
Expired activity
Please go to the PowerPak
homepage and select a course.
Type 2 Diabetes – Pathophysiology and Pharmacology Review (Article)
INTRODUCTION
The number of medications and combination products available for the treatment of type 2 diabetes mellitus (T2DM) has increased dramatically in recent years. This rapid expansion of pharmacologic options is due, at least in part, to a better understanding of the pathophysiology of T2DM. Traditionally, T2DM has been described by the “triumvirate”: 1) impaired insulin secretion due to declining pancreatic β-cell function; 2) insulin resistance leading to decreased glucose uptake by peripheral tissues (i.e., muscle and adipose); and 3) increased glucose production by the liver due to augmented gluconeogenesis.1 Since the late 1980’s, however, the “triumvirate” has expanded to include additional core defects. While the list of pathophysiologic defects continues to grow, the “ominous octet” provides a representation of 8 key defects found in T2DM (Figure 1).2 New classes of medications, such as the dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and sodium-glucose cotransporter- 2 (SGLT-2) inhibitors, specifically target some of the more recently identified defects described in the “ominous octet” to help manage uncontrolled hyperglycemia.
Figure 1. The Ominous Octet: Multiple Pathophysiologic Defects in Type 2 Diabetes Mellitus (T2DM)2
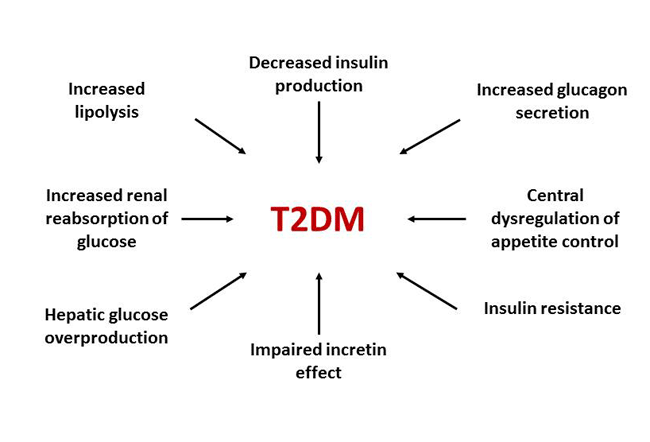
This review will focus on incretin-based therapies (DPP-4 inhibitors and GLP-1 receptor agonists) and SGLT-2 inhibitors currently approved for the treatment of T2DM. The module will emphasize the pharmacology and key clinical effects of these drugs and describe how each medication class fits into treatment paradigms currently outlined in clinical recommendations established by the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology (ACE).
PATHOPHYSIOLOGY AND PHARMACOLOGY OF T2DM
People with T2DM present with a variety of core organ defects that contribute to hyperglycemia. Given the multiple pathophysiologic targets that exist in the setting of T2DM, it is common to encounter patients who require the use of multiple antihyperglycemic agents to successfully reach treatment goals. Indeed, treatment recommendations from the ADA and AACE/ACE outline evidence-based strategies for the use of combination therapy to meet patient-specific treatment goals.3,4 Incretin-based therapies and SGLT-2 inhibitors have an ever- increasing emphasis within clinical guideline recommendations and are, therefore, increasingly utilized in clinical practice.
The incretin effect in T2DM
Hormonal regulation of glucose metabolism extends well beyond the physiologic effects of insulin and glucagon. Incretin (INtestinal seCRETion of INsulin) hormones, namely GLP-1 and gastric inhibitory peptide (GIP), have long been recognized as playing key roles in glucose homeostasis. The term incretin effect is used to describe the phenomenon that oral glucose induces a more robust insulin response than intravenous glucose administration.5 This observation supports the notion that signals originating from the gut in response to the oral ingestion of nutrients are instrumental in the stimulation of mealtime insulin release. In the 1970’s, GIP was identified as the first incretin hormone. Upon study, GIP was shown to stimulate insulin secretion from pancreatic β-cells.6,7 GLP-1 was later identified in the 1980’s as another primary incretin hormone responsible for the stimulation of insulin release to cover carbohydrate utilization following a meal.8 In total, the effects of incretin hormones are believed to be responsible for up to 60% of postprandial insulin release.9 In light of the important contribution of the incretin effect to prandial insulin release, augmentation of this system in people with T2DM has been shown to be an important pharmacologic approach to glycemic management.
