
Expired activity
Please go to the PowerPak
homepage and select a course.
Pharmacists' Role in the Comprehensive Care of Heart Failure in Long-Term Care Settings-Article
INTRODUCTION
As the population ages, the number of patients with heart failure (HF) will increase. In 2012, an estimated 6 million Americans were living with HF, and by 2030 this number is projected to increase by 46%.1 The total cost for treating patients with HF will also increase from $30.7 billion in 2012 to $69.7 billion in 2030. The major driving force behind treatment cost is hospitalizations (80% of the cost). In addition to cost and hospitalization, there is a high risk of mortality associated with HF, with 5-year rates of roughly 50%. Annual mortality rates can vary from as low as 2% or 3% to as high as 50% depending upon the population.2 Given the significant morbidity and mortality associated with HF, it is imperative that we effectively treat patients with HF living in long-term care (LTC) settings. This article discusses guideline-directed medical therapy (GDMT) for the treatment of HF, key evaluation and monitoring parameters for therapeutic classes, the challenges of managing patients in LTC settings.
Heart Failure and the Long-Term Care Setting
Long-term care facilities can encompass a variety of settings from “nursing homes” to assisted living or custodial care. LTC facilities are often defined by the level of nursing care provided and reimbursement. For the purpose of this monograph LTC facilities or skilled nursing facilities will be defined as Medicare-
certified post-hospital care units or long-term care facilities that provide at least 16 hours per day of licensed nursing care for 7 days a week.3 Another definition for LTC facilities is one that provides “24-
hour on-site nursing care to persons with complex chronic illnesses or disabilities, and for whom community care provision is no longer feasible.”4 The prevalence of HF in LTC facilities has been estimated to be in the range of 20% to 37%.3-5 A recent prospective cohort study (GOLD-HF) followed 546 newly admitted residents from 42 LTC facilities over a one-year period and the HF prevalence in this study was 21%.4 Morbidity and mortality associated with HF in LTC appear to be significantly higher. In an observational analysis of 15,459 Medicare beneficiaries discharged from the hospital with the diagnosis of HF in 2005 and 2006, 24.1% of patients were admitted to the LTC setting. All-cause 30-day rehospitalization rates were 27% for LTC patients and 23.5% for patients not admitted to LTC setting (P < 0.0001).6 In the GOLD-HF study 31% of HF patients were hospitalized within one year and 42% of HF patients had died within one year, versus 19% of patients without HF (P < 0.0001).4 Other studies have found similar one-year mortality rates (46%–53%).3,6 High morbidity and mortality in HF patients in LTC adds to the challenge and importance of optimizing therapy in these patients.
Heart Failure Patients in Long-Term Care Settings
It is important to tailor management of HF in LTC for the individual patient. Patient characteristics in LTC may differ markedly compared with the general HF population. These patients are typically older (>80 years), have 3 to 5 comorbidities, and take an average of 9 medications.3,4,6 Geriatric syndrome is an important consideration in HF management and may include increased frailty, functional decline, cognitive impairment (delirium), and psychiatric comorbidities.7-9 Complications associated with geriatric syndrome can increase morbidity and mortality in HF patients. Cognitive impairment is common in patients with HF in general (affecting 25% to 75%) and even more common among patients in LTC. One of the concerns is symptom awareness. Careful assessment for signs and symptoms of worsening HF is essential, including less-common signs. Frail elderly patients may present with less-common symptoms of HF such as dyspnea on exertion or orthopnea (Table 1).7 In addition to monitoring for worsening symptoms it is essential to monitor laboratory values for HF decompensation, including:
- Brain natriuretic peptide (BNP) or NT-proB-type brain natriuretic peptide (Pro-BNP) levels – Increase from baseline levels suggests elevated myocardial wall tension due to increased fluid volume and worsening HF.
- Serum creatinine – Increase in levels can suggest either worsening HF or dehydration. Dehydration can also lead to decreased cardiac output and worsening HF.
- Liver function tests – Liver enzymes may increase due to decreased cardiac output and increased fluid volumes.
- Hemodilution – Decrease in hemoglobin, hematocrit, albumin, and/or sodium levels may suggest hypervolemia and need for diuresis.
| Table 1. Signs and Symptoms of Heart Failure7 |
Classic Sign and Symptoms
- Dyspnea
- Orthopnea
- Paroxysmal nocturnal dyspnea
- Fatigue, weakness
- Exercise intolerance
- Edema (ankle)
- Cough
- Weight gain
- Pulmonary rales/crackles
- Loss of appetite
- Increase jugular venous distension
Nonspecific Sign and Symptoms in LTC Patients
- Delirium
- New or worsening cognitive impairment
- Falls
- Sudden functional decline
- Sleep disturbances
- Incontinence, nocturia
- Sacral edema
- Cool extremities
|
Guideline-Directed Medical Therapy for HF in LTC
In the last 35 years a number of large randomized controlled trials have supported development of guideline-directed medical therapy (GDMT) to significantly reduce symptoms, hospitalizations, and mortality risk in patients with HF and reduced ejection fraction (HFrEF).2,10 The primary clinical practice guidelines used to direct care of HF in the U.S. are the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) and the European Society of Cardiology (ESC) guidelines.2,10,11 The ACCF/AHA guideline is organized, in part, according to left ventricular (LV) function status (Table 2) and New York Heart Association (NYHA) classification of HF severity (Table 3). This monograph will focus on management of patients in LTC with HFrEF Stages B and C and HF with preserved ejection fraction (HFpEF).
