spinal cord MRI, or positive
cerebrospinal fluid (CSF).4 For further information, please refer to Diagnosis section.
Progressive-Relapsing (PRMS)
PRMS is characterized by a steady disease progression, in addition to clear-cut periods of
exacerbations of MS. This is the rarest form of the disease, affecting approximately 5% of
patients.2
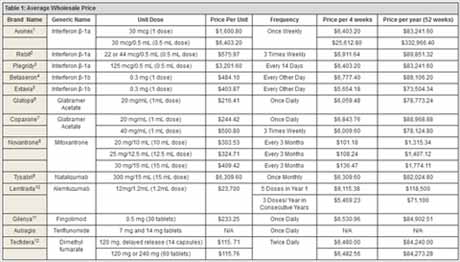
BURDEN OF DISEASE
In the U.S., total all-cause health care costs for MS have been reported to range from $8528 to
$54,244 per patient per year, based on a review of the literature from 2007 to 2012. However, at
present, the average wholesale price for a year of medication exceeds these figures (Table 1).
Therefore, total costs are believed to be higher. On average, direct costs comprised 77% of total
costs. Direct medical costs can include costs such as prescription drugs, physician services,
hospital stays, and nursing home stays. Direct nonmedical costs can include home and
automobile modifications, informal care provided by family and friends, and most home and
community-based services. Prescription medications accounted for the majority of direct costs.
As shown in Table 1, it is important to not only consider the unit cost, but also the frequency and
the method of administration, which will also play a role in the annual cost. Indirect costs address the labor productivity losses and wages associated with a withdrawal from the workforce
by people with MS and the impact of disease progression on employment and health for the
family and friends who are closest to them. On average, indirect costs comprised 23% of total
costs. There are also intangible costs that impact the patient and caregiver’s quality of life.6
Diagnosis
MS is diagnosed by clinical presentation, as well as by imaging and laboratory data. It is also
imperative that an alternative diagnosis is considered and excluded. The diagnosis requires at
least 2 documented clinical exacerbations separated by time and space, as well as 2 distinct MRI
lesions separated by time and space.4 Dissemination in time (DIT) refers to the simultaneous
presence of gadolinium-enhancing lesions (representing inflammation and disease activity) and
non-enhancing lesions, or a new lesion on a follow-up MRI when compared with a previous
MRI. Dissemination in space (DIS) refers to distinctly different anatomical lesions on imaging
occurring in areas known to be affected by MS (i.e., periventricular, juxtacortical, infratentorial,
or spinal cord). Based on the 2010 revised McDonald Criteria, at least 1 attack must be
corroborated by findings on neurological examination, visual evoked potential response in
patients reporting prior visual disturbance, or MRI consistent with demyelination in the area of
the CNS implicated in the historical report of neurologic symptoms.4 PPMS is diagnosed after 1
year of disease progression and if the patient meets 2 of the following criteria: DIS in the brain,
DIS within the spinal cord, and/or positive CSF.4 CIS is diagnosed after 1 exacerbation and 1
lesion, while the clinician awaits a second exacerbation and lesion to be able to make the
diagnosis of MS.4 Positive cerebrospinal fluid testing (CSF) can aid in diagnosing clinically
definite MS.4
TREATMENT
Goals of Treatment
The goals of treatment for MS include improving quality of life and minimizing long-term
disability.1 Treatment approaches include managing exacerbations and use of disease-modifying
therapies to slow or halt disease progression.7 Treatment should be chosen on an individual
patient basis after considerations, such as disease characteristics, treatment response, adverse
effects and tolerability, adherence, and cost or access issues.7 Medications can also be used to
manage specific symptoms and physical therapy; speech, therapy, and exercise can also play a
role.
Managing Acute Relapses
Relapses or exacerbations are managed by treatment with steroids. Corticosteroids decrease
inflammation by suppressing migration of polymorphonuclear leukocytes and reversing capillary
permeability. Methylprednisolone sodium succinate (Solu-Medrol) is given as 1 gram
intravenously (IV) for 3 to 5 days and may or may not be followed by a prednisone taper.1,8 High-dose oral corticosteroids may also be used.8 Alternatively, Acthar gel is an
adrenocorticotropic hormone (ACTH) analog used as a repository injection of corticotropin. It
may be given intramuscularly or subcutaneously in doses of 80 and 120 units daily for 2 to 3
weeks. It may be useful for patients with poor venous access, patients who are unable to tolerate adverse effects of high-dose corticosteroids, or patients who have been treated unsuccessfully
with corticosteroids.8
A 2012 Cochrane Review of 5 trials concluded that there was no substantial difference in the
proportion of disability improvement or gadolinium enhancement at 4 weeks between patients
treated with oral corticosteroids versus those treated with IV corticosteroids in a review of 5
trials. However, the authors also acknowledge that evidence for using oral corticosteroids is
lacking compared with evidence for using IV corticosteroids; but, nonetheless,
they also comment on
the substantial decrease in the cost of oral corticosteroids versus those administered an IV
injection. It should be noted that the review was limited by small sample size, that information
was not captured regarding patients who required retreatment for a relapse, and patients were
able to enter eligible trial for up to 1 month post-relapse, which may have confounded early
data.9 Although 1250 mg of oral prednisone is believed to be bioequivalent to 1 gram of IV
methylprednisolone, most neurologists continue to favor IV corticosteroids. The Optic Neuritis
Treatment Trial (ONTT) showed oral prednisolone was an ineffective treatment and increased
the risk of new episodes of optic neuritis.10,11
DISEASE-MODIFYING MEDICATIONS
Currently Available Therapies (Table 2)
Prior to 2010 there were 6 medications approved for the treatment of RRMS. These included
interferon beta, glatiramer acetate, natalizumab, and mitoxantrone.All of these medications had
to be administered parenterally, by injection or infusion, and compliance may have been an issue
for patients, particularly as patients developed injection fatigue.12 In 2010, fingolimod entered
the market as the first oral agent and was soon followed by teriflunomide and dimethyl fumarate.