Following oral nutrient intake, GLP-1 is secreted from intestinal L-cells located in the ileum and colon. Multiple mechanisms contribute to the glucoregulatory effects of GLP-1. Principally, GLP-1 induces glucose-dependent insulin secretion from pancreatic β-cells,10,11 decreases plasma glucagon concentrations,11 and delays gastric emptying.12 The delayed gastric emptying induced by GLP-1 helps decrease postprandial hyperglycemia and induces a feeling of fullness, which is believed to contribute to a reduced appetite and, therefore, reduced food intake. GLP-1 is additionally believed to induce satiety via a direct effect within the central nervous system.13
While native GLP-1 has been targeted as a therapeutic agent due to its favorable effects on glycemia, its clinical utility is limited due to its extremely short half-life.13 Both endogenously secreted GLP-1 and GIP are quickly cleared following release due to both renal clearance and rapid metabolism by the enzyme DPP-4.14 Two therapeutic strategies currently exist to overcome the short half-life of incretin hormones and pharmacologically augment the incretin effect: 1) inject GLP-1 receptor agonists that are resistant to degradation by the DPP-4 enzyme and 2) orally administer DPP-4 inhibitors that delay the enzymatic degradation of naturally secreted incretin hormones (Figure 2).3,4 Both strategies are utilized to augment the incretin effect, but there are key clinical differences among the DPP-4 inhibitors and GLP-1 receptor agonists. Table 1 provides a general clinical comparison of these medication classes for the treatment of T2DM.15,16
Figure 2. Currently Available Incretin-based Therapies3,4
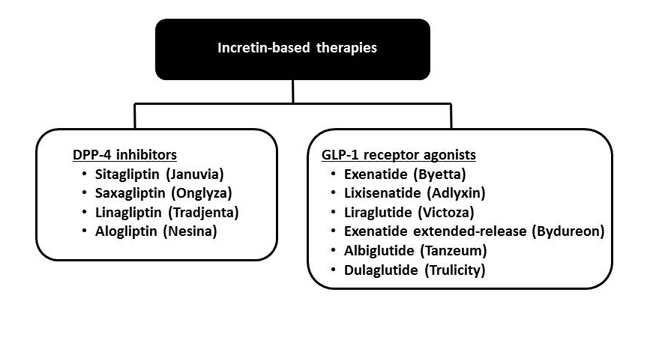
Abbreviations: DPP-4 = dipeptidyl peptidase-4; GLP-1 = glucagon-like peptide-1.
| Table 1. Select Clinical Properties of GLP-1 Receptor Agonists and DPP-4 Inhibitors15,16 |
| |
DPP-4 inhibitors |
GLP-1 receptor agonists |
| Slow gastric emptying? |
No |
Yes* |
| Reduce postprandial hyperglycemia? |
Yes |
Yes |
| Effect on weight |
Weight neutral |
Weight loss |
| Common side effects |
Headache, sinusitis, rhinorrhea |
Nausea, vomiting, diarrhea |
| Route of administration |
Oral |
Subcutaneous injection |
| Associated with hypoglycemia when used as monotherapy? |
No |
No |
Abbreviations: DPP-4 = dipeptidyl peptidase-4; GLP-1 = glucagon-like peptide-1.
*Short-acting GLP-1 receptor agonists. |
DPP-4 inhibitors
DPP-4 inhibitors are orally administered medications that prevent the inactivation of incretin hormones; this, in turn, increases the amount of available, active incretin hormones.17 By increasing the pool of active incretin hormones, DPP-4 inhibitors increase glucose-dependent stimulation of insulin secretion and inhibit glucagon secretion.18 DPP-4 inhibitor therapy is not associated with weight loss, which is attributed to a negligible effect of these agents on gastric emptying.15,16 As such, the DPP-4 inhibitors are generally considered “weight neutral” antihyperglycemic medications. DPP-4 inhibitors are generally well tolerated, with a low risk of contributing to hypoglycemia when used as monotherapy or in combination with metformin due to their glucose-dependent mechanism of action. There are currently 4 DPP-4 inhibitors approved by the United States (U.S.) Food and Drug Administration (FDA): sitagliptin (Januvia), saxagliptin (Onglyza), linagliptin (Tradjenta), and alogliptin (Nesina).19-22 The DPP-4 inhibitor class of medications is particularly suited for addressing postprandial glycemic excursions, with an expected hemoglobin A1c (A1C) reduction falling in the range of approximately 0.4% to 0.9% (Table 2).19-23
| Table 2. Key Clinical Characteristics and Dosing Recommendations for Currently Available DPP-4 Inhibitors*19-23 |
| |
Sitagliptin (Januvia) |
Saxagliptin (Onglyza) |
Linagliptin (Tradjenta) |
Alogliptin
(Nesina) |
| Hypoglycemia risk |
Low |
Low |
Low |
Low |
| Dose (normal renal function) |
100 mg daily |
5 mg daily |
5 mg daily |
25 mg daily |
| Dose adjustment for renal impairment |
- CrCl < 50 mL/min:
50 mg daily
- CrCl ≤ 30 mL/min:
25 mg daily
|
- CrCl ≤ 50 mL/min:
2.5 mg daily
|
No renal dose adjustment recommended |
- CrCl < 60 mL/min:
12.5 mg daily
- CrCl < 30 mL/min:
6.25 mg daily
|
| Average A1C lowering* |
0.65% to 0.79% |
0.36% to 0.82% |
0.50% to 0.69% |
0.47% to 0.85% |
| Average weight change* |
+0.3 to +1.2 kg |
-0.51 to +1.3 kg |
+0.33 to +1.1 kg |
+0.14 to +0.51 kg |
Abbreviations: A1C = hemoglobin A1c; CrCl = creatinine clearance; DPP-4 = dipeptidyl peptidase-4.