| Table 2. Heart Failure Definition2,10,11 |
| Heart failure with reduced ejection fraction (HFrEF) |
Heart failure with preserved ejection fraction (HFpEF) |
HFpEF borderline (AHA)
Heart failure with mid-range reduced ejection fraction – HFmrEF (ESC guidelines) |
| LVEF ≤ 40% |
LVEF ≥ 50% |
LVEF 41-49% |
| Most clinical studies performed in this type of patients. |
Also referred to as diastolic failure |
Gray area – characteristics and outcomes appear most similar to HFpEF patients. Still defining |
| Therapies for improving hospitalizations and mortality -DEFINED |
Therapies for improving hospitalizations and mortality – NOT DEFlNED |
Therapies for improving hospitalizations and mortality – NOT DEFlNED |
| Table 3. Heart Failure New York Heart Association (NYHA) Classification and American Heart Association Staging2 |
| NYHA I |
NYHA II |
NYHA III |
NYHA IV |
| No limitation of physical activity. Ordinary physical activity does not cause symptoms of HF. |
Slight limitation of physical activity. Comfortable at rest, but ordinary physical activity results in symptoms of HF. |
Marked limitation of physical activity. Comfortable at rest, but less than ordinary activity causes symptoms of HF. |
Unable to carry on any physical activity without symptoms of HF, or symptoms of HF at rest. |
| Stage A |
Stage B |
Stage C |
Stage D |
| At high risk for HF but without structural heart disease or symptoms of HF |
Structural heart disease, but without signs or symptoms of HF |
Structural heart disease with prior or current symptoms of HF |
Refractory HF requiring specialized interventions |
| NYHA - None |
NYHA I (asymptomatic patients with reduced EF) |
NYHA I to IV |
NYHA IV |
| Treat risk factors (i.e., hypertension, lipids) |
Guideline directed medication therapy |
Guideline directed medication therapy. |
LVAD, transplant, palliative consideration |
| HF=heart failure; EF=ejection fraction; NYHA=New York Heart Association; LVAD=left ventricular assist device. |
GDMT should be considered for every patient presenting with HF to reduce the risk of hospitalization and mortality. A number of therapeutic classes are utilized for the management of patients with HFrEF (Tables 4 and 5). For initial management of patients with Stage B HFrEF, the two therapeutic classes that must be initially considered are angiotensin-converting enzyme (ACE) inhibitors and beta blockers (Table 5).2 In patients with Stage C HF, the 4 therapeutic classes that should be initially considered are diuretics, ACE inhibitors, beta blockers, and aldosterone antagonists.2,10 Additional therapies may need to be considered for individual patients (Table 5).10
Although GDMT was not necessarily derived from studies of geriatric patients or those residing in LTC settings, GDMT is recommended for consideration in all patients with HF. A number of studies demonstrate benefits of GDMT such as ACE inhibitors, digoxin, and beta blockers have benefits in geriatric patients.12-14 Challenges for individualizing therapy in LTC patients include:7-9
- Higher rates of cognitive impairment
- Higher risk of falls
- Pharmacokinetic changes (e.g., decreased drug clearance, change in volume of distribution and drug absorption)
- Pharmacodynamic changes (e.g., orthostatic risk, SA/AV node conduction defects, increased drug sensitivity)
- Increased risk for hypotension, kidney injury, electrolyte abnormalities
- Polypharmacy
| Table 4. Therapeutic Classes for Treatment of HFrEF |
| Drug Class |
Morbidity |
Mortality |
| Diuretics |
↓symptoms |
? |
| ACE inhibitors |
↓hospitalizations and symptoms |
↓ mortality |
| Angiotensin receptor blockers |
↓hospitalizations and symptoms |
↓ mortality |
| Angiotensin receptor/neprilysin inhibitor |
↓hospitalizations and symptoms |
↓ mortality |
| Beta adrenergic receptor blockers |
↓hospitalizations and symptoms |
↓ mortality |
| Aldosterone antagonists |
↓hospitalizations and symptoms |
↓ mortality |
| Vasodilators (hydralazine and isosorbide dinitrate) |
↓hospitalizations and symptoms |
↓ mortality |
| If channel blocker (ivabradine) |
↓hospitalizations |
neutral |
| Inotrope (digoxin) |
↓hospitalizations and symptoms |
neutral |
| Omega-3 polyunsaturated fatty acid |
|
↓mortality |
| Table 5. Heart Failure Treatment for Patients with Reduced Ejection Fraction2,10 |
| Drug/Drug Class |
ACC/AHA Stage B |
ACC/AHA Stage C |
ACC/AHA Stage D |
| ACE inhibitors* |
Yes (or ARB) |
Yes (or ARB) |
Yes |
| Angiotensin receptor-neprilysin inhibitor |
No |
Yes |
Yes |
| β-adrenergic blockers |
Yes |
Yes |
Yes |
Aldosterone
receptor antagonist* |
No |
Consider |
Yes |
| Diuretics |
No |
Yes |
Yes |
| Hydralazine/nitrate |
No |
Consider |
Consider |
| Ivabradine |
No |
Consider |
No |
| Digoxin |
No |
Consider |
Consider |
| Omega 3 polyunsaturated fatty acid |
Consider |
Consider |
Consider |
The individual patient’s treatment goals should be considered. GDMT has the potential to improve symptoms, reduce hospitalizations, improve mortality, reverse HF progression (to some degree), and promote physiological changes such as cardiac remodeling (Table 4). For some people, the goals of cardiac remodeling and decreased mortality risk may need to be balanced with improving symptoms and quality of life. That is, feeling better may be a better individualized goal than extending life expectancy, particularly for a person in LTC. Extensive review of GDMT can be found elsewhere.2,10,11 This article will highlight key concepts for recommended drug classes in the LTC setting. When evaluating patients for GDMT, key concepts to consider are:
- All patients with HFrEF should be considered for therapy with either an ACE inhibitor, angiotensin receptor blocker (ARB) or angiotensin receptor/neprilysin inhibitor (ARNI); beta blocker (BB); and aldosterone antagonist (AA) to reduce morbidity and mortality
- Diuretic therapy is essential to manage fluid accumulation
- For patients who tolerate ACE/ARB, consideration should be made to switch to ARNI
- Titrate patients to target doses if appropriate
- Being on lower doses of multiple GDMT rather than a high dose of a single GDMT is preferred
- Patients should be evaluated for other therapies including ivabradine, hydralazine and isosorbide dinitrate, digoxin, and omega-3 polyunsaturated fatty acid (PUFA), where appropriate
Diuretics
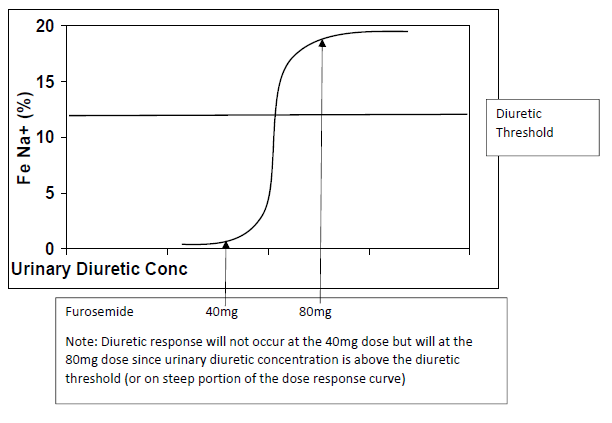