The most recent disease-modifying medication to enter the market was alemtuzumab, approved
in 2014.
Injectable Medications
Interferon beta and glatiramer acetate are considered first-line, disease-modifying agents for the
treatment of RRMS. Each has demonstrated comparable efficacy for decreasing relapses, by
approximately 30%.13 Comparative studies have been completed assessing the efficacy of both
agents for decreasing relapse rates, as well as for other outcome measures, such as a reduction in
either MRI lesions or disability progression as measured by the Expanded Disability Status Scale
(EDSS). No substantial differences have been identified between either agent in regard to relapse
rate, disability progression, or MRI outcomes, including lesion number or volume. However, a
slight advantage was seen with the use of interferon beta in reducing the number of gadolinium-enhancing lesions, as compared with glatiramer acetate.14 Based on the similar rate of efficacy
for these agents, selection is typically based on patient preference and side-effect profile.
Interferon Beta
Interferon beta, a first-line treatment for MS, is available in 5 formulations as beta-1a,
intramuscular injection given once a week (Avonex), beta-1a, subcutaneous injection given 3
times a week (Rebif), beta-1a, subcutaneous injection every 14 days (Plegridy), and beta-1b,
subcutaneous injection given every other day (Betaseron, Extavia). The exact mechanism of
action of interferon beta in the prevention of MS disease progression is unknown.12 Common
side effects of interferon therapy include flu-like symptoms after injection, depression, and liver
enzyme abnormalities.14 Plegridy is pegylated interferon, which refers to polyethylene glycol
attached to interferon molecules to allow them to maintain their biologic effects in the body for a
longer period of time.8,15
Glatiramer Acetate
Glatiramer acetate, another first-line treatment for MS, is available as a subcutaneous injection
given daily as 20 mg (i.e., either Glatopa or Copaxone). Copaxone is also available as a 40 mg
dose, given 3 times weekly.8 The mechanism of action for glatiramer acetate in the treatment of
MS is not fully elucidated; however, it is thought to be related to the alteration of T-cell
activation and differentiation.12 This medication more commonly causes injection-site reactions,
including indurations and masses, as compared with interferon beta.14 This medication is an
appropriate choice for patients who are unable to tolerate flu-like symptoms or have coexisting
psychiatric illnesses.
Infused Medications
Mitoxantrone
Mitoxantrone, available as Novantrone, is an immunosuppressive agent chemically related to the
antineoplastic agents doxorubicin and daunorubicin. It works by intercalating with DNA strands
and causing breaks, as well as inhibiting DNA repair through topoisomerase II.12,16 It affects
rapidly dividing cells and, therefore, has secondary effects on the immune system, including
antigen presentation, pro-inflammatory cytokine expression, and decreased leukocyte
migration.12 Mitoxantrone is not a first-line agent because of its cardiotoxicity at cumulative
doses greater than 100 mg/m2. There is a limit on lifetime cumulative exposure to this agent. It
also causes severe bone marrow suppression, necessitating that hemoglobin levels, white blood
cell count, and platelet counts be monitored before each infusion.16 There is no datum on efficacy,
either clinically or on MRI, compared with interferon beta or glatiramer acetate. Mitoxantrone is
recommended to be used as a second-line treatment in patients with very active relapsing disease
who have failed other therapies.12,16
Natalizumab
Natalizumab, available as Tysabri, is a humanized monoclonal antibody that antagonizes α4-integrin of the adhesion molecule very late activating antigen (VLA)-4 on leukocytes. Inhibition
of VLA-4 is responsible for blockade of T cells across the blood-brain barrier.12,17 Natalizumab is very effective at decreasing relapse rates and slowing disease progression. A phase II study
showed a 50% reduction in relapses and a 92% reduction in gadolinium-enhancing lesions on
MRI compared with placebo.18 Additional trials have confirmed these results, showing a 42%
reduction in sustained disability and a 68% reduction in relapses. Compared with once-weekly
interferon beta-1a, natalizumab reduced relapses by 56% and decreased gadolinium-enhancing
lesions by 87%18; however; it carries with it the risk of progressive multifocal
leukoencephalopathy (PML). PML is an occasionally fatal opportunistic infection caused by the
John Cunningham polyomavirus (JCV).12 This virus is a common infection in the general
population and remains latent in many individuals. In those patients receiving natalizumab, it
may become reactivated. This JCV causes a demyelinating condition similar to MS; however,
with this disease, myelin cannot regenerate as it does for patients with MS. Since the drug was
allowed back into the U.S. market in 2006, 440 cases of PML have been reported.19 There is a
system called the TOUCH Prescribing Program, the goal of which is to monitor for PML and
patients, prescribers, and infusion centers must be enrolled in this before natalizumab infusions
can be administered.17 More common adverse reactions include infusion and hypersensitivity
reactions, infections including respiratory tract or urinary tract, depression, headache, fatigue,
diarrhea, cholelithiasis, and arthralgia.17 Despite its efficacy, because of the potential to develop
PML, natalizumab is considered a second-line therapy for patients who are unresponsive or
intolerant to first-line therapies. The following 3 factors can increase one’s risk of developing
PML: testing positive for antibodies to JCV, prior use of certain immunosuppressant
medications, and using natalizumab for more than 2 years.20
Alemtuzumab
Alemtuzumab (Lemtrada) is a humanized monoclonal antibody approved for relapsing forms of
MS in 2014.21,22 It is thought to exert therapeutic benefit by binding to CD52, a cell surface
antigen and T and B lymphocytes, natural killer cells, monocytes, and macrophages, resulting in
antibody-dependent cellular cytolysis and complement mediated lysis. In 2 Phase 3 trials, it was
shown to decrease the annualized relapse rate more effectively than interferon beta-1a.