*Baseline characteristics of study groups and concomitant medications varied among the studies. |
Dosing and administration: All DPP-4 inhibitors currently approved for use in the U.S. are administered orally once daily, although the specific dosing parameters for each individual agent varies, as summarized in Table 2.19-22 With the exception of linagliptin, the DPP-4 inhibitors are primarily cleared by the kidneys and, thus, require renal dose adjustment.23
Adverse reactions and tolerability: The DPP-4 inhibitor class of medications is generally very well tolerated, although post-marketing reports have described serious adverse events (see Warnings and precautions section). Some commonly reported side effects of DPP-4 inhibitors noted in clinical trials include upper respiratory tract infection, urinary tract infection (UTI), nasopharyngitis, and headache (Table 3).19-22
| Table 3. Common Side Effects of DPP-4 Inhibitors Noted in Clinical Trials19-22 |
| Agent (brand name) |
Common side effects* |
| Sitagliptin (Januvia) |
- Upper respiratory tract infection
- Nasopharyngitis
- Headache
|
| Saxagliptin (Onglyza) |
- Upper respiratory tract infection
- Urinary tract infection
- Headache
|
| Linagliptin (Tradjenta) |
|
| Alogliptin (Nesina) |
- Upper respiratory tract infection
- Nasopharyngitis
- Headache
|
Abbreviations: DPP-4 = dipeptidyl peptidase-4.
* Side effects occurred in ≥ 5% of study participants in studies of sitagliptin, saxagliptin, and linagliptin and in ≥ 4% of participants in studies of alogliptin. |
Warnings and precautions: Several post-marketing reports of serious adverse events associated with DPP-4 inhibitors have led to the addition of several warnings and precautions to the U.S. labels for these medications. Table 4 provides a summary of these warnings and precautions that are noted in the prescribing information for these products.19-22
| Table 4. Warnings and Precautions for Currently Available DPP-4 Inhibitors19-22 |
| |
Sitagliptin
(Januvia) |
Saxagliptin
(Onglyza) |
Linagliptin
(Tradjenta) |
Alogliptin
(Nesina) |
| Pancreatitis |
X |
X |
X |
X |
| Acute renal failure |
X |
|
|
|
| Hypoglycemia (when used in combination with SU or insulin) |
X |
X |
X |
X |
| Allergic/hypersensitivity reactions |
X |
X |
X |
X |
| Severe arthralgia |
X |
X |
X |
X |
| Bullous pemphigoid |
X |
X |
X |
X |
| Heart failure |
|
X |
|
X |
| Hepatic failure |
|
|
|
X |
| Abbreviations: DPP-4 = dipeptidyl peptidase-4; SU = sulfonylurea. |
GLP-1 receptor agonists
GLP-1 receptor agonists continue to grow in popularity for the treatment of T2DM due to their beneficial effects on glycemia and weight. There are several important clinical considerations when choosing 1 of the 6 currently available GLP-1 receptor agonists for a patient.23 Factors such as convenience (e.g., frequency of administration) and tolerability are certainly important, but the choice of agent may also be determined by the individualized glycemic needs of the patient. Currently available GLP-1 receptor agonists have variable effects on fasting and postprandial blood glucose levels: short-acting GLP-1 receptor agonists have a greater effect on postprandial glucose and long-acting agents have a greater relative effect on fasting glucose (Table 5).16 These differences may lead a clinician to choose a short-acting GLP- 1 receptor agonist for a patient with predominant postprandial hyperglycemia. Compared to DPP-4 inhibitors, GLP-1 receptor agonists have the potential for more robust A1C reductions (0.5% to 2.0%) and weight loss.