Effective diuretic therapy is the important approach to manage volume overload and subsequently keep patients with HF of the hospital. In addition, patients feel better when they are euvolemic. Diuretics are recommended therapy for all Stage C HFrEF patients to manage fluid accumulation.2 In the LTC setting, monitoring weight and net urine output is important. Once patients are stable and euvolemic the goal is to maintain dry weight and to balance fluid intake and output. The amount of fluid intake should be similar to fluid output over a 24-hour period. To help guide and recommend effective diuretic therapy it is important to understand the pharmacokinetic and pharmacodynamic relationship with loop diuretics.15 Perhaps the most important concept for loop diuretics is that the diuretic threshold needs to be reached. As shown in Figure 1, loop diuretics have a steep dose-response curve.15 In order to achieve significant diuresis, urinary concentrations of diuretics need to reach the upper portion of the curve. Clinically, this means that high doses of diuretics may be needed to induce diuresis, especially in patients with poor renal function. The common starting and ceiling doses of loop diuretics are shown in Table 6.15 The most important assessment for diuretic therapy is to determine what happens after diuretic administration. If there is a change in response—such as a decrease in urine output or if urine output is not increased over a 6-hour period (usually 1 or 2 hours)—patients need be evaluated for “diuretic resistance.” Diuretic resistance can be attributed to a number of factors, one of which is the loop diuretic dose. As mentioned, patients need to achieve the diuretic threshold to achieve a good response. This concept is especially important in patients on low doses of loop diuretics, for whom increasing the dose (e.g., doubling dose of furosemide) should be a considered. Making sure patients are at their diuretic threshold is especially important during transitions from the hospital back to the LTC setting. If an effective dose is not established, re-hospitalization is likely.16
| Table 6. Common Dosing Guidelines for Loop Diuretics15 |
| Usual daily dose (PO) |
| Furosemide |
Bumetanide |
Torsemide |
| 20–160 mg/d |
0.5–4 mg/d |
10–80 mg/d |
Ceiling dosea
Normal renal function
CLCR 20–50 mL/min
CLCR <20 mL/min |
80–160 mg
160 mg
400 mg |
1–2 mg
2 mg
8–10 |
20–40 mg
40 mg
100 mg |
| Bioavailability |
10%–100%
Average: 50% |
80%–90% |
80%–100% |
| Affected by food |
Yes |
Yes |
No |
Bumetanide conversion ~ 40:1 furosemide to bumetanide
Torsemide conv ~ 2 - 4:1 furosemide to torsemide |
| Figure 1. Dose Response Curve for Loop Diuretics15 |
 |
Another factor to consider is a change in absorption that may occur, especially with furosemide, when patients begin to decompensate. Specifically, as the gut becomes edematous there is delayed absorption resulting in a decrease in Cmax. If this occurs, the diuretic threshold will not be reached. In these cases, the diuretic dosage needs to be increased or the patient should be switched from furosemide to a diuretic with better bioavailability and more consistent absorption, such as torsemide.17
A third factor to consider is diet. Patients who are not on a low to moderate sodium diet (which may be difficult to achieve in some LTC settings) may have symptoms despite having a good diuretic response. For example, if a patient has an increase in sodium intake, a single daily dose of diuretic cannot sufficiently excrete the excess sodium, so fluid retention may occur. For patients where sodium restriction is difficult, increasing the frequency of dosing (assuming the dose is at the patient’s diuretic threshold) can manage the excess fluid.
Another consideration for decreased diuretic effectiveness is drug interactions, especially with nonsteroidal anti-inflammatory drugs (NSAIDs), which can lead to decreased renal function and sodium retention. The final consideration is the induction of distal tubular hypertrophy, which may occur in patients receiving chronic therapy with loop diuretics. It is hypothesized that cells in the distal tubular section will hypertrophy in response to exposure to high levels of urinary sodium caused by the administration of loop diuretics.18 These enlarged cells become more efficient in reabsorbing sodium, such that the effects of loop diuretics in blocking sodium reabsorption in the loop of Henle is negated by enhanced reabsorption of sodium in the distal segment. To overcome the effects of distal tubular hypertrophy a combination of a loop diuretic and a thiazide-type diuretic is recommended to increase urinary output. The effect of a thiazide diuretic to increase diuretic responsiveness appears to be a class effect. Hydrochlorothiazide (usual dose range 25 to 100 mg once or twice daily) and metolazone (usual dose range 2.5 mg to 10 mg once daily) are thiazide diuretics commonly used in combination with loop diuretics. Hydrochlorothiazide and metolazone differ in duration of action; the long duration of action of metolazone may make it more effective than hydrochlorothiazide in some patients. However, if patient is over-diuresed, then the longer duration of action for metolazone may become detrimental. Key concepts for overcoming diuretic resistance are outlined in Table 7.19 Key concepts for the use of diuretics in HF are outlined below:
Evaluation and Monitoring, Diuretics
- Evaluate for symptom relief (e.g., shortness of breath, orthopnea)
- Weight and signs of fluid retention (e.g., edema). In general, weight gain > 2 lbs overnight or 5 lbs in a week is a cause for concern and possible intervention.
- Urine output: Monitor urine output to include acute response following diuretic dosing and 24-hour net urine output. In general, the goal is to balance fluid in and out once a patient is euvolemic.
- Electrolytes: In particular, monitor for hypokalemia, hypomagnesemia, and hyponatremia.
- Blood pressure: If patients experience hypotension, evaluate for dehydration.
- Renal function: Diuretics, especially with aggressive therapy may increase serum creatinine levels. Increase in serum creatinine levels may also be seen in patients who become dehydrated.
- Uric acid: Monitor for hyperuricemia/gout
- Sodium restriction if feasible (< 2–3 grams/day)
| Table 7. Key Concepts for Overcoming "Diuretic Resistance" |
- Evaluate dietary sodium level, maintain low sodium diet if feasible.