Recommended dosing is 12 mg/day IV over 4 hours, for 2 treatment courses. The first treatment
course is 12 mg/day for 5 consecutive days and the second treatment course is 12 mg/day for 3
consecutive days administered 12 months after the first treatment course. Because of its safety
profile, it is generally reserved for people who have had an inadequate response to 2 or more MS
therapies. Patients can be retreated again for another 3 days after 12 months.23
Alemtuzumab can cause serious, sometimes fatal autoimmune conditions, such as immune thrombocytopenia (2%) and antiglomerular basement membrane disease (0.3%). Other autoimmune cytopenias have also occurred. Therefore, monitoring of complete blood count (CBC) with differential, serum creatinine, and urinalysis is recommended prior to treatment and monthly until 48 months after the last dose was administered.23
Because alemtuzumab causes cytokine release syndrome, infusion-related reactions were seen in 92% of patients in clinical studies, with 3% of patients experiencing serious reactions. Patients must be monitored for up to 2 hours after their infusion and corticosteroids (i.e., 1000 mg methylprednisolone or equivalent) should be used both as premedication and for 3 days of each treatment course. Antihistamines and antipyretics may also be used. Alemtuzumab may also cause an increased risk of malignancies, including thyroid cancer, melanoma, and lymphoproliferative disorders. Baseline and yearly skin exams are recommended. Autoimmune thyroid disorders occurred in 34% of patients in clinical studies. Thyroid function tests should be obtained prior to treatment and every 3 months until 48 months after the last infusion or longer if indicated. Patients taking alemtuzumab may be at increased risk of infections and should not receive live vaccines following a course of alemtuzumab. Patients who do not have antibodies to herpes zoster should not receive alemtuzumab until 6 weeks after vaccination. Concurrent use of antineoplastic or immunosuppressive therapies can increase the risk of immunosuppression. One should consider screening patients for hepatitis B and C because carriers may be at risk for liver damage as the result of virus reactivation. Herpes viral infection developed in 16% of patients treated with alemtuzumab, compared with 3% of interferon beta-1a patients. Antiviral prophylaxis for herpetic viral infections should be administered starting on the first day of each treatment course and continue for a minimum of 2 months following treatment or until the CD4+ lymphocyte count is ≥ 200 cells per microliter, whichever occurs later. Cervical human papillomavirus (HPV) infection occurred in 2% of patients treated with alemtuzumab and annual
screening is recommended for women. In clinical trials, the most common adverse reactions (i.e., incidence ≥ 10%) with alemtuzumab versus interferon beta-1a were as follows: rash, headache, pyrexia, nasopharyngitis, nausea, urinary tract infection, fatigue, insomnia, upper respiratory
tract infection, herpes viral infection, urticaria, pruritus, thyroid gland disorders, fungal infection, arthralgia, pain in extremity, back pain, diarrhea, sinusitis, oropharyngeal pain, paresthesia, dizziness, abdominal pain, flushing, and vomiting.23
Oral Medications
Fingolimod
Fingolimod, available as Gilenya, was the first oral drug marketed in 2010 for the treatment of
MS and it is indicated for relapsing forms of MS. It is an orally administered immunomodulator
that acts on the sphingosine-1-phosphate receptor. Fingolimod is phosphorylated to its active
form, FTY720-P, and binds to sphingosine-1-phosphate (S1P) receptors S1P1 and S1P3-5 on the
surface of lymphocytes.13,24 It, thereby, depletes both CD4+ and CD8+ T lymphocytes in the
blood stream, up to 75% below baseline.13,25 CD4+ cells are decreased to a greater extent than
CD8+ cells. It also inhibits lymphocyte release from lymphatic organs, decreasing the overall
numbers in circulation.23 It does not, however, inhibit lymphocyte recruitment.23,24 It has been
shown to substantially decrease annualized relapse rate more effectively than placebo and interferon beta-1a. A substantially higher percentage of patients were relapse free after a placebo
or treatment with interferon beta-1a. Fewer new lesions or enlarging lesions were seen in patients
treated with fingolimod as compared with placebo or interferon beta-1a. Fingolimod is dosed 0.5
mg once daily.26
Because of cardiac adverse effects and the first-dose effect of bradycardia, several precautions
must be taken. Patients should receive an electrocardiogram (ECG) prior to fingolimod being
prescribed. On the day of their first dose, patients must receive a predose ECG and vitals and
then have their vitals taken hourly for at least 6 hours following their first dose, as well as an
ECG 6 hours after first dose. Patients who develop a heart rate slower than 45 beats per minute
(BPM), second degree or higher atrioventricular (AV) block, or patients whose heart rate reaches
a nadir at 6 hours should be monitored until resolution. Patients who develop symptomatic
bradycardia should begin continuous ECG monitoring and may require pharmacologic
intervention with continued monitoring overnight, as well as repeat first-dose monitoring for
their second dose. Patients that are at higher risk of symptomatic bradycardia or heart block,
have prolonged QTc, or are taking drugs with the risk for torsades de pointes should be observed
overnight. This drug is contraindicated for those with recent myocardial infarction, unstable
angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring
hospitalization, and Class III or Class IV heart failure. It is also contraindicated for those with a
history of Mobitz type II, 2nd or 3rd degree AV block or sick sinus syndrome (unless the patient
has a pacemaker), as well as for those with a baseline QTc faster than 500 milliseconds or for
those taking concurrent treatment with Class Ia or Class II antiarrhythmic medications. The
clinical pharmacist may review patient charts to monitor medications and perform first-dose
monitoring activities. Patients eventually become tolerant to this first-dose effect, but if the
patient misses more than 1 day of treatment during the first 2 weeks, 1 week during the second 2
weeks, or more than 2 weeks at one time, thereafter, first-dose monitoring should be repeated.
Therefore, the pharmacist can play a key role in monitoring compliance and refill history.26
Fingolimod may increase the risk of infections and, therefore, CBC should be monitored.