| Table 5. Comparison of Currently Available GLP-1 Receptor Agonists 16,23-32 |
| |
Exenatide (Byetta) |
Lixisenatide (Adlyxin) |
Liraglutide (Victoza) |
Exenatide extended-release (Bydureon) |
Albiglutide (Tanzeum) |
Dulaglutide (Trulicity) |
| Administration frequency |
Twice daily |
Once daily |
Once daily |
Once weekly |
Once weekly |
Once weekly |
| Duration of action |
Short-acting |
Short-acting |
Long-acting |
Long-acting |
Long-acting |
Long-acting |
| Effect on gastric emptying rate |
Slows |
Slows |
No effect |
No effect |
No effect |
No effect |
| Effect on fasting blood glucose levels |
Modest reduction |
Modest reduction |
Strong reduction |
Strong reduction |
Strong reduction |
Strong reduction |
| Effect on postprandial blood glucose levels |
Strong reduction |
Strong reduction |
Modest reduction |
Modest reduction |
Modest reduction |
Modest reduction |
| Average A1C reduction* |
0.5% to 1.0% |
0.6% to 1.0% |
0.83% to 1.36% |
1.5% to 2.0% |
0.76% to 1.04% |
0.55% to 1.48% |
| Average weight change (kg)* |
-0.3 to -3.1 |
-0.2 to -2.96 |
+0.8 to -2.6 |
-2.3 to -4.7 |
+0.3 to -0.21 |
+0.9 to -2.9 |
Abbreviations: A1C = hemoglobin A1c; GLP-1 = glucagon-like peptide-1.
*Baseline characteristics of study groups and concomitant medications varied among studies. |
Dosing and administration: Currently, GLP-1 receptor agonists are only available in injectable form and require administration via subcutaneous injection.24-29 (An oral GLP-1 receptor agonist is currently in clinical development.) Table 6 provides a summary of dosing recommendations for currently available GLP-1 receptor agonists.
| Table 6. Dosing Recommendations for Currently Available GLP-1 Receptor Agonists24-29 |
| Agent (brand name) |
Recommended dosing and titration schedule |
| Exenatide (Byetta) |
- Initially, 5 mcg twice daily within 60 minutes of the morning and evening meals
- May be increased to 10 mcg twice daily after 1 month of therapy according to clinical response
|
| Lixisenatide (Adlyxin) |
- Initially, 10 mcg once daily within 1 hour before the first meal of the day for 14 days
- Increase to 20 mcg once daily starting on day 15 of treatment
|
| Liraglutide (Victoza) |
- Initially, 0.6 mg once daily independent of meals
- After 1 week, increase the dose to 1.2 mg once daily
- If the 1.2-mg dose does not result in acceptable glycemic control, the dose can be increased to 1.8 mg once daily
|
| Exenatide extended-release (Bydureon) |
- 2 mg once weekly (every 7 days) independent of meals
|
| Albiglutide (Tanzeum) |
- Initially, 30 mg once weekly (every 7 days) independent of meals
- May be increased to 50 mg once weekly if the glycemic response to the 30-mg dose is inadequate
|
| Dulaglutide (Trulicity) |
- Initially, 0.75 mg once weekly (every 7 days) independent of meals
- May be increased to 1.5 mg once weekly for additional glycemic control
|
| Abbreviations: GLP-1 = glucagon-like peptide-1. |
Adverse reactions and tolerability: Since they are administered via injection and affect gastric emptying (particularly in the case of shorter-acting agents), GLP-1 receptor agonists present some tolerability concerns. The dose-limited side effects for shorter-acting agents such as exenatide, lixisenatide, and liraglutide are largely gastrointestinal in nature. Table 7 provides a summary of common side effects noted in clinical trials of GLP-1 receptor agonists.24-29
| Table 7. Common Side Effects of GLP-1 Receptor Agonists Noted in Clinical Trials24-29 |
| Agent (brand name) |
Common side effects* |
| Exenatide (Byetta) |
- Nausea, hypoglycemia, vomiting, diarrhea, feeling jittery, dizziness, headache, dyspepsia, constipation, and asthenia
|
| Lixisenatide (Adlyxin) |
- Nausea, vomiting, headache, diarrhea, dizziness, and hypoglycemia
|
| Liraglutide (Victoza) |
- Nausea, diarrhea, headache, and vomiting
|
| Exenatide extended-release (Bydureon) |
- Nausea, diarrhea, headache, vomiting, constipation, injection-site pruritus, injection-site nodules, and dyspepsia
|
| Albiglutide (Tanzeum) |
- Upper respiratory tract infection, diarrhea, nausea, injection-site reactions, cough, back pain, arthralgia, sinusitis, and influenza
|
| Dulaglutide (Trulicity) |
- Nausea, diarrhea, vomiting, abdominal pain, and decreased appetite
|
Abbreviations: GLP-1 = glucagon-like peptide-1.