- Discontinue interacting drugs (e.g., NSAIDs)
- Make sure drug is administered at threshold dose
- Increase dose if not at threshold
- Increase frequency if at threshold (e.g., change twice daily dosing to three times daily)
- ↓ absorption? – change to torsemide or bumetanide
- Add thiazide-type diuretic if distal tubular hypertrophy is suspected.
|
ACE Inhibitors, Angiotensin Receptor Blockers, and Angiotensin Receptor/Neprilysin Inhibitors
For Stage B patients, guidelines recommend use of ACE inhibitors to prevent symptomatic HF and to reduce mortality.2 In patients who cannot tolerate an ACE inhibitor (most likely due to cough), ARBs can be utilized unless contraindicated. For patients with Stage C HFrEF, the updated 2017 guidelines recommend that either an ACE inhibitor, ARB, or ARNI be used to reduce morbidity and mortality in conjunction with other GDMT (beta blockers and aldosterone antagonists).10 In addition, there are limited data suggesting that ACE inhibitor therapy may improve cognitive function to some degree. The guidelines further state that in patients with chronic symptomatic HFrEF who tolerate either an ACE inhibitor or ARB, the ARNI should be used in place of these drugs (ACE inhibitor or ARB) to further reduce morbidity and mortality.10
In general, the ability of both ACE inhibitors and ARBs to reduce morbidity or mortality by blocking the neurohormonal effects of the renin-angiotensin system appears to be a class effect. Starting doses, target doses, and doses achieved in clinical trials where appropriate are shown in Table 8. Commonly used ACE inhibitors include lisinopril and enalapril. When beginning therapy, start with low doses and titrate increasing dose levels at approximately 2- to 4-week intervals.
With regard to ARNI, the currently available agent in this class is a combination of sacubitril and valsartan. Dosage and administration for this agent is outlined in Table 8. Hypotension may be more common with ARNI (vs. ACE inhibitor or ARB) based on the results of PARADIGM-HF trial which compared sacubitril/valsartan to enalapril.20 Careful blood pressure monitoring and symptom assessment is warranted, especially in higher-risk patients.
| Table 8. Dosing Guidelines2,10,11 |
| Drug |
Initial Daily Dose |
Target or Common Dose |
Mean Dose Achieved in Clinical Trials |
| ACE inhibitors |
| Captopril |
6.25 mg tid |
50 mg tid |
122.7 mg/d |
| Enalapril |
2.5 mg bid |
10 mg bid |
16.6 mg/d |
| Fosinopril |
5 to 10 mg qd |
40 – 80 mg qd |
|
| Lisinopril |
2.5 to 5 mg qd |
20 mg qd |
32.5 to 35.0 mg/d |
| Perindopril |
2 mg qd |
8 to 16 mg qd |
|
| Quinapril |
5 mg bid |
10 -20 mg bid |
|
| Ramipril |
1.25 to 2.5 qd |
10 mg qd |
|
| Trandolapril |
1 mg qd |
4 mg qd |
|
| Angiotensin receptor blockers |
| Candesartan |
4 to 8 mg qd |
32 mg qd |
24 mg/d |
| Losartan |
25 to 50 mg qd |
150 mg qd |
129 mg/d |
| Valsartan |
20 - 40 mg bid |
160 mg bid |
254 mg/d |
| Angiotensin receptor neprilysin inhibitor |
| Sacubitril/valsartan |
49/51 mg bid
24/26mg bid for patients not currently taking ACE inhibitor or ARB or in patients with severe renal impairment (eGFR < 30 mL/min/1.73 m)2 |
97/103 mg bid |
375 mg/d |
| Aldosterone antagonists |
| Eplerenone |
25 mg qd |
50 mg qd |
42.6 mg/d |
| Spironolactone |
12.5 to 25 mg qd |
25 mg qd |
26 mg/d |
| Beta blockers |
| Bisoprolol |
1.25 mg qd |
10 mg qd |
8.6 mg/d |
| Carvedilol |
3.125 mg bid |
25mg bid |
37 mg/d |
| Carvedilol CR |
10 mg qd |
80 mg qd |
|
| Metoprolol succinate extended release (metoprolol CR/XL) |
12.5 to 25 mg qd |
200 mg qd |
159 mg/d (447) |
| Vasodilator |
|
|
|
| Fixed dose combination |
37.5 mg hydralazine/
20 mg isosorbide dinitrate tid |
75 mg hydralazine/
40 mg isosorbide dinitrate tid |
~142 mg hydralazine/76 mg isosorbide dinitrate |
| Hydralazine and isosorbide dinitrate |
Hydralazine: 25 to 50 mg, tid or qid and isosorbide dinitrate:
20 to 30 mg tid or qid |
Hydralazine 75 mg tid to qid and isosorbide dinitrate 40 mg daily tid to qid |
|
| If channel blocker |
|
|
|
| Ivabradine |
5 mg bid |
7.5 mg bid |
6.5 mg bid |
| Inotrope |
|
|
|
| Digoxin |
0.125 to 0.25 mg qd |
Target to digoxin concentration range of 0.5 to 0.9 ng/mL |
|
| Polyunsaturated fatty acid (PUFA) |
|
|
|
| Omega 3 PUFA |
1 gram qd of omega-3 PUFA (850 mg to 882 mg EPA/DHA) |
1 gram qd of omega-3 PUFA (850 mg to 882 mg EPA/DHA) |
1 gram qd of omega-3 PUFA (850 mg to 882 mg EPA/DHA) |
ACE=angiotensin converting enzyme; tid=three times daily; bid=two times daily; qd=once daily;
qid=four times daily; eGFR=estimated glomerular filtration rate |
ARNI, the most recently approved HF therapy, is a combination of sacubitril and the ARB valsartan.10 Sacubitril is an inhibitor of neprilysin, a neutral endopeptidase responsible for the breakdown of peptides, including beneficial vasoactive peptides such as brain natriuretic peptide (BNP). BNP is a counter-regulatory vasoactive peptide that promotes vasodilation and natriuresis. It is hypothesized that blocking the effects of angiotensin II while increasing counter-regulatory hormone levels (i.e., BNP), is superior to blocking only angiotensin II. The PARADIGM-HF trial tested this hypothesis by comparing enalapril to sacubitril/valsartan in HF patients already treated with an ACE inhibitor.20 The results of demonstrated significant improvement in the primary endpoint of reduced cardiovascular death and HF hospitalizations by approximately 20%. Individual composite endpoints also demonstrated significant reductions in cardiovascular death (approximately 20%) and HF hospitalizations (21%). Treatment with sacubitril/valsartan was associated with a lower incidence of cough, serum creatinine elevation, and hyperkalemia relative to enalapril. However, the ARNI was associated with increased symptomatic hypotension. The majority of patients were receiving diuretics, beta blockers, and aldosterone antagonists. Based on the results of this trial, reduced mortality and decrease hospitalizations, ARNI therapy is now recommended for treatment of Stage C HFrEF patients.10
Evaluation and Monitoring, ACE, ARB, ARNI
- Reach target doses when appropriate. However, reaching target doses may not be possible in LTC patients. Lower doses will also provide benefit.