Patients without antibodies who wish to become vaccinated against varicella zoster should wait
30 days after vaccination before receiving fingolimod; patients taking fingolimod should not
receive live vaccines. Macular edema can develop with this medication and patients should
receive an eye exam both before starting treatment and then again 3 to 4 months after treatment
is initiated.24 Patients with a history of uveitis and patients with diabetes are at increased risk for
developing macular edema and should have regular follow-up. Effects on pulmonary function
have also been observed and evaluation may be performed if clinically indicated. There have
been rare cases of posterior encephalopathy syndrome (PRES) reported. Fingolimod may also
increase blood pressure. The most common adverse effects (i.e., greater than 10% and greater
than placebo) include the following: headache, liver transaminase elevation, diarrhea, cough,
influenza, sinusitis, back pain, abdominal pain, and pain in extremities.23 Patients should be counseled to avoid pregnancy for two months after stopping fingolimod. Patients taking
fingolimod should be monitored for PML because 3 cases of PML have been reported.26,27
Teriflunomide
Teriflunomide is an orally administered immunomodulatory agent thought to possess anti-inflammatory and antiproliferative properties useful for the treatment of MS. It is currently
available as Aubagio and is indicated for relapsing forms of MS.28 Teriflunomide is the active
metabolite of leflunomide, a medication commonly used to treat rheumatoid arthritis.29 It is a
dihydroorotate dehydrogenase inhibitor, and blocks pyrimidine synthesis in rapidly dividing
cells, such as T cells and B cells.29,30 It has also been shown to inhibit protein tyrosine-kinase and
cyclooxygenase-2 activity and decrease the ability of antigen-presenting cells to activate T
cells.27 It is dosed 7 or 14 mg daily. Teriflunomide 7 or 14 mg daily substantially reduced the
annualized relapse rate, lesion volume, and gadolinium-enhancing lesions more effectively than
placebo and 14 mg reduced the relative risk of disability progression in clinical studies.28
Because severe liver injury has been seen with the use of leflunomide, transaminase and bilirubin
levels should be monitored within 6 months before initiation of teriflunomide and at least
monthly for 6 months thereafter. Because major birth defects have been reported, teriflunomide
is contraindicated for pregnant women or women of childbearing age who are not using reliable
contraception. It has also been detected in semen and men wishing to father a child are
recommended to discontinue teriflunomide and undergo accelerated elimination. Teriflunomide
may decrease white blood cell count and a recent CBC should be measured within 6 months
before initiating therapy. Patients should also be screened for latent tuberculosis prior to
initiating therapy. Blood pressure may increase and should be monitored. The most common side
effects when taking teriflunomide include the following: headache, diarrhea, nausea, hair
thinning or loss, and abnormal liver test results. Teriflunomide may increase exposure to drugs
metabolized by CYP2C8, OAT3, BCRP and OATP1B1/B3, as well as to ethinyl estradiol and
levonorgestrel. The dose of rosuvastatin should not exceed 10 mg for patients using
teriflunomide. Teriflunomide may decrease international normalize ratio (INR) for patients
taking warfarin. Teriflunomide has a long half-life and can remain in the blood for up to 2 years.
Elimination can be accelerated by the administration of cholestyramine 8 g every 8 hours or 50 g
activated charcoal every 12 hours for 11 days.28
Dimethyl Fumarate
Dimethyl fumarate, available as Tecfidera, is an orally administered immunomodulatory agent
shown to induce T-helper type 2-like cytokines (including interleukins 4, 5, and 10) to cause
apoptosis in activated T cells. It also causes downregulation of intracellular adhesion molecules,
leading to reduced migration of lymphocytes.29,30 It is approved to treat relapsing forms of MS.33 It is dosed 120 mg twice daily for 7 days and then 240 mg twice daily. In clinical studies, dimethyl fumarate reduced annualized relapse rate and number of new or newly enlarging
lesions.33
The most common adverse reactions associated with dimethyl fumarate versus placebo are
flushing and gastrointestinal events, including abdominal pain, diarrhea, and nausea, all of which
typically decrease with continued treatment.33 Temporary dose reductions to 120 mg twice daily
can be considered in cases where individuals cannot tolerate the maintenance dose; 240 mg twice
daily should be resumed within 4 weeks. The incidence of flushing may be reduced by
administration with food and the incidence or severity of flushing may be reduced by
administering non-enteric coated aspirin 30 minutes prior to dosing. This drug can cause
lymphopenia and a CBC should be ordered prior to initiation, 6 months after initiation, and every
6 to 12 months thereafter. Dimethyl fumarate can cause angioedema or anaphylaxis. One case of
PML has been reported to date. Dimethyl fumarate has caused an increase in liver function tests
and a transient increase in eosinophilia.33
NEW THERAPIES IN DEVELOPMENT
Emerging Therapies—Immunomodulators
Laquinimod
Laquinimod is an orally-administered immunomodulator being studied for the treatment of
RRMS and SPMS. While the mechanism of action has not been fully elucidated, it is proposed
that laquinimod acts by affecting the T helper 1 to T helper 2 cytokine shift.34,35 Initially in the
Phase 3 trial Safety and Efficacy of Orally Administered Laquinimod Versus Placebo for
Treatment of Relapsing Remitting Multiple Sclerosis (RRMS) (ALLEGRO), laquinimod 0.6 mg
reduced annualized relapse rate, disability progression, mean cumulative number of enhancing
lesions, and new or enlarging lesions more effectively than placebo.36 However in the Study of
the Efficacy and Safety of Ranibizumab Injection in Patients With Macular Edema Secondary to
Branch Retinal Vein Occlusion (BRAVO) trial, laquinimod 0.6 mg failed to reduce relapse rates
compared with placebo; but, the 2 groups were not well-matched for disease severity.
Laquinimod did reduce annualized relapse rate, risk of disability progression, and reduced brain
volume loss compared with placebo once groups were adjusted for baseline differences.37 The
results from the pivotal Phase 3 trial Safety and Tolerability of Laquinimod in Subjects With
Relapsing and Remitting Multiple Sclerosis (CONCERTO) involving laquinimod 1.2 and 0.6 mg
versus placebo are expected in 2017.38 The most common adverse events included elevated
levels of liver enzymes, abdominal pain, back pain, and cough.36
Emerging Therapies—Monoclonal Antibodies
Monoclonal antibodies are biologic agents that recognize specific target antigens exclusively.