* Side effects occurred in ≥ 5% of study participants. |
Warnings and precautions: Table 8 provides a summary of warnings and precautions noted in the GLP-1 receptor agonist labels.24-29 GLP-1 receptor agonists have been associated with post- marketing reports of pancreatitis. These agents are also associated with a minimal risk of hypoglycemia when used as monotherapy or as add-on to metformin monotherapy, but they can contribute to hypoglycemia when used in combination with medications associated with hypoglycemia, such as insulin and insulin secretagogues.
| Table 8. Warnings and Precautions for Currently Available GLP-1 Receptor Agonists24-29 |
| |
Exenatide (Byetta) |
Lixisenatide (Adlyxin) |
Liraglutide (Victoza) |
Exenatide extended-release (Bydureon) |
Albiglutide (Tanzeum) |
Dulaglutide (Trulicity) |
| Pancreatitis |
X |
X |
X |
X |
X |
X |
| Hypoglycemia (when used in combination with SU or insulin) |
X |
X |
X |
X |
X |
X |
| Renal impairment/AKI |
X |
X |
X |
X |
X |
X |
| Severe GI disease |
X |
|
|
X |
|
X |
| Hypersensitivity reactions |
X |
X |
X |
X |
X |
X |
| Immunogenicity (anti-drug antibody production) |
|
X |
|
|
|
|
| Thyroid C-cell tumors |
|
|
X |
X |
X |
X |
| Injection-site reactions |
|
|
|
X |
|
|
| Abbreviations: AKI = acute kidney injury; GI = gastrointestinal; GLP-1 = glucagon-like peptide-1; SU = sulfonylurea. |
Role of SGLT-2 in T2DM
Each day, approximately 180 grams of glucose is filtered in the kidneys of a normal healthy adult. Typically, all of this glucose is reabsorbed, with less than 1% of filtered glucose ultimately being excreted in the urine in a person without T2DM.33 The reabsorption of glucose from the tubule is accomplished by sodium-glucose cotransporters (SGLTs).34 SGLTs encompass a family of membrane proteins that are responsible for the transport of a variety of substances across the membrane of the proximal renal tubules and the intestinal epithelium.35 Within the kidneys, there are 2 salient SGLTs: SGLT-1 and SGLT-2. SGLT-1 is a low-capacity, high-affinity sodium-glucose transporter found primarily in the gastrointestinal tract, but it is also found in the S3 segment of the proximal tubule within the nephron.35 SGLT-1 is primarily responsible for glucose absorption in the gastrointestinal tract and only accounts for approximately 10% of glucose reabsorption in the kidney. SGLT-2, in contrast, is a high-capacity, low-affinity transporter expressed primarily in the kidney and is predominantly located in the S1 (early) segment of the proximal tubule. SGLT-2 accounts for the majority of glucose reabsorption in the kidney and has, thus, surfaced as a therapeutic target for the treatment of T2DM.35
As mentioned, in the absence of T2DM, nearly all glucose filtered through the glomeruli is reabsorbed via SGLTs. However, when the glucose load exceeds the renal “glucose threshold,” as is the case in people with uncontrolled hyperglycemia, glucose begins to appear in the urine – a clinical finding termed glucosuria.34 The most common cause of glucosuria is T2DM, and the average patient will not “spill” glucose into their urine until his or her blood glucose concentration exceeds approximately 180 mg/dL.34
SGLT-2 inhibitors
Since SGLT-2 activity accounts for the majority of glucose reabsorption in the kidney, inhibition of SGLT-2 effectively blocks the reabsorption of filtered glucose, leading to a pharmacologically augmented glucosuria. Clinically, SGLT-2 inhibition can lead to improvements in glycemic control in addition to a loss of approximately 200 to 300 kilocalories per day, which provides a potential benefit of weight loss.34 There are currently 3 SGLT-2 inhibitors available in the U.S.: canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance).36-38
Dosing and administration: Currently available SGLT-2 inhibitors are administered orally once- daily.36-38 Table 9 lists specific dosing information for each available agent. Because the mechanism of action of SGLT-2 inhibitors is dependent on kidney function, these agents are not recommended for patients with diminished renal function. Table 9 provides dosing recommendations in the presence and absence of renal impairment.36-38
| Table 9. Key Clinical Characteristics and Dosing Recommendations for Currently Available SGLT-2 Inhibitors36-38 |
| |
Canagliflozin
(Invokana) |