- Blood pressure. Evaluate for symptomatic hypotension, use caution as systolic blood pressure decreases to < 90mmHG. In older and frail patients in the LTC setting avoiding low blood pressure (< 100–110 mmHg systolic blood pressure) may be reasonable. In patients with lower blood pressure switching to an ARNI may provide additional challenges and need to be carefully considered whether or not to switch patients.
- Monitor potassium at the start of therapy, upon dosage change, and routinely. Hyperkalemia can occur, especially when used in combination with an aldosterone antagonist and in patients with poor or declining renal function.
- Renal function. Minor increase in serum creatinine may occur at start of therapy or change in dose – often no action is necessary and may resolve.
- Cough. Often can resolve over a few months, if persistent and bothersome, can switch to ARB.
- Sacubitril/valsartan may decrease Cmax for furosemide. Monitor for diuretic effectiveness upon initiation or up-titration.
- Biomarkers in patients receiving sacubitril/valsartan: measure NT-pro BNP; BNP levels will be elevated following sacubitril/valsartan therapy due to inhibition of neprilysin.
Beta Blockers
Guidelines recommend that beta blockers should be used in all Stage B patients with HFrEF to block sympathetic effects on the myocardium and minimize HF symptoms.2 For patients with Stage C HFrEF, guidelines particularly recommend the use of one of three beta blockers: bisoprolol, carvedilol, or metoprolol succinate.2 Unlike ACE inhibitors and ARBs, guidelines do not recognize a class effect for beta blockers, so only those agents with evidenced-based benefits are recommended for Stage C HFrEF. The benefits of beta-blocker therapy in HF suggest that inhibition of multiple pathways (in this case, different neurohormones) is involved in reducing progression of HF.21
Starting doses, target doses, and doses of beta blockers achieved in clinical trials are shown in Table 8. When beginning or up-titrating beta-blocker therapy, patients must be stable and relatively euvolemic. Patients should be started on the recommended initial dose and titrated upwards in 2- to 4-week intervals. More typical up-titration with beta blockers occurs over months. When starting beta-blocker therapy or increasing the dose, some patients may experience worsening symptoms of HF for a period of time. Symptoms include fluid retention and increase in fatigue. It may take weeks or months for patients to feel better. In some cases, up-titration may not be possible. In these cases, the patient should return to the previously tolerated dose. The decompensation that may occur during these times is due to inhibition of the sympathetic drive to the heart, resulting in a decrease in cardiac output. Over time (weeks to months) as the heart adapts to less sympathetic drive (norepinephrine), the heart will remodel and become more efficient, with an improvement in cardiac output and ejection fraction and improvement in outcomes.21
Evaluation and Monitoring, Beta Blockers
- Must start low and titrate upward no more frequently than every 2 weeks. May take months to up-titrate.
- Patients need to be clinically stable and euvolemic before starting or increasing dose.
- Reach target doses when appropriate. However, in LTC patients reaching target doses may not be possible. In these cases, lower doses will also provide benefit and patients should be on therapy.
- In some patients, upon initiation or increased dose, HF sign and symptoms (dyspnea, fatigue, and edema) may worsen. This is often transient and can be managed by increasing the diuretic dose or frequency. If persistent, beta blocker dose may need to be reduced. In some LTC patients where the priority is maximizing quality of life the initiation or up-titration may not be a priority and in some cases stopping therapy may be appropriate.
- Symptom improvement may take months
- Heart rate, PR intervals (monitor for bradycardia, AV block)
- Blood pressure (hypotension)
- HF patients need to be on metoprolol succinate and not metoprolol tartrate
Mineralocorticoid Receptor Antagonists (Aldosterone Receptor Antagonists)
For patients with HFrEF Stage C who are symptomatic (NYHA Class II-IV) and who have EF ≤35%, guidelines recommend the addition of an aldosterone receptor antagonist (also called mineralocorticoid receptor antagonist or MRA) to reduce morbidity and mortality.2 In patients with a history of acute myocardial infarction and EF ≤40% who develop symptoms of HF or who have a history of diabetes mellitus, the addition of an aldosterone receptor antagonist is recommended.2 Similar to ACE inhibitors and beta blockers, the approach to inhibiting aldosterone is based on the concept that the pathophysiology of HF is driven, in part, by neurohormonal activation including angiotensin II, norepinephrine, and aldosterone. Starting doses, target doses, and doses achieved in clinical trials are shown in Table 8. When beginning therapy, renal function and potassium levels need to be considered. Guidelines state that “serum creatinine should be ≤ 2.5 mg/dL in men or ≤ 2.0 mg/dL in women (or estimated glomerular filtration rate [eGFR] >30 mL/min/1.73m2) and potassium should be < 5.0 mEq/L.”2 Patients must be monitored closely for changes in renal function and potassium upon starting therapy and during dose titration and then on a routine basis. Up-titration can occur over 4 to 8weeks or longer.
Evaluation and Monitoring, Aldosterone Receptor Antagonist
- Potassium should be checked upon initiation and rechecked within 2 to 3 days and again after 7 days.2,11
- Additional potassium monitoring will be patient-dependent but should occur at least monthly for the first 3 months and every 3 months afterwards or sooner if warranted.2,11
- If patient is on potassium supplements, re-evaluate need and/or consider discontinuing.
- Can up-titrate dose after 4 to 8 weeks or longer.
- Increase diuresis. May need to adjust diuretic therapy in some patients.
- Renal function. Increase in serum creatinine may occur. May be associated with increased diuresis seen with aldosterone antagonists. May need to reduce loop diuretic therapy.
- Monitor blood pressure for hypotension
- Gynecomastia may occur with spironolactone (consider switching to eplerenone).
- Contraindications - Serum creatinine > 2.5 mg/dL in men or > 2.0 mg/dL in women (or eGFR <30 mL/min/1.73m²), and/or potassium > 5.0 mEq/L.