They are used to selectively bind to targets and cause immune responses, including cellular
apoptosis or inhibition of ligand-receptor binding. Daclizumab, alemtuzumab, ocrelizumab, and
natalizumab are humanized monoclonal antibodies. These are less immunogenic to the immune
system than either the murine or chimeric monoclonal antibodies because the only nonhuman
portion of the antibody is the complement sequence.18
Daclizumab
Daclizumab is a humanized monoclonal antibody that antagonizes the α subunit of interleukin-2
(IL-2) on activated lymphocytes, inhibiting CD25-IL-2 complex formation.3,36 IL-2 is
responsible for upregulation of the immune system, apoptosis of T cells, and inhibition of T-helper 17 cells. It has been proposed that the CD25-IL-2 complex in patients with MS is
abnormal, and daclizumab may be effective by inhibiting abnormal lymphocyte complexes from
becoming activated.3,39 Daclizumab, has been used to treat rheumatoid arthritis and other
autoimmune disorders. It has also been FDA-approved as brand name Zenapax for inhibiting
rejection of organ transplants.
Daclizumab high-yield process (HYP) is a newer formulation of daclizumab in development for
MS. In a recent Phase 3 study, daclizumab HYP 150 mg given subcutaneously every 4 weeks
reduced relapse rates and reduced new lesions on MRI more effectively than interferon beta-1a.
One of the most promising actions of this agent is activation of CD56 bright NK (natural killer)
cells, which may be a biomarker of MS activity. The side effect profile is of concern with all
monoclonal antibodies because they have the ability to deplete cell lines and lead to serious
infections. Adverse events reported were serious infections, skin rash, and abnormalities in liver
enzymes.3,40 This drug is expected to be reviewed for market approval.
Ocrelizumab
Ocrelizumab is a humanized monoclonal antibody targeting CD20 B cells. It has been shown to
substantially reduce relapse rates, disability progression, and disease activity on MRI for patients
with RRMS and SPMS. Infusions were given every 6 months during 2 Phase 3 trials, A Study of
Ocrelizumab in Comparison With Interferon Beta-1a (Rebif) in Patients with Relapsing Multiple
Sclerosis (OPERA) I and OPERA II, which are ongoing. Additionally, it has been shown to
reduce disability progression, time required to walk 25 feet, volume of brain lesions, and whole
brain volume loss for patients with PPMS in A Study of Ocrelizumab in Patients With Primary
Progressive Multiple Sclerosis (ORATORIO), a Phase 3 trial. This is the first large-scale trial to
show positive results for patients with PPMS. The most common adverse events were infusion
reactions. Patients were premedicated with steroids, antipyretics, and antihistamines. Genentech
is expected to seek market approval in 2016.41
SYMPTOM MANAGEMENT
In addition to treating relapses and also using disease-modifying medications, there remains a
role for managing the individual symptoms of MS.
Spasticity, feelings of stiffness and involuntary muscle spasms, is one of the more common MS
symptoms. It can be treated with antispasticity medications, such as the following: baclofen,
tizanidine or dantrolene, benzodiazepines, such as diazepam or clonazepam, and botulinum
toxin.8
Dalfampridine (Ampyra) is the only medication to be approved for the treatment of a specific
symptom of MS, to improve walking. It is a broad-spectrum potassium channel blocker, shown
to increase the conduction of action potentials in demyelinated axons. It is administered as 10 mg
twice daily and is contraindicated for patients with a history of seizure or moderate-to-severe
renal impairment. Adverse reactions include the following: asthenia, balance disorder, dizziness,
headache, insomnia, paresthesia, nasopharyngitis, pharyngolaryngeal pain, constipation,
dyspepsia, nausea, back pain, and urinary tract infection.1,42
Bladder issues can occur in at least 80% of patients with MS. Symptoms can include overactive
bladder or urinary retention. Anticholinergic medications, such as oxybutynin, solifenacin,
darifenacin, trospium, hyoscyamine, fesoterodine, propantheline, and dicyclomine, have been
used. Desmopressin acetate (DDAVP) has been used as a synthetic analog of the natural pituitary
hormone arginine vasopressin, an antidiuretic hormone affecting renal water conservation.
Patients have also used prazosin and tamsulosin as inhibitors of alpha-adrenergic receptors to
help with urinary dysfunction. Other patients have used botulinum toxin and some patients
require catheterization.1,8 Mirabegron (Myrbetriq) is a novel selective human beta-3 adrenergic
receptor agonist that relaxes the detrusor smooth muscle and increases bladder storage capacity.