Dapagliflozin
(Farxiga) |
Empagliflozin
(Jardiance) |
| Hypoglycemia risk (monotherapy or in combination with metformin) |
Low |
Low |
Low |
| Dose (normal renal function) |
- 100 mg daily before breakfast
- Increase to 300 mg daily if needed
|
- 5 mg daily in the morning
- Increase to 10 mg daily if needed
|
- 10 mg daily in the morning
- Increase to 25 mg daily if needed
|
| Dose adjustment for renal impairment |
- eGFR ≥ 60 ml/min/1.73 m2: No dosage adjustment needed
- eGFR 45 - 59 ml/min/1.73 m2: Do not exceed 100 mg/day
- eGFR < 45 ml/min/1.73 m2: Do not initiate; discontinue in patients currently receiving drug
|
- eGFR < 60 mL/min/1.73 m2: Do not initiate; discontinue in patients currently receiving drug
|
- eGFR ≥ 45 ml/min/1.73 m2: No dosage adjustment needed
- eGFR < 45 ml/min/1.73 m2: Do not initiate; discontinue in patients currently receiving drug
|
| Effect on weight |
Loss |
Loss |
Loss |
| Abbreviations: CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; SGLT-2 = sodium-glucose cotransporter-2. |
Adverse reactions and tolerability: The most common adverse reactions associated with SGLT- 2 inhibitor therapy include an increased risk of certain infections and volume status-related events. As a class, SGLT-2 inhibitors are associated with an increased risk of UTIs and genital mycotic infections.39 A meta-analysis of SGLT-2 inhibitor therapy reported an odds ratio of 3.5 for the development of genital infections in patients treated with SGLT-2 inhibitors versus placebo.40 In clinical trials, SGLT-2 inhibitor therapy was associated with reductions of systolic blood pressure in the range of 2 to 10 mmHg.41 Likewise, hypotensive events secondary to volume depletion with SGLT-2 inhibitor therapy have been reported.36-38 Low rates of hypoglycemia have been reported in clinical trials of SGLT-2 inhibitors, except in patients receiving therapy with background insulin or insulin secretagogues.39
Recent case reports of euglycemic diabetic ketoacidosis (DKA) in patients receiving treatment with SGLT-2 inhibitors have been reported. In a recent FDA drug safety communication regarding SGLT-2 inhibitor-associated DKA, hypovolemia and acute renal impairment were listed as potential factors that may contribute to the development of a high anion gap metabolic acidosis.42 Health care providers should be cognizant of this potential risk and monitor patients appropriately. In the same FDA drug safety communication, post-marketing cases of urosepsis and pyelonephritis were discussed, leading to the addition of warnings of severe infections to the prescribing information for these products.42
Warnings and precautions: Warnings and precautions for currently available SGLT-2 inhibitors are largely the same, with only a few small exceptions. Table 10 summarizes the warnings and precautions listed in the labels for SGLT-2 inhibitors currently marketed in the U.S.36-38
| Table 10. Warnings and Precautions for Currently Available SGLT-2 Inhibitors36-38 |
| |
Canagliflozin
(Invokana) |
Dapagliflozin
(Farxiga) |
Empagliflozin
(Jardiance) |
| Ketoacidosis |
X |
X |
X |
| Renal impairment/AKI |
X |
X |
X |
| Hyperkalemia |
X |
|
|
| Urosepsis and pyelonephritis |
X |
X |
X |
| Hypoglycemia (when used in combination with SU or insulin) |
X |
X |
X |
| Genital mycotic infections |
X |
X |
X |
| Hypersensitivity reactions |
X |
|
|
| Bone fracture |
X |
|
|
| Increased LDL-C |
X |
X |
X |
| Hypotension |
|
X |
X |
| Bladder cancer |
|
X |
|
| Abbreviations: AKI = acute kidney injury; LDL-C = low-density lipoprotein cholesterol; SGLT-2 = sodium-glucose cotransporter-2; SU = sulfonylurea. |
CURRENT GUIDELINE RECOMMENDATIONS: ROLES OF INCRETIN-BASED THERAPIES AND SGLT-2 INHIBITORS
The use of currently available treatments for T2DM are guided by clinical recommendations from several organizations, including the ADA and the AACE/ACE. There are slight differences between these guidelines, but a key similarity is that they both emphasize diet and lifestyle management as the cornerstone of T2DM treatment plans.3,4
ADA Standards of Medical Care in Diabetes