Hydralazine and Isosorbide Dinitrate
For patients with Stage C HFrEF (NYHA III–IV) who are self-described as African American, guidelines recommend optimal therapy with ACE inhibitors and beta blockers and consideration of hydralazine plus isosorbide dinitrate to reduce morbidity and mortality.2 In addition, for patients with current or prior symptomatic HFrEF who cannot be given an ACE inhibitor or ARB due to drug intolerance, a combination of hydralazine and isosorbide dinitrate can be useful.2 Starting doses, target doses, and doses achieved in clinical trials are shown in Table 8. Patients may be prescribed either a fixed-dose combination of hydralazine and isosorbide dinitrate or single agents.
The A-HeFT trial was conducted based on a post-hoc analysis of previous studies showing benefits of hydralazine and isosorbide dinitrate in African Americans with HF. The trial evaluated a fixed-dose combination hydralazine and isosorbide dinitrate on top of standard therapy (ACE inhibitor/ARB and beta blocker) in 1,050 self-identified African American patients.22 The results demonstrated a 43% reduction in mortality beyond standard therapies compared to placebo. Based on these findings, guidelines recommend that every patient self-identifying as African American should be considered for hydralazine and isosorbide dinitrate in addition to optimized standard therapy of ACE inhibitors/ARB and beta blocker.
Evaluation and Monitoring, Hydralazine and Isosorbide Dinitrate
- Blood pressure (hypotension, postural hypotension, dizziness)
- Headaches (can be common)
- Heart rate (tachycardia)
- Systemic lupus erythematosus-like symptoms (including glomerulonephritis)
- Although measures of quality of life were improved, headaches and dizziness were common adverse effects. If adverse effects are persistent need to consider balance between quality of life and mortality benefit in appropriate LTC patients.
Ivabradine
Ivabradine can be beneficial in reducing HF-related hospitalizations for Stage C patients with HFrEF (EF≤ 35%) and NYHA class II–III receiving standard therapy (including a beta blocker at maximum tolerated doses unless contraindicated).10 Other indications for ivabradine include sinus rhythm with a heart rate of >70 bpm at rest. Starting doses, target doses, and doses achieved in clinical trials are shown in Table 8. If after initiation and stabilization the heart rate is > 60 bpm, the dose may be increased to 7.5 mg twice daily. If after initiation the heart rate decreases to < 50 bpm, the dose should be decreased to 2.5 mg twice daily. If the dose is already at 2.5 mg then the medication should be discontinued.
Elevated heart rate is associated with poor outcomes in several cardiovascular conditions including HF. High heart rates can lead to increased oxygen demand, oxidative stress, and ischemia. Based on these findings, ivabradine was evaluated in the SHIFT trial to determine if lowering heart rate in patients with HF with elevated heart rates would be beneficial.23 Ivabradine inhibits the If current in the sinoatrial node causing a decrease in the slope for diastolic depolarization leading to reduced heart rate. Importantly, ivabradine does not alter myocardial contractility or blood pressure. The SHIFT trial evaluated over 6,000 HF patients with heart rate ≥ 70 bpm and demonstrated a significant 18% reduction (P < 0.0001) in the primary endpoint of CV death and HF hospitalizations compared to placebo.[19] However, the primary endpoint was driven mainly by HF hospitalizations (26% reduction, P < 0.0001). Based on this finding, the recommendation is for reduction in hospitalizations only. In the SHIFT trial the greatest benefit was seen in patients who had the greatest reduction in heart rate (> 10 bpm). This finding suggest that up-titration to maximum dose may be warranted to achieve the greatest reduction in heart rate.
Evaluation and Monitoring, Ivabradine
- Heart rate: treatment must be reduced or stopped if the resting heart rate decreases persistently below 50 bpm or if symptoms of bradycardia occur.
- If a patient develops persistent/continuous atrial fibrillation during therapy with ivabradine, the drug should be stopped.
- Luminous phenomena (phosphenes – visual color spots). Onset is generally within the first 2 months and often transient. Phosphenes may occur in approximately 2% of patients receiving the 5 and 2.5 mg doses.
- In patients >75 years old, a lower starting dose should be considered (2.5 mg twice daily).
- Drug interactions
- Pharmacodynamic interactions
- QT prolonging drugs
- SA node blockers - beta blockers, non-dihydropyridine calcium channel blockers, digoxin, amiodarone
- Pharmacokinetic interactions
- CYP3A4 inhibitors – azole antifungals, HIV protease inhibitors, grapefruit juice, non-dihydropyridine calcium channel blockers, etc.
- CYP3A4 inducers – rifampicin, barbiturates, phenytoin, Hypericum perforatum (St John’s Wort)
- Contraindications/Precautions
- Blood pressure less than 90/50 mmHg
- Sick sinus syndrome, sinoatrial block or 3rd degree AV block, unless a functioning demand pacemaker is present. Importantly, older patients often have impaired SA/AV node conduction (sick-sinus syndrome), monitoring for excessive bradycardia is important when using this therapy.
- Resting heart rate less than 60 bpm prior to treatment
- Severe hepatic impairment
- Pacemaker dependence (heart rate maintained exclusively by the pacemaker)
Digoxin
For Stage C patients with reduced ejection fraction, digoxin may be beneficial in decreasing hospitalizations for HF.2 Today, digoxin is not often considered as first-line therapy. In most cases, digoxin is often considered in patients who have been aggressively treated to guideline recommendations (ACE inhibitor/ARB, beta blocker, aldosterone receptor antagonist, diuretic) but still remain symptomatic. Dosing for digoxin is shown in Table 8. Therapy is commonly initiated and maintained at a dose of 0.125 to 0.25 mg daily. Lower doses (0.125 mg QD or every other day) are often initiated in geriatric patients and patients with poor renal function. The maintenance dose is determined by plasma levels. Plasma concentration in the range of 0.5 to 0.9 ng/mL is suggested. Given digoxin’s long half-life (approximately 24–48 hours, up to 5 days in patients with poor renal function) the dose should not be increased more frequently than every few weeks.
Digoxin at lower plasma levels has a mild inotropic effect (inhibits sodium/potassium ATPase) and improves baroreceptor sensitivity which can improve cardiac output. Improving cardiac output can make patients feel better. In addition, there are limited data suggesting that digoxin therapy may improve cognitive function to some degree. The major outcome trial for digoxin is the Digitalis Investigation Group (Dig Trial).24 In this trial of over 7,000 patients, digoxin was found to have a neutral effect on mortality and a significant 25% reduction in hospitalizations as compared to placebo in well-
treated HF patients. In a secondary analysis of the Dig Trial, digoxin concentrations between 0.5 to 0.9 ng/mL were shown to have the greatest benefit in regard to outcomes.25 Based on this secondary analysis it is usually recommended to monitor digoxin levels and maintain concentrations between 0.5 to 0.9 ng/mL.