It has been shown to be efficacious in a small study involving patients with MS who had
previously experienced low efficacy after treatment with antimuscarinics.43
Paresthesias and neuropathic pain can also affect patients with MS. Fifty-five percent of patients
have been shown to have substantial pain at least some of the time, with 48% experiencing
chronic pain. Antiepileptic medications, such as carbamazepine, oxcarbazepine, gabapentin,
pregabalin, and lamotrigine, as well as serotonin and norepinephrine reuptake inhibitors (SNRIs),
such as duloxetine and tricyclic antidepressants have been useful.1,8
For the management of fatigue, cognitive issues, and emotional issues, such as depression,
patients may turn to selective serotonin reuptake inhibitors (SSRIs), SNRIs, amantadine,
modafinil, methylphenidate, or dextroamphetamine. Pseudobulbar affect (PBA) disorder occurs
as a result of neurologic disorders whereby the patient experiences uncontrollable laughing
and/or crying. About 10% of patients who experience this disorder. Nuedexta is FDA approved
for the treatment of PBA and contains 20 mg of dextromethorphan (i.e., an uncompetitive N-Methyl-D-aspartate (NMDA) antagonist and sigma-1 agonist) and 10 mg of quinidine, which is
used to inhibit the metabolism of dextromethorphan via CYP2D6. It is dosed once daily for 7
days and then twice daily thereafter.44,45
Cannabinoids and MS Symptom Management46
According to Koppel and colleagues, the American Academy of Neurology recently published a
systematic review of literature from 1948 to 2013 addressing efficacy and safety data in regard to
cannabis use as a treatment for patients with MS and several other neurologic conditions.46 Orally administered products, such as oral cannabis extracts (OCE) (including brand name
Cannador, dronabinol [Marinol], and nabilone [Cesamet]), as well as oromucosal sprays (such as
nabiximols [Sativex]), and smoked or vaporized marijuana, provided the availability of THC and
cannabidiol in a variety of strengths. Symptom management of MS endpoints addressed included
spasticity, central pain or painful spasms (excluding neuropathic pain), urinary dysfunction, and
tremor.46
There is strong evidence to support the efficacy of OCE for the reduction of patient-reported
spasticity scores and moderate evidence to support the efficacy of THC and nabiximols (Sativex) for patient-reported spasticity scores. There is strong evidence to support the efficacy of OCE for
the reduction of central pain and moderate evidence to support the efficacy of THC or
nabiximols for the treatment of pain or painful spasms caused by MS. There is only moderate
evidence to support the efficacy of nabiximols for a reduction in bladder voiding; therefore, THC
and OCE would not be responsible for a reduction in bladder complaints. Moderate evidence
supports that THC and OCE are most likely ineffective for the treatment of tremors associated
with MS. There are insufficient data to support or refute the efficacy of smoked marijuana for the
relief of spasticity, pain, and painful spasms associated with MS.46
In summary, cannabinoids may be effective for aiding with spasticity, MS-related pain, painful
spasm, and bladder voiding. Although, while cannabinoids may be effective for the treatment of
MS symptoms and management, the psychoactive effects of cannabinoid consumption, such as
cognitive impairment, can deter product use, particularly for patients already experiencing
cognitive difficulties.46
Adherence to MS Medications
Adherence has been linked with improved outcomes for patients with MS, including fewer
relapses, hospital visits, hospital admissions, costs, and absences from work.47,48,49 According to
a 2014 Express Scripts Drug Trend Report, 23% of patients are not adherent to MS
medications.50 Other studies have shown that patient adherence to MS disease-modifying
medications varies from 61% to 87% and adherence to injectables ranged from 61% to 64%.
Patients tend to over-report their adherence as well.49 Similarly, a survey of patients at 17
neurology clinics found a patient-reported adherence rates between 36% and 39%.51
Factors affecting patient reported nonadherence include the following: forgetting to administer
medications, injection-site reactions, injection fatigue, side effects, frequency of administration,
cognition and complexity of regimen, monitoring requirements, presence of active disease
symptoms, patient self-efficacy, patient-clinician relationships, quality of life, patient perception
of the injectable medication, hope, depression, and degree of support.49,51 Cost may be another
major barrier to adherence, with an average cost per prescription for MS medications being
approximately $4500.48 Costs for MS medications have increased substantially over the last few
years, with increases of 9.2% in 2014 alone.50,52 It has been predicted that the costs for these
medications will only continue to increase, making insurance coverage, access to patient
assistance programs, and the availability of generic formulations even more important (Table 1).
Strategies to minimize barriers to adherence can include adherence aids, such as calendars,
alarms, journals, or cell phone applications. Patients and their caregivers may also benefit from
education about their disease and medications, as well as support groups. Choosing a medication
based on patient-specific factors, such as disease characteristics, treatment response, adverse
effects, and tolerability, cost or access issues, and choosing a medication to which a patient is
willing to be adherent and one that fits into their lifestyle is important. Using medications available in pre-filled syringes, auto-injectors, or pens can increase a patient’s comfort level with
an injectable medication, but may also increase direct costs of medication, as shown in Table 1.
Patients and providers should also be educated on the availability of patient assistance programs.
APPROVAL PROCESS FOR NON-INNOVATOR MS MEDICATIONS
There are currently 2 approval pathways in the United States for non-innovator medications.
These include the abbreviated new drug application (ANDA) approval process for generic
versions of reference listed drugs. This information is listed in the FDA Orange Book of
approved drug products, which contains therapeutic equivalence evaluations. There is also the
biosimilar approval process for reference drugs, which is listed in the FDA Purple Book, which
contains licensed biological products with the reference product exclusivity and biosimilarity or
interchangeability evaluations.53-55 The appropriate pathway for approval of a non-innovator
medication is based on whether the innovator is listed in the Orange Book or the Purple Book.
None of the currently approved MS medications is a branded biologic product that would require
FDA approval of a biosilimiar or interchangeable formulation through the abbreviated licensure
pathway for biologic drugs; rather, they could be approved as traditional generic formulations
through the ANDA pathway.
Case Study: Generic Glatiramer Acetate (Glatopa)
Glatopa was approved through the ANDA pathway because the innovator glatiramer acetate
product (Copaxone) is listed in the Orange Book.56 The approval of Glatopa has garnered some
controversy because of citizen and competitor manufacturer concerns about the appropriateness
of the ANDA pathway for amino acid and peptide polymer medication formulations, such as
Copaxone. However, these concerns seem largely based on misinformation about the
physicochemical characteristics of Copaxone and the ability to demonstrate active ingredient
sameness between Glatopa and Copaxone. Copaxone demonstrates conservation and variation of
certain characteristics from batch to batch. The FDA thoroughly evaluated active ingredient
sameness and performed its own analytical testing before determining the equivalence of these 2
products. Glatopa was determined to have equivalent fundamental reaction scheme, equivalent
physicochemical properties, including composition, and equivalent structural signatures for
polymerization and depolymerization.56-58
CONCLUSION
There is no cure for MS. Treatment approaches include managing exacerbations and the use of
disease-modifying therapies to slow or halt disease progression. Medications can also be used for
the management of specific symptoms. Newer medications are highly efficacious and offer
alternative routes and frequencies of administration; but these medications may also require more
complex monitoring for possible adverse effects. Treatment should be chosen based on
individual patient characteristics and patient willingness to be adherent.