The 2017 ADA Standards of Medical Care in Diabetes offer the ADA’s general recommendations for the use of antihyperglycemic therapy in T2DM. The guidelines suggest initiating lifestyle interventions in combination with metformin at the time of diagnosis (Figure 3).3 If initial therapy with metformin and lifestyle efforts are not adequate to achieve individualized glycemic targets, the guidelines recommend that patients should progress to dual therapy by adding either another oral agent, basal insulin, or an injectable GLP-1 receptor agonist. The addition of basal insulin to metformin for dual therapy is considered the “highest efficacy” option according to the ADA Standards of Care.3 If A1C goals are not achieved after 3 months of dual therapy, triple therapy is subsequently recommended. For patients not achieving A1C goals after approximately 3 months of triple therapy, combination injectable therapy is the recommended next step. The ADA recommends considering initial dual therapy in individuals with an A1C greater than 9.0%; combination injectable therapy should be considered as initial therapy for patients with a blood glucose of 300 mg/dL or higher, patients with an A1C of 10% or higher, and patients who are markedly symptomatic.3
Figure 3. General Recommendations for Antihyperglycemic Therapy in Type 2 Diabetes Mellitus3
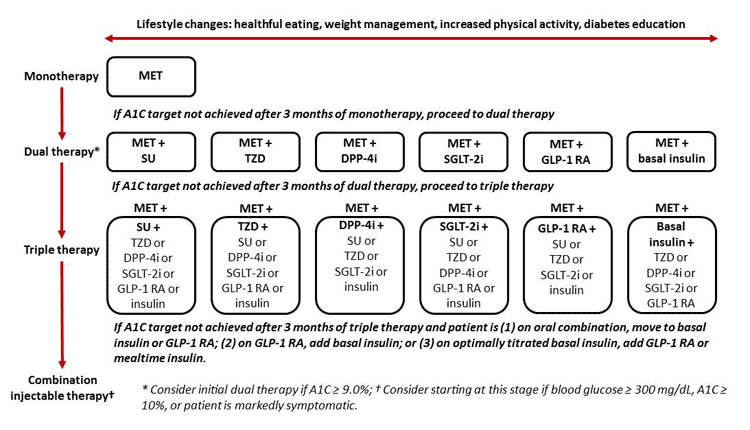
Abbreviations: A1C = hemoglobin A1c; DPP-4i = dipeptidyl peptidase-4 inhibitor;
GLP-1 RA = glucagon-like peptide-1 receptor agonist; MET = metformin;
SGLT-2i = sodium-glucose cotransporter-2 inhibitor; SU = sulfonylurea; TZD = thiazolidinedione.
DPP-4 inhibitors, GLP-1 receptor agonists, and SGLT-2 inhibitors are included as options for combination therapy with metformin for patients who require dual therapy to meet glycemic goals. Likewise, as patients progress and require triple therapy, these medication classes comprise options in combination with a variety of other antihyperglycemic medication classes. Notably, once a patient transitions to combination injectable therapy, the ADA recommends the addition of a GLP-1 receptor agonist for patients who require improved postprandial glycemic control despite basal insulin optimization.3
Empagliflozin (Jardiance) and liraglutide (Victoza) have been shown to reduce cardiovascular and all-cause mortality when added to standard care. As a result, a recommendation was included in the 2017 ADA Standards of Medical Care in Diabetes stating that these agents should be considered in patients with long-standing suboptimally controlled T2DM and established cardiovascular disease.3
The 2017 ADA Standards of Medical Care in Diabetes is readily available at the ADA Web site.
AACE/ACE Comprehensive Type 2 Diabetes Management Algorithm
Like the ADA, the AACE/ACE 2017 Comprehensive Type 2 Diabetes Management Algorithm provides guidance on medication use in T2DM.4 The AACE/ACE management algorithm stratifies antihyperglycemic recommendations according to entry A1C levels. For patients with an A1C less than 7.5%, monotherapy is recommended. The AACE/ACE, in contrast to the ADA, provides several monotherapy options that are recommended in the following order according to the strength of each recommendation: metformin, GLP-1 receptor agonist, SGLT-2 inhibitor, DPP-4 inhibitor, thiazolidinedione, α-glucosidase inhibitor, and an insulin secretagogue (a sulfonylurea or meglitinide).4 For patients with an entry A1C of 7.5% or higher, the algorithm recommends dual therapy consisting of metformin plus an additional antihyperglycemic agent. Again, a variety of options are presented, with GLP-1 receptor agonists, SGLT-2 inhibitors, and DPP-4 inhibitors listed as the top recommendations for use in combination with metformin. Like the ADA, the AACE/ACE algorithm recommends intensification from monotherapy to dual therapy and beyond for patients not achieving glycemic goals after 3 months of therapy with a given antihyperglycemic regimen.4 Interestingly, the AACE/ACE algorithm additionally makes non-insulin recommendations for patients who are optimized on basal insulin and require additional postprandial glycemic control. While the ADA recommends considering a GLP-1 receptor agonist or the addition of mealtime insulin,3 the AACE/ACE algorithm additionally recommends SGLT-2 inhibitors and DPP-4 inhibitors as potential therapies that can be added to basal insulin, if they are not already incorporated into the medication regimen.4
The 2017 AACE/ACE Comprehensive Type 2 Diabetes Management Algorithm is readily available at the AACE Web site.