Evaluation and Monitoring, Digoxin
- Digoxin concentrations (range 0.5 to 0.9 ng/mL), should be routinely monitored for LTC patients
- Symptom improvement
- Digoxin toxicity – nausea/vomiting, visual disturbances, confusion
- Heart rate and PR interval (bradycardia and AV-block)
- Potassium levels (low K+ may enhance digoxin toxicity)
- Renal function - change in function can alter digoxin clearance, need to monitor renal function on a frequent basis, especially as patients decompensate
- Drug interactions – P-glycoprotein inhibitors can raise digoxin levels and pharmacodynamic interactions with other drugs that may affect SA/AV node conduction (e.g. amiodarone, diltiazem, beta blockers)
Omega-3 Polyunsaturated Fatty Acid (Fish Oil)
For Stage C patients with reduced ejection fraction and NYHA class II-IV symptoms, omega-3 polyunsaturated fatty acid (PUFA) is reasonable to use as adjunct therapy to reduce mortality and cardiovascular hospitalizations.2 Recommended dosing is 1 gram daily of omega-3 PUFA (850 mg to 882 mg EPA/DHA). Dosing recommendations comes from the GISSI-HF study which randomized nearly 7,000 patients with HF NYHA class II-IV to either placebo or 1 gram of omega-3 PUFA.26 The results showed a decrease in mortality and hospitalizations as compared to placebo. The mechanism behind omega-3 PUFA benefit is not clear but may be secondary to proposed anti-inflammatory effects. Although omega-3 PUFA is relatively safe, caution may be advised in patients with bleeding complications or taking warfarin or antiplatelet therapy. Patients may complain of a fishy aftertaste. Taking with meals or changing products may help.
Guideline Based Treatment Recommendations for HFpEF and HF mid-range ejection fraction (HFmrEF)
In contrast to HFrEF, there are no randomized control trials of GDMT that show improved mortality or reduced hospitalizations in patients with preserved or mid-range EF.2,10,11 At this time the cornerstone of management is treating cardiovascular risk factors such as hypertension and dyslipidemia. Recommendations for controlling hypertension and lipids should be based on current clinical guidelines. ACE inhibitors, ARBs and beta blockers can be recommended for treatment of hypertension in HFpEF patients. Diuretic therapy may potentially be beneficial for symptom relief due to volume overload and is often needed. ARBs may also be considered to decrease hospitalizations in HFpEF. This recommendation is based in part from data from the CHARM-Preserved trial which suggested that candesartan may reduce hospitalizations.27 A recent HFpEF study with spironolactone (TOPCAT) in over 3,000 patients showed a 17% decrease in hospitalizations for HF (P=0.042) as compared to placebo.28 However, there was no significant difference in the primary outcome (composite endpoint of cardiovascular mortality, aborted cardiac arrest, or hospitalizations) between spironolactone and placebo in the study. These results may support the use of spironolactone in treating hypertension in appropriate HFpEF patients.10 The ongoing PARAGON-HF trial will examine ARNI therapy compared with valsartan in patients with preserved LVEF, with the composite endpoint of CV death and total (first and recurrent) HF hospitalizations.29
Pharmacists’ Roles and Challenges in Managing Patients with HF in the LTC Setting
The pharmacist’s roles for managing HF patients in the LTC setting may include:
- Recommendations for individualized GDMT
- Therapeutic drug monitoring
- Medication reconciliation
- Documentation of process of care
- Prevention of adverse events and medication errors
Time Management
Pharmacists often encounter significant challenges when evaluating and monitoring patients with HF in the LTC setting. Among these are time limitations. Patients are seen on average once a month. LTC pharmacists need to be able to rapidly triage patients with HF, meaning rapid assessment of key monitoring parameters. Ideally, monitoring parameters should be assessed for the past week and compared with the results from the previous visit. Evaluating both short-term (current week) and long-
term (prior 30 days) gives both acute and chronic assessments. Although the pharmacist’s time limitations may make chronic assessment difficult, these assessments are important to detect subtle changes in patient’s status.
Critical laboratory values (e.g., BNP, renal function, electrolytes, drug levels) present another challenge in LTC. Lab values may not be readily available or current for a given patient and may take 24 hours to be reported. In addition, there may be gaps in documentation assessing a patient’s HF symptoms or therapeutic effectiveness (e.g., weight, urine output, blood pressure, and heart rate). Having this documentation done, and done correctly, in the LTC setting can be a challenge, so ongoing staff education is key. Pharmacists can provide assessment tools that can be modified to the LTC setting to help staff assess patients with HF and develop documentation templates with key monitoring parameters that can be filled in at time of patient assessment.30-32
Transitions of Care
Transitions of care are a particularly important time to manage and monitor patients with HF. However, the pharmacist may not be on site when a patient transitions from the hospital to the LTC setting. Soon after the transition, daily monitoring for fluid retention is essential. Patients are often discharged from the hospital without being completely diuresed and the effectiveness of oral diuretic therapy is not clearly established. If the patient’s weight goes up, symptoms of fluid retention increase, and/or the patient is not diuresing, a change in diuretic therapy needs to be implemented as soon as possible. Again, this is where education of nursing and medical staff is key, especially in regard to managing diuretic therapy. Many physicians do not understand how to manage diuretic therapy and diuretic resistance. Since decisions about diuretics often need to be made when a pharmacist in not on site, staff education is essential. In addition to education, developing a diuretic protocol or using an existing one can help guide management in this acute period.33,34
Polypharmacy
Patients with HF, especially older patients, usually have multiple comorbidities and are on a large number of medications. Medication reconciliation is an important tool for the pharmacist to ensure that medication regimens are appropriate and to evaluate for drug interactions. For patients with HF, there are a number of medications that should be avoided or used with caution. These include:
- Nonsteroidal anti-inflammatory drugs – May retain sodium and water and may worsen renal function
- Steroid therapy – May retain sodium and water and promote edema o Verapamil, nifedipine, diltiazem – Potential negative inotropic effects and edema (nifedipine)
- Tricyclic antidepressants, citalopram – May cause orthostatic hypotension, and may prolong QT interval and arrhythmia potential
- Thiazolidinediones – May cause fluid retention and increase risk of HF, should not be prescribed in patients with HF
- Metformin – Metformin can be safely used in chronic HF patients; however, renal function needs to be monitored. Metformin is contraindicated in patients with an eGFR below 30 mL/minute/1.73 m².