REFERENCES
- Bainbridge JL, Miravalle A, Corboy JR. Chapter 39. “Multiple Sclerosis.” In: Dipiro JT,
Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A
Pathophysiologic Approach. 9 Ed. New York, NY: McGraw-Hill;
2014. http://accesspharmacy.mhmedical.com/content/aspx?bookid=689&Sectionid=4531048. Accessed June, 08, 2015.
- National Multiple Sclerosis Society. What is MS? National Multiple Sclerosis Society Web
site. http://www.nationalmssociety.org/What-is-MS/. Accessed December 9, 2015.
- Kim SE. Daclizumab treatment for multiple sclerosis. Pharmacotherapy. 2009;29(2):227-235.
- Polman CH, Reingold SC, Banwell B, et al; Diagnostic criteria of multiple sclerosis: 2010
revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302.
- National Multiple Sclerosis Society. Clinically Isolated Syndrome.
National Multiple Sclerosis Society Web site.
http://www.nationalmssociety.org/Symptoms-Diagnosis/Clinically-Isolated-Syndrome-(CIS).
Accessed December 9, 2015.
- Adelman G, Rane SG, Villa KF. The cost burden of multiple sclerosis in the United States: a
systematic review of the literature.J Med Econ. 2013;16(5):639-647.
- National Multiple Sclerosis Society. The Use of Disease-Modifying Therapies in Multiple
Sclerosis: Principles and Current Evidence. National Multiple Sclerosis Society Web
site. http://www.nationalmssociety.org/NationalMSSOciety/media/MSNationalFiles/Brochur
es/DMT_Consensus_MS_Coalition.pdf. Accessed June 8, 2015.
- National Multiple Sclerosis Society. Medications. National Multiple Sclerosis Society Web
site. http://www.nationalmssociety.org/Treating-MS/Medications. Accessed December 9,
2015.
- Burton JM, O’Connor PW, Hohol M, Beyene J. Oral versus intravenous steroids for
treatment of relapses in multiple sclerosis. Cochrane Database Syst Rev. 2009;(3):CD006921.
- Beck RW, Cleary PA, Anderson MM Jr, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 1992;326(9):581-588.
- National Multiple Sclerosis Society. Vision Problems. National Multiple Sclerosis Society
Web site. http://www.nationalmssociety.org/What-is-MS/. Accessed December 9, 2015.
- Menge T, Weber MS, Hemmer B, et al. Disease-modifying agents for multiple sclerosis:
recent advances and future prospects. Drugs. 2008;68(17):2445-2468.
- O’Connor P, Comi G, Montalban X, et al; Oral fingolimod (FTY720) in multiple sclerosis:
two-year results of a phase II extension study. Neurology 2009;72:73-79.
- Goodin D. Comparative studies of glatiramer acetate and interferon beta. Int MS J. 2008;15(2):39-41.
- Plegridy (peginterferon beta-1a) injection for subcutaneous injection [package insert].
Cambridge, MA: Biogen Idec; 2015
- Morrissey SP, Le Page E, Edan G. Mitoxantrone in the treatment of multiple sclerosis. Int
MS J. 2005;12(3):74-87.
- Thompson JP, Noyes K, Dorsey ER, et al. Quantitative risk-benefit analysis of natalizumab. Neurology. 2008;71(5):357-364.
- Cree B. Emerging monoclonal antibody therapies for multiple sclerosis.Neurologist. 2006;12(4):171-178.
- Goodman B. New clues to Link Between MS Drug Tysabri and Rare Brain Disease. WebMD
News Archive. March 25, 2014. http://www.webmd.com/multiple-sclerosis/news/20140325/new-clues-to-link-between-ms-drug-tysabri-and-rare-brain-disease.
Accessed November 12, 2015.
- Tysabri.com. Fight with knowledge: Important Safety Information. Biogen Web
site. http://www.tysabri.com/about/safety. Accessed November 12, 2015.
- Osborne, R. Buzz around Campath proof-of-concept trial in MS. Nature Biotechnol. 2009;27(1):6-8.
- Coles AJ, Compston DA, Selmaj KW, et al; for the CAMMS223 Investigators.
Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359(17):1786-1801.
- Lemtrada (alemtuzumab) [prescribing information]. Cambridge, MA: Genzyme Corporation;
2014.
- Foster CA, Howard LM, Schweitzer A, et al. Brain penetration of the oral
immunomodulatory drug FTY720 and its phosphorylation in the central nervous system
during experimental autoimmune encephalomyelitis: consequences for mode of action in
multiple sclerosis. J Pharmacol Exp Ther. 2007;323(2):469-476.
- Mehling M, Brinkmann V, Antel J, et al. FTY720 therapy exerts differential effects on T cell
subsets in multiple sclerosis. Neurology. 2008;71(16):1261-1267.
- Gilenya (fingolimod) capsules, for oral use [prescribing information]. East Hanover, NJ:
Novartis Pharmaceuticals Corporation; 2015.
- Brooks M. Third case of PML With Fingolimod (Gilenya) in MS. Medscape Medical News.
August 18, 2015. Medscape Web site. http://www.medscape.com/viewarticle/849677.
Accessed November 12, 2015.
- Aubagio (teriflunomide) tablets, for oral use [prescribing information]. Cambridge, MA:
Genzyme Corporation; 2014.
- Tallantyre E, Evangelou N, Constantinescu CS. Spotlight on teriflunomide. Int MS J.
2008;15(2):62-68.
- O’Connor PW, Li D, Freedman MS, et al; for the Teriflunomide Multiple Sclerosis Trial
Group and the University of British Columbia MS/MRI Research Group. A Phase II study of
the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology. 2006;66(6):894-900.