CONCLUSION
The number of medications approved for the treatment of T2DM continues to expand. Given the complex pathophysiology of T2DM, dual and triple therapy regimens are often needed for patients to meet individualized glycemic goals. Use of DPP-4 inhibitors, GLP-1 receptor agonists, and SGLT-2 inhibitors is on the rise owing, at least in part, to their glycemic benefits, lack of weight gain, and complementary mechanisms of action when used in combination with metformin or other antihyperglycemic medication classes. Additionally, liraglutide (Victoza) and empagliflozin (Jardiance) offer potential cardiovascular benefits, which is important for many patients with T2DM. Use of any of these antihyperglycemic medications is not without risk, however, and the choice to use any of these agents in a patient should consider both potential risks and benefits of therapy.
REFERENCES
- DeFronzo RA. Lilly lecture 1987. The triumvirate: beta cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37(6):667-687.
- DeFronzo RA, Triplitt CL, Abdul-Ghani M, Cersosimo E. Novel agents for the treatment of type 2 diabetes. Diabetes Spectr. 2014;27(2):100-112.
- American Diabetes Association. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2017;40(Suppl 1):S64-S74.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2017 executive summary. Endocr Pract. 2017;23(2):207-238.
- Elrick H, Stimmler L, Hlad CJ Jr, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076-1082.
- Creutzfeldt W. The incretin concept today. Diabetologia. 1979;16(2):75-85.
- Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973;37(5):826-828.
- Bell GI, Santerre RF, Mullenbach GT. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature. 1983;302(5910):716-718.
- Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63(2):492-498.
- Holst JJ, Orskov C, Nielsen OV, Schwartz TW. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 1987;211(2):169-174.
- Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2(8571):1300-1304.
- Wettergren A, Schjoldager B, Mortensen PE, et al. Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci. 1993;38(4):665-673.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696-1705.
- Orskov C, Wettergren A, Holst JJ. Biological effects and metabolic rates of glucagonlike peptide-1 7-36 amide and glucagonlike peptide-1 7-37 in healthy subjects are indistinguishable. Diabetes. 1993;42(5):658-661.
- Campbell RK. Clarifying the role of incretin-based therapies in the treatment of type 2 diabetes mellitus. Clin Ther. 2011;33(5):511-527.
- Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728-742.
- Ahren B. Clinical results of treating type 2 diabetic patients with sitagliptin, vildagliptin or saxagliptin – diabetes control and potential adverse events. Best Pract Res Clin Endocrinol Metab. 2009;23(4):487-498.
- Deacon CF. Therapeutic strategies based on glucagon-like peptide 1. Diabetes. 2004;53(9):2181-2189.
- Januvia [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2017.
- Onglyza [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2017.
- Tradjenta [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2017.
- Nesina [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc.; 2016.
- Neumiller JJ. Incretin-based therapies. Med Clin North Am. 2015;99(1):107-129.
- Byetta [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015.
- Adlyxin [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2016.
- Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc.; 2016.
- Bydureon [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015.
- Tanzeum [package insert]. Wilmington, DE: GlaxoSmithKline LLC; 2016.
- Trulicity [package insert]. Indianapolis, IN: Eli Lilly and Company; 2017.
- McCarty D, Coleman M, Boland CL. Lixisenatide: a new daily GLP-1 agonist for type 2 diabetes management. Ann Pharmacother. 2017;51(5):401-409.
- Shaefer CF Jr. Lixisenatide: a new member of the glucagon-like peptide 1 receptor agonist class of incretin therapies. Clin Diabetes. 2016;34(2):81-85.
- Jendle J, Grunberger G, Blevins T, et al. Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: a comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program. Diabetes Metab Res Rev. 2016;32(8):776-790.
- Wright EM. Renal Na(+)-glucose cotransporters. Am J Physiol Renal Physiol. 2001;280(1):F10-18.
- Neumiller JJ, White JR, Campbell RK. Sodium-glucose co-transport inhibitors: progress and therapeutic potential in type 2 diabetes mellitus. Drugs. 2010;70(4):377-385.
- Bakris GL, Fonseca V, Sharma K, et al. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75(12):1272-1277.
- Invokana [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2017.
- Farxiga [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2017.
- Jardiance [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2016.
- White JR. Sodium glucose cotransporter 2 inhibitors. Med Clin North Am. 2015;99(1):131-143.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159(4):262-274.
- Chao E. SGLT-2 inhibitors: a new mechanism for glycemic control. Clin Diabetes. 2014;32(1):4-11.
- U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. https://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Published December 4, 2015. Accessed April 8, 2017.
Back to Top