- Fluoxetine and paroxetine – Both drugs are strong CYP2D inhibitors and may significantly affect the metabolism of carvedilol and metoprolol, leading to higher serum concentrations and greater beta-blocking effects.
- Antacids – Evaluate for sodium content and magnesium content, especially in patients with impaired renal function
- Laxatives – Monitor for dehydration and electrolyte disturbances o SGLT2 inhibitors (e.g., empagliflozin) – Drugs in this class may have beneficial cardiovascular effects including reduction in HF hospitalizations. However, these drugs may increase diuresis, evaluate for dehydration may need to adjust diuretic dosing.
- Acetylcholinesterase inhibitors (e.g., donepezil) – May cause bradycardia and may have pharmacodynamics interactions with other drugs that affect SA and AV node conduction (e.g., beta blockers).
Overall, the LTC pharmacist has unique challenges in managing and helping patients with HF in the LTC setting. Creating a standard triage approach may help with the time constraints faced by the pharmacist. In addition, education and the use of a variety of tool sets, templates, and protocols can help assist in the management of the HF patient.
REFERENCES
- Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606-619.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e327.
- Jurgens CY, Goodlin S, Dolansky M, et al. Heart Failure Management in Skilled Nursing Facilities. A Scientific Statement From the American Heart Association and the Heart Failure Society of America. J Card Fail 2015;21:263-299.
- Foebel AD, Heckman GA, Ji K, et al. Heart Failure – Related Mortality and Hospitalizations in the Year Following Admission to a Long Term Care Facility: The Geriatric Outcomes and Longitudinal Decline in Heart Failure (GOLD-HF) Study. J Card Fail 2013;19:468-477.
- Daamen MA, Schols JM, Jaarsma T, Hamers JP. Prevalence of heart failure in nursing homes: a systematic literature review. Scand J Caring Sci 2010;24:202-8.
- Allen LA, Hernandez AF, Peterson ED, et al. Discharge to a skilled nursing facility and subsequent clinical outcomes among older patients hospitalized for heart failure. Circ Heart Fail 2011;4:293-300.
- Heckman GA, Boscart VM, McKelvie RS. Management considerations in the care of elderly heart failure patients in long-term care facilities. Future Cardiol 2014;10:563-77.
- Vetrano DL, Lattanzio F, Martone AM, et al. Treating Heart Failure in Older and Oldest Patients. Curr Pharm Des 2015;21:1659-44.
- Bader F, Atallah B, Brennan LF, et al. Heart failure in the elderly: ten peculiar management considerations. Heart Fail Rev 2017;22:219-228.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. April 28, 2017. Available at: http://circ.ahajournals.org/content/136/6/e137.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129-2200.
- Sanam K, Bhatia V, Bajaj NS, et al. Renin-Angiotensin System Inhibition and Lower 30-Day All-Cause Readmission in Medicare Beneficiaries with Heart Failure. Am J Med 2016;129:1067-73.
- Sheriff HM, Thogaripally MR, Panjrath G, et al. Digoxin and 30-Day All-Cause Readmission in Long-Term Care Residents Hospitalized for Heart Failure. J Am Med Dir Assoc 2017 May 11. pii: S1525-8610(17)30187-1. doi: 10.1016/j.jamda.2017.03.016. [Epub ahead of print].
- Bhatia V, Bajaj NS, Sanam K, et al. Beta-blocker use and 30-day All-cause Readmission in Medicare Beneficiaries with Systolic Heart Failure. Am J Med 2015;128:715-21.
- Brater DC. Pharmacology of diuretics. Am J Med Sci. 2000;319:38-50.
- Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol. 2013;61:391-403.
- Bleske BE, Welage LS, Kramer WG, Nicklas JM. Pharmacokinetics of torsemide in patients with decompensated and compensated congestive heart failure. J Clin Pharmacol. 1998;38:708-714.
- Kim GH. Long-term adaptation of renal ion transporters to chronic diuretic treatment. Am J Nephrol. 2004 Nov-Dec;24(6):595-605.
- De Bruyne LK. Mechanisms and management of diuretic resistance in congestive heart failure. Postgrad Med J. 2003 May;79(931):268-271.
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004.
- Kotecha D, Manzano L, Altman DG, et al. Beta-Blockers in Heart Failure Collaborative Group. Individual patient data meta-analysis of beta-blockers in heart failure: rationale and design. Syst Rev. 2013 Jan 18;2:7.
- Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004; 351:2049-2057.
- Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875-885.
- The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997 ;336:525-533.
- Adams KF Jr, Patterson JH, Gattis WA, et al. Relationship of serum digoxin concentration to mortality and morbidity in women in the digitalis investigation group trial: a retrospective analysis. J Am Coll Cardiol. 2005 Aug 2;46:497-504.
- GISSI-HF investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223-1230.
- Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction. Lancet. 2003;362:777–781.
- Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014; 370:1383-1392.
- Solomon SD, Rizkala AR, Gong J, et al. Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON-HF Trial. JACC Heart Fail. 2017 Jul;5(7):471-482.
- Bleske BE, Dillman NO, Cornelius D, et al. Heart failure assessment as the community pharmacy level: a feasibility pilot study. J Am Pharm Assoc. 2014;54(6):634-41.
- American Heart Association. Get With the Guidelines® – Heart Failure Clinical Tools. http://www.heart.org/HEARTORG/
- Agency for Healthcare Quality and Research (AHRQ). Red-Yellow-Green Congestive Heart Failure (CHF) Tool. Updated 2008. Available at: https://innovations.ahrq.gov/qualitytools/red-yellow-green-congestive-heart-failure-chf-tool.
- Veilleux RP, Wight JN, Cannon A, et al. Home Diuretic Protocol for Heart Failure: Partnering with Home Health to Improve Outcomes and Reduce Readmissions. Perm J 2014;18:44-48.
- http://www.kcmetrophysicians.com/images/Guidelines/Home_Health_Diuretic_Protocol-Standing_Order.pdf
Back to Top