- Schimrigk S, Brune N, Hellwig K, et al; Oral fumaric acid esters for the treatment of active
multiple sclerosis: an open-label, baseline-controlled pilot study. European Journal of
Neurology 2006;13:604-610.
- National Institutes of Health. Dimethyl fumarate. Clinical Trials Web
site. http://www.clinicaltrials.gov/ct2/results?term=dimethyl+fumarate. Accessed June 25,
2009.
- Tecfidera (dimethyl fumarate) delayed-release capsules, for oral use [prescribing informtion].
Cambridge, MA: Biogen Inc.; 2013.
- Comi G, Pulizzi A, Rovaris M, et al; for the LAQ/5062 Study Group. Effect of laquinimod
on MRI-monitored disease activity in patients with relapsing-remitting multiple sclerosis: a
multicentre, randomized, double-blind, placebo-controlled phase IIb study. Lancet. 2008;371(9630):2085-2092.
- Weiner HL. Oral laquinimod for treatment of relapsing-remitting multiple sclerosis. Lancet
Neurol. 2008;7(8):672-673.
- Comi G, Jeffery D, Kappos L, et al; for the ALLEGRO Study Group. Placebo-controlled trial
of oral Laquinimod for multiple sclerosis. N Engl J Med. 2012;366(11):1000-1009.
- Jeffrey S. Laquinimod Trial Misses Primary Endpoint in MS. Medscape Medical News.
August, 1, 2001. Medscape Web site. http://www.medscape.com/viewarticle/747361.
Accessed November 12, 2015.
- Teva and Active Biotech Announce Completion of Patient Enrollment in Laquinimod Phase
III CONCERTO Trial. June 25, 2015. Business Wire Web site. June 25,
2015. http://www.businesswire.com/news/home/20150625005155/en/. Accessed November
12, 2015.
- Oh U, Blevins G, Griffith C, et al. Regulatory T cells are reduced during anti-CD25 antibody
treatment of multiple sclerosis. Arch Neurol. 2009;66(4):471-479.
- Kappos L, Wiendl H, Selmaj K, et al; Daclizumab HYP versus Interferon Beta-1a in
relapsing multiple sclerosis. N Engl J Med. 2015;373(15):1418-1428.
- National Multiple Sclerosis Society. News. National Multiple Sclerosis Society Web
site http://www.nationalmssociety.org/About-the-Society/News/Positive-Results-Announced-from-Two-Phase-III-Clin. Accessed November 12, 2015.
- Ampyra (dalfampridine) extended release tablets, for oral use [prescribing information].
Ardsley, NY: Acorda Therapeutics, Inc.; 2014.
- Risi O, Cito L, Andretta E, et al. Mirabegron in treatment of neurological overactive bladder
in multiple sclerosis patients. International Continence Society Web
site. http://www.ics.org/Abstracts/Publish/218/000566.pdf. Accessed December 11, 2015.
- National Multiple Sclerosis Society. MS Symptoms. National Multiple Sclerosis Society Web
site. http://www.nationalmssociety.org/Symptoms-Diagnosis/MS-Symptoms/Emotional-Changes. Accessed December 11, 2015.
- Nuedexta (dextromethorphan hydrobromide and quinidine sulfate) capsules, for oral use
[prescribing information]. Aliso Viejo, CA: Avanair Pharmaceuticals, Inc.; 2015.
- Koppel B, Brust J, Fife T, et al. Systematic review: efficacy and safety of medical marijuana
in selected neurologic disorders: report of the Guideline Development Subcommittee of the
American Academy of Neurology. Neurology. 2014;82(17):1556-1563.
- Steinberg SC, Faris RJ, Chang CF, et al. Impact of adherence to interferons in the treatment
of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig.
2010;30(2):89-100.
- Tan H, Cai Q, Agarwal S, et al. Impact of adherence to disease-modifying therapies on
clinical and economic outcomes among patients with multiple sclerosis. Adv Ther.
2011;28(1):51-61.
- Remington G, Rodriguez Y, Logan D, et al. Facilitating medication adherence in patients with multiple sclerosis. Int J MS Care. 2013;15(1):36-45.
- Express Scripts. 2014 Drug Trend Report. http://lab/express-scripts.com/drug-trend-report/.
Accessed June 1, 2015
- Treadaway K, Cutter G, Salter A, et al. Factors that influence adherence with disease-modifying therapy in MS. J Neurol. 2009;256(4):568-576.
- Hauser SL, Johnston SC. Multiple sclerosis drugs: sticker shock. Ann Neurol. 2012;71(5):A5-A6.
- U.S. Food and drug Administration (FDA). Orange Book: Approved Drug Products with
Therapeutic Equivalence Evaluations. FDA Web site.
http://www.accessdata.fda.gov/scripts/cder/ob/default.cfm. Accessed December 8, 2015.
- U.S. Food and Drug Administration (FDA). Purple Book: Lists of Licensed Biological
Products with Reference Product Exclusivity and Biosimilarity or Interchangeable
Evaluations. FDA Web site.
http://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/
approvalapplications/therapeuticbiologicapplications/biosimilars/ucm411418.htm. Accessed
December 15, 2015.
- U.S. Food and Drug Administration (FDA). Guidances (Drugs): Biosimilars. FDA Web
site. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm
290967.htm. Accessed June 8, 2015.
- Woodcock J. Citizen Petition Denial Letter From CDER to Teva Pharmaceuticals.
Regulations.gov Web site. http://www.regulations.gov/#!documentDetail;D=FDA-2015-P-1050-0012. Accessed December 15, 2015.
- Anderson J, Bell J, Bishop, et al. Demonstration of equivalence of a generic glatiramer
acetate (GlatopaTM). J Neurol Sciences. 2015;359(1-2):24-34.
- D’Alessandro JS, Duffner J, Pradines J, et al. Equivalent gene expression profiles between
Glatopa and Copaxone. PLoS One. 2015;10(10):e0140299.
Back to